Introduction to Cerebral Angiography and Its Role in Modern Diagnostic Imaging
Unlike most parts of this website, this page is primarily intended neither for the patient, nor for an angiographic trainee / practitioner. Its target is a medical professional, or a well-informed layman, who is already familiar with capabilities and indications for noninvasive cerebrovascular imaging, as exemplified by MR and CT techniques. Within this ever growing segment of professionals, the role of cerebral (and spinal) angiography may perhaps be better defined, through illustration of its anatomical and functional capabilities. Finally, comparative analysis of catheter and non-invasive imaging techniques often rewards the reader with a more comprehensive, functional approach to evaluation of vascular imaging in general.
Throughout this presentation, a few points will be repeatedly emphasized:
1) Angiography is a lumen-based imaging technique. Unlike CT and MR, there is less information available on the anatomy of the vessel outside the lumen
2) Circulation is a dynamic state. Angiography affords unique capabilities of evaluating dynamic and functional anatomy of cerebral and spinal circulation
3) The spatial and temporal resolution of angiography is, and will remain, far greater than that of non-invasive imaging, due to intrinsic limitations of MR and CT techniques
Technique
Angiographic images are acquired through contrast injection of the vessel of interest. Like most arterial angiograhic procedures, intra-arterial access is obtained by placement of a catheter ( a hollow plastic tube), most often, into the superficial femoral artery. It is an ideal vessel for access, since it is large, superficial, compressible, and not responsible for supply of a vital organ. From the SFA, the catheter is navigated, under x-ray guidance, into the descending aorta, and from there into the great vessels. A variety of catheters and wires are available for safe catheterization of appropriate vessels. Needless to say, angiographic safety and diagnostic yield are extremely operator-dependent. It is usually not necessary to place the catheter into the brain vessels themselves; rather, the catheter is maintained within a vessel in the neck. An x-ray dye, usually a non-ionic, near-isosmolar x-ray contrast agent, is then injected through the catheter and carried by blood flow into the vessels of the brain. As the x-ray dye (the same dye as used for CT angiography) passes through the arteries, capillaries, and veins of the brain, a series of x-ray images are made, usually by two cameras located at the front and on the side of the patient’s head. The frequency of image acquisition can be varied, as directed by necessity to obtain dynamic information while minimizing radiation exposure. Typical cerebral angiography involves catheterization of both carotid arteries and at least one vertebral artery, though exact protocol depends on the indication. Diagnostic angiogrpahy is usually performed awake or under mild sedation (the inside of blood vessels has no nerve endings, and therefore the patient does not, for most part, feel the cathetheter once it is inside the body). Local anesthesia (typically lidocaine) is administered at the groin prior to cannulation of the SFA. For most patients, this is all that is required. The advantage of performing angiography in awake state is that the patient may be given instructions in anticipation of particular diagnostic maneuvers, and is better able to hold still during injections, which produces higher quality images. The actual injection of contrast is not painful, but rather perceived by most people as a warm sensation in the distribution of the selected vessel. Other sensations, such as flashes of lights behind one or both eyes, intermittent dizziness, or taste changes, may be experienced depending on the vessel of interest. Most diagnostic angiograms last less than 1 hour of actual catheter time, but may vary widely based on indication, vascular health, and other considerations. At conclusion of the study, the catheter is removed from the groin and hemostasis achieved by direct manual compression or deployment of a vascular closure device. Subsequent recovery depends primarily on the particular method of closing the groin puncture site.
Cerebral angiography is an invasive technique. Its risks include stroke, infection, bleeding, renal issues, access site (groin) hematomas, and others. The risks are highly institution-specific, and their estimates vary widely in the literature. The risks of any procedure should be weighed against potential benefits and possible alternatives to the procedure in question. Finally, the perception of risk is highly individual-specific.
Images
Angiographic images consist of two-dimensional snapshots of contrast dye transit through the cerebral vasculature. Several images of the head are acquired immediately prior to injection of contrast, which then serve as “mask” images. This mask image of the skull prior to dye injection is then subtracted from one made during dye injection, so that all osseous and soft tissue structures are subtracted out, and only the dye within the vessels is visible. In this way, both “subtracted” and “native” images can be generated. Most angiographic images consist of discrete arterial, capillary, and venous phases. The transit time of contrast from arteries to arterioles to capillaries to venules to veins of the brain depends on the patient’s blood pressure, cardiac output, intracranial pressure, and any mechanical impedance to contrast flow. Generally, it takes about 4 seconds for the dye to move from arteries to veins. Thus, contrast injection duration of 1-2 seconds allows for adequate separation of arterial and venous phases, so that the by the time dye reaches the veins, there is no longer contrast still present in the arteries to confuse the picture. An appreciation of normal contrast flow allows for detection of pathologic states where such considerations are altered.
INDICATIONS
Both pathologic and practical considerations play a role in deciding whether a cerebral angiogram might be considered. Factors apart from disease itself include local practice preference, angiographic availability and angiographer experience, and presence of non-invasive capabilities such as multislice CT or modern MRI equipment. For example, dynamic MRA or CTA may obviate need for angiography in some cases, whereas proximity to a high-volume, experienced angiograhic center suggests a lower threshold for referral, should angiography be indicated. At our institution, common indications for angiography include:
1 ) Cerebral aneurysm — institutional practice varies, with many surgeons now operating based on CTA or less often MRA data alone. Our practice is to obtain a diagnostic angiogram prior to consideration of aneurysm treatment, endovascular or surgical.
2 ) Brain Arteriovenous Malformation (AVM) — pretty much all AVMs visualized on MRI or CT are then evaluated by catheter angiography.
3 ) Dural Arteriovenous Fisula — CT and MR are still quite limited in their capability to detect fisulas (dynamic MRA/CTA is changing this, but in my opinion are not yet sensitive and reliable and safe enough[from CTA radiation exposure standpoint]).
4 ) Cryptogenic Intracerebral Hemorrhage — patients with atypical demographics, unusual hemorrhage locations, and other potentially non-standard situations should be considered for angiography — small fistulas, AVMs, venous thromboses, mycotic aneurysms, are among etiologies which may not be adequately excluded by CT or MR techniques. A particular kind of common cryptogenic hemorrhage is CTA-negative subarachnoid hemorrhage. At our institution, all subarachnoid hemorrhage patients with no etiology found on CT angiography are referred for catheter angiography. Diagnostic yield in these cases varies with hemorrhage pattern and other factors, but on the whole at least 10% of patients are thus found to have a reason for their bleeding.
5 ) Dissections — many cervical carotid and vertebral dissections can now be adequately evaluated with CT and MR. Most dissections do not lead to a neurologic event, such as stroke, and might come to medical attention as a result of pain. In our practice, any dissection with a neurologic event, such as TIA or stroke, is indication for angiogrpahic evaluation — see below. Another reason may be a high-grade dissection with suspicion of inadequate collaterals based on CT or MR techniques — evaluation of collateral potential based on CT or MR requires expert knowledge of neurovascular anatomy, which usually implies consultation with a highly experienced neuroradiologist, vascular neurologist, or vascular neurosurgeon.
6 ) High-grade intracranial stenosis — at least requires a consultation with a neuro-angiographer. The issues of intracranial stenosis are complex, both in terms of pathophysiology and adequate management. Practitioners dealing with intracranial stenoses should be familiar with results of the SAMPRIS trial, which demonstrated overall superiority of medical management over intracranial stenting for patients with symptomatic intracranial stenosis. Individual patients may, nevertheless, benefit from an angiographic evaluation based on unique considerations.
7 ) Acute stroke — not technically a referral for angiography, but rather for angiography and stroke treatment, such as thrombolysis or thrombectomy (clot removal). Recent substantial advances in mechanical clot retrieval have greatly increased the technical success of thrombectomy. Translating this into clinical improvement is a complex process which requires public education, perfection of triage and logistics, etc. Ultimately, most patients with ischemic stroke are, unfortunately, still not eligible for interventional stroke treatment or intravenous t-PA.
8 ) Cerebral vasculitis — remains a difficult diagnosis to make. Multiple subcortical strokes are the hallmark of cerebral vasculitis, either primary or as part of a systemic disorder, such as Lupus. Referral depends on practice patterns of local rheumatologists, neurologists, and other physicians dealing with this difficult disease. It is possible to have a normal angiogram and still suffer from cerebral vasculitis, so that a normal angiogram does not exclude the diagnosis. On the other hand, if vasculitis treatment will be offered regardless of angiographic findings, there may be no reason to perform the angiogram, given that compelling evidence for vasculitis exists based on other considerations.
9 ) Preoperative Tumor Embolization — also not technically an angiogram, but rather angiogram and embolization. Vascular neoplasms such as meningiomas, renal cell carcinomas, glomus tumors, JNAs, among others, can be de-vascularized by particle embolization prior to surgical resection to limit intraoperative blood loss and improve the overall success and safety of subsequent surgery.
10 ) Cerebral venous thrombosis — a treacherous condition, often requiring high level of suspicion, which is most often made based on non-invasive imaging such as CT and MR. Suspected cases of cortical venous thrombosis or those considered for catheter – based thrombolysis require an angiogram.
Additional procedures coupled with cerebral angiography include Wada activation testing (usually performed for various surgical considerations), venous sinus sampling (pituitary issues), venous sinus stenosis (occasionally associated with pseudotumor cerebri, and possibly requiring treatment), among others.
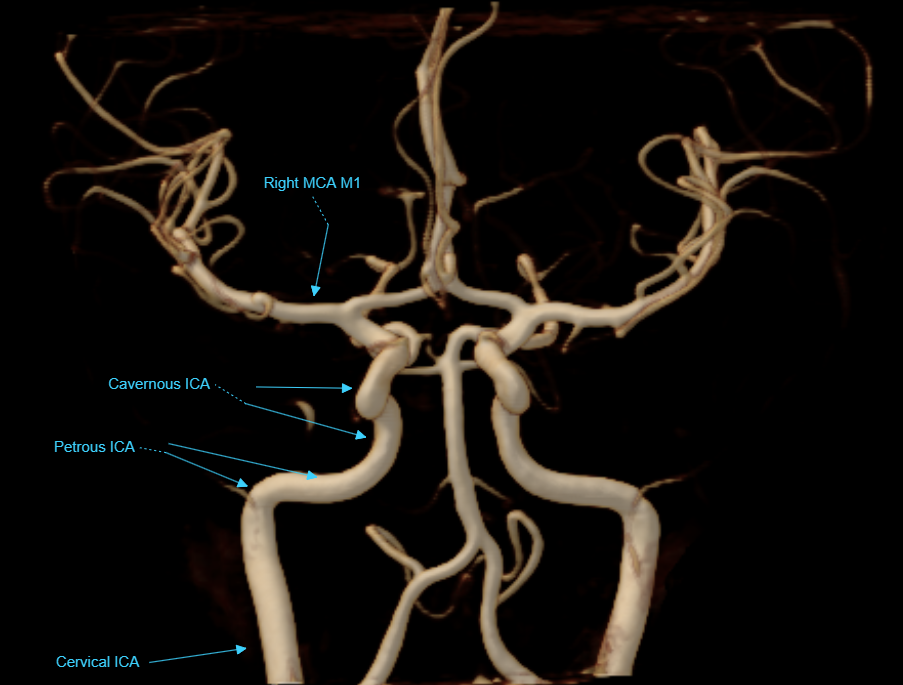
Vascular Angiographic Anatomy
This section, again, is not intended as an anatomical tutorial of cerebral vasculature — other sections of this website, particularly the “Anatomy and Variants” portion, and countless other internet and print sources, exist for this purpose. Instead, it serves as illustration of ways in which angiographic anatomical information may be different (and thus more or less “accurate”) from that of other techniques known to the reader.
As mentioned above, angiography is a lumen-based method. What you see is dye inside vessel lumen.
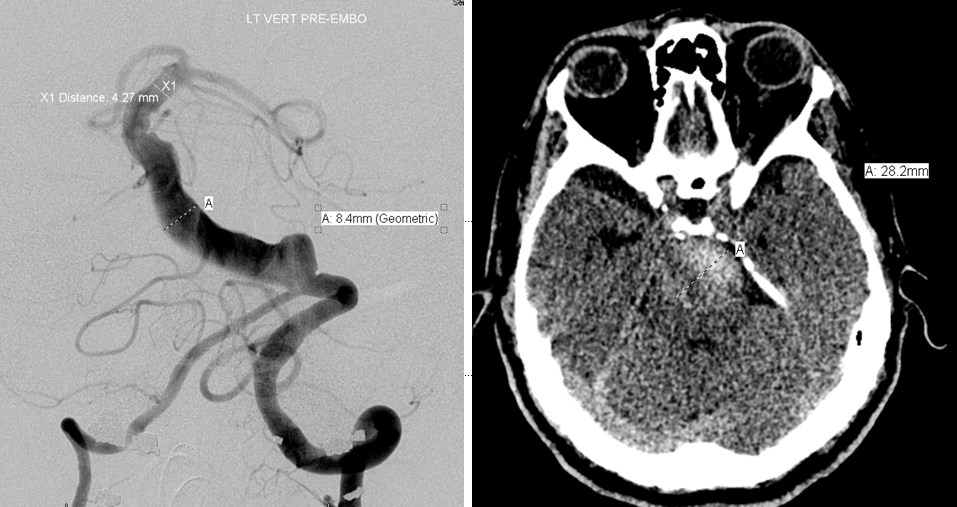
CT and angiographic discrepancy in open lumen and total size of a partially thrombosed fusiform basilar aneurysm. The patient presented with symptoms of brainstem mass effect and obstructive hydrocephalus.
T2 MRI and angiographic right ICA frontal and lateral projection views; both studies were obtained within 1 month of each other. The patient presented with progressive right-sided visual loss.
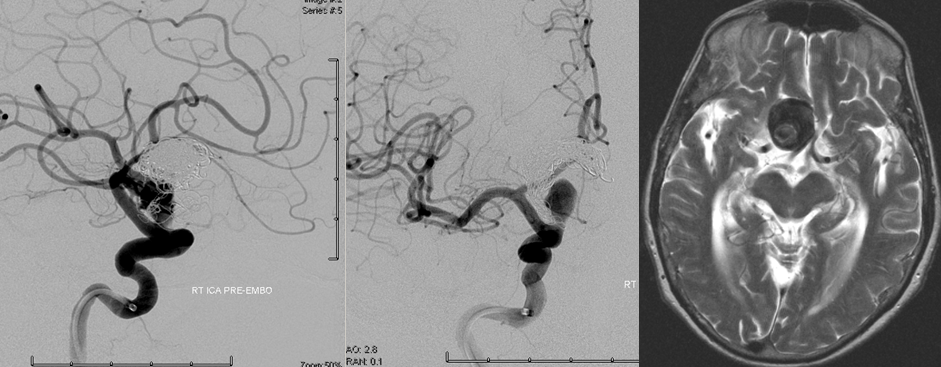
Complex terminal ICA aneurysm, status post repeat coiling and multiple recurrences, in a patient presenting wtih visual loss.
Dynamic capability / Time/ Temporal resolution
Angiography is a dynamic technique, capable of imaging the transit of contrast through the arteriovenous circulation in real time, with a temporal (time) resolution of up to 30 frames / second — similar to the now old analog movies with their 24 frames/second. This is extremely useful when evaluating lesions that are characterized by abnormal flow patterns. For example, an arteriovenous malformation (AVM) or arteriovenous fistula are short circuits between arteries and veins, whereby arterial blood directly flows into the venous system. This is best seen by a dynamic technique, such as angiography (more below on dynamic CTA and MRA).
In this patient, an AVM was suspected based on this MRI, with the draining vein shown by the blue arrow
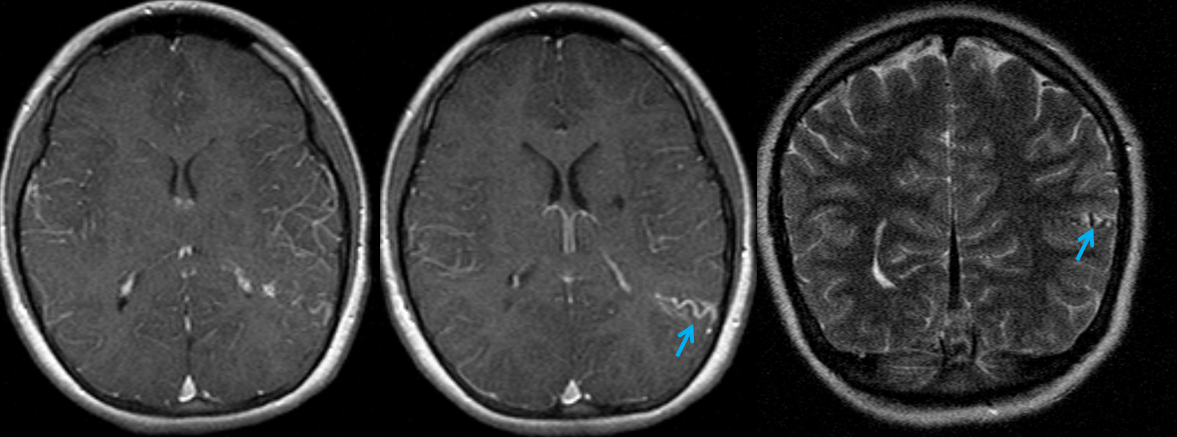
CTA did not resolve the question, in this instance
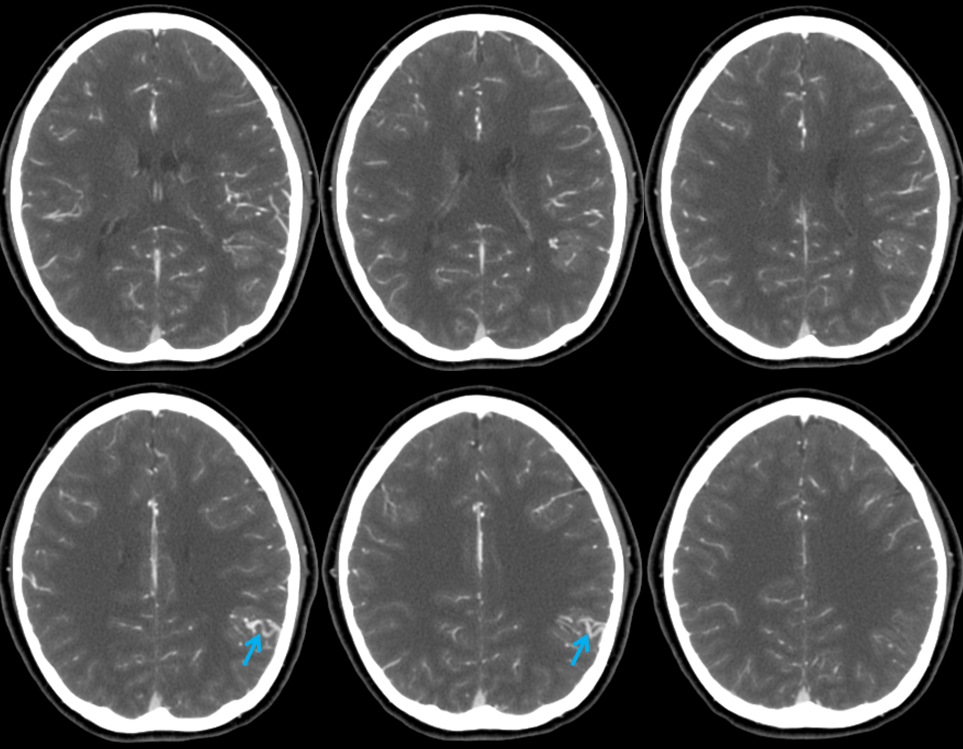
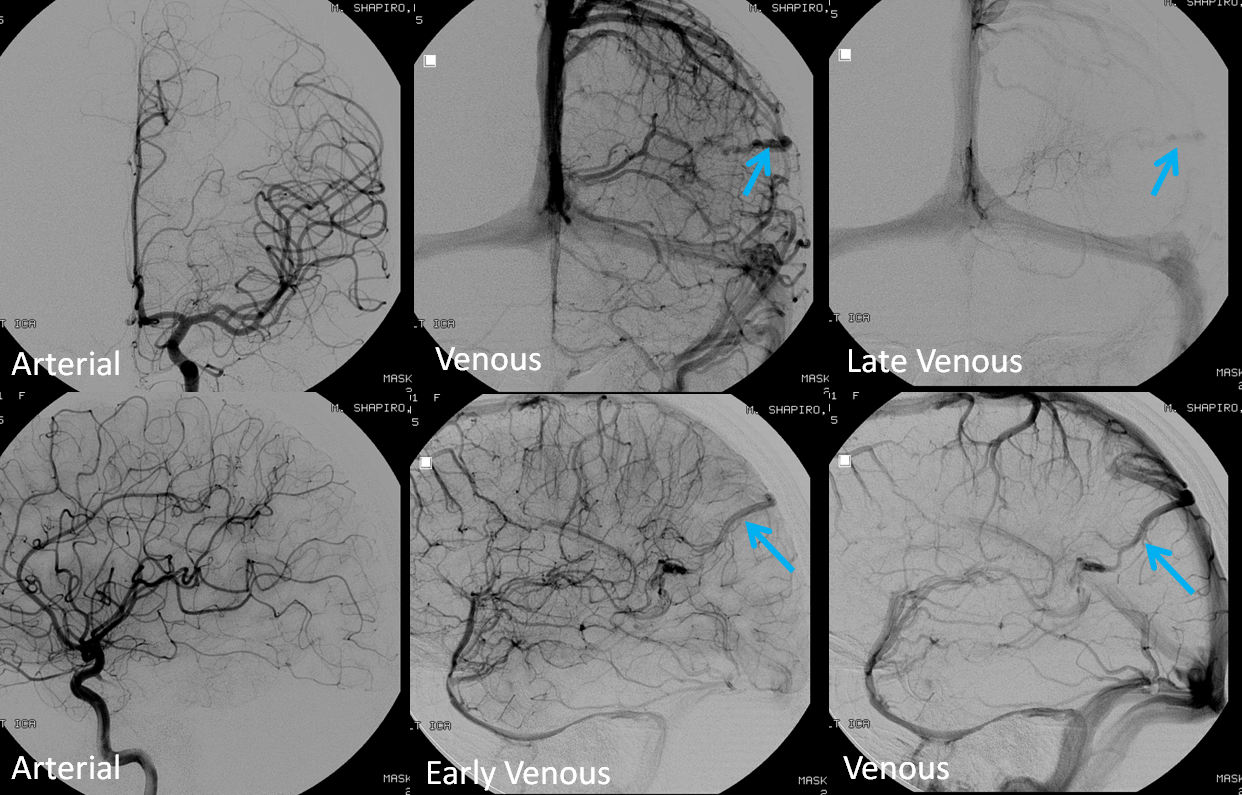
The angiogram below shows that there vein is not seen in the arterial phase — which means there is no “shunt”. This proves that the lesion is not an AVM, but a Developmental Venous Anomaly (DVA)
Spatial resolution and Functional Capability
Catheter angiography continues to enjoy superior spatial resolution, when compared to CT and MR techniques. Secondly, its capacity to demonstrate functional, hemodynamic significance of a given anatomical disposition is unmatched. The following case serves as illustration.
The 50+ patient presented with momentary right-sided weakness, which quickly resolved, followed several days later by dysarthria, which remained. MRI and MRA were obtained.
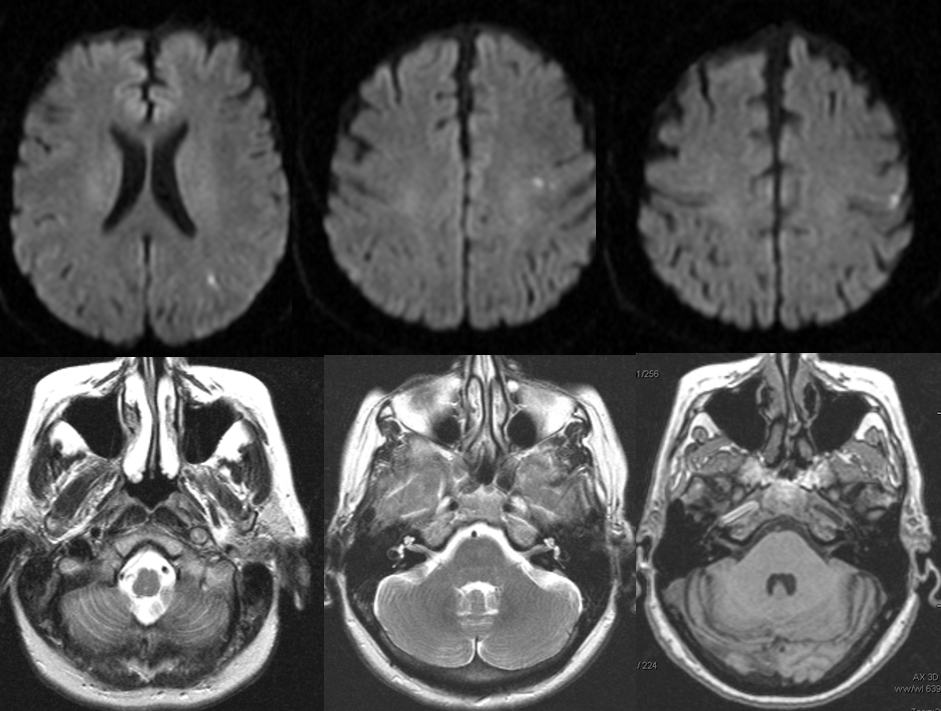
Diffusion-weighted images (top), demonstrating foci of acute ischemia in the left MCA distribution. T2 and T-1 weighted images, showing near-complete obliteration of petrous carotid flow void (left image), preservation of flow void in the horizontal petrous segment (middle image), and lack of flow-related enhancement on 3-D T1 weighted image (MP-RAGE). These image imply:
– most likely etiology is dissection of the left ICA with severe secondary stenosis
– the lumen must be patent (not occluded), since horizontal petrous segment flow void is preserved (and no functional collateral is present to re-constitute the petrous carotid from below)
– flow in the horizontal petrous ICA, while present, is slow, as flow-related enhancement is diminished on T1 study (right image)
– in this way, much functional information can be gathered from static MRI images
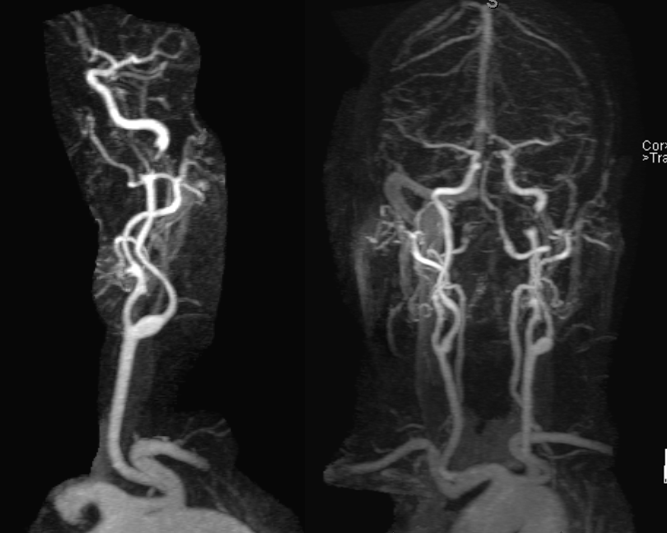
Severe stenosis / occlusion (we know it is stenosis, as above) of the distal cervical left ICA. This is an example of cervical dissection with associated neurologic manifestation. Catheter angiography is indicated, in our opinion.
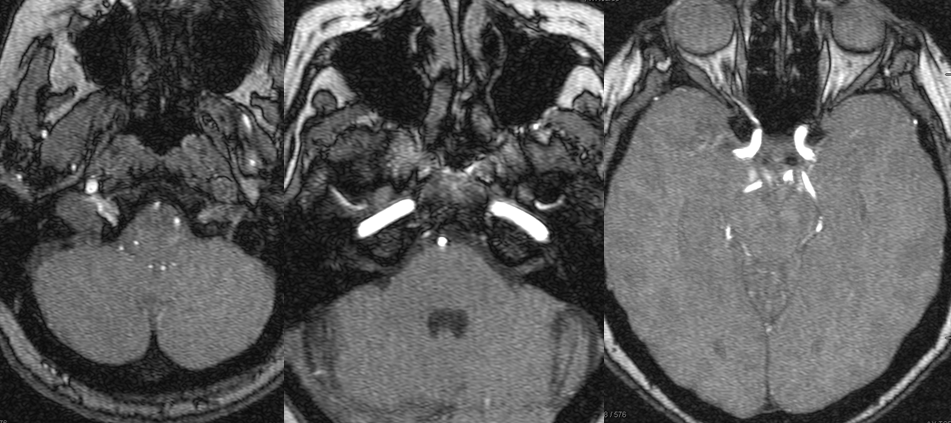
MRA (3T) showing preserved antegrade horizontal petrous flow (middle image). The ophthalmic artery is not visualized on the right image. The likely cause is reversal of ophthalmic artery flow.
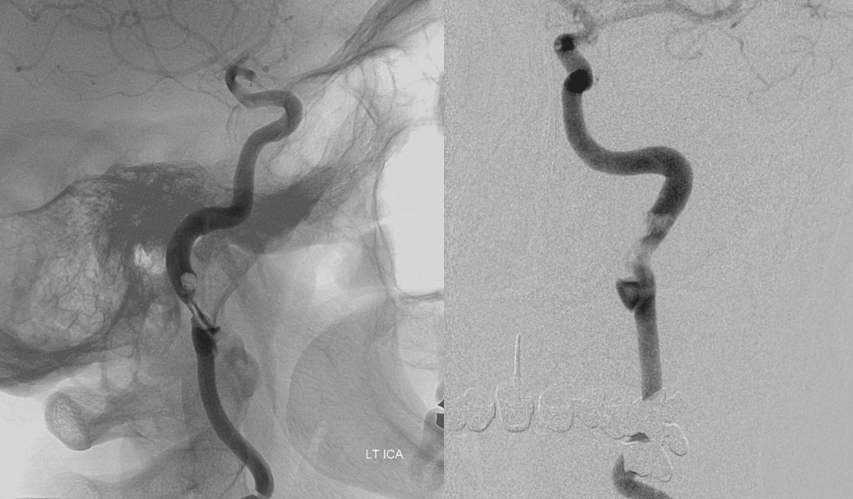
Frontal and lateral projection Lt ICA views, showing a dissection flap, severe luminal narrowing, and actual thrombus (fluffy filling defect within the ICA immediately above the dissection) sitting above the dissected segment, and most likely source of patient’s embolic events. The clot does not embolize “en toto” because of dissection-related limitation in distal ICA flow. Sequential frames at 2FPS actually demonstrate the thrombus to be mobile, with each heartbeat, within the parent artery. In visualizing this thrombus and its tenuous hold on the ICA, the superior spatial and temporal resolution of angiography is shown.
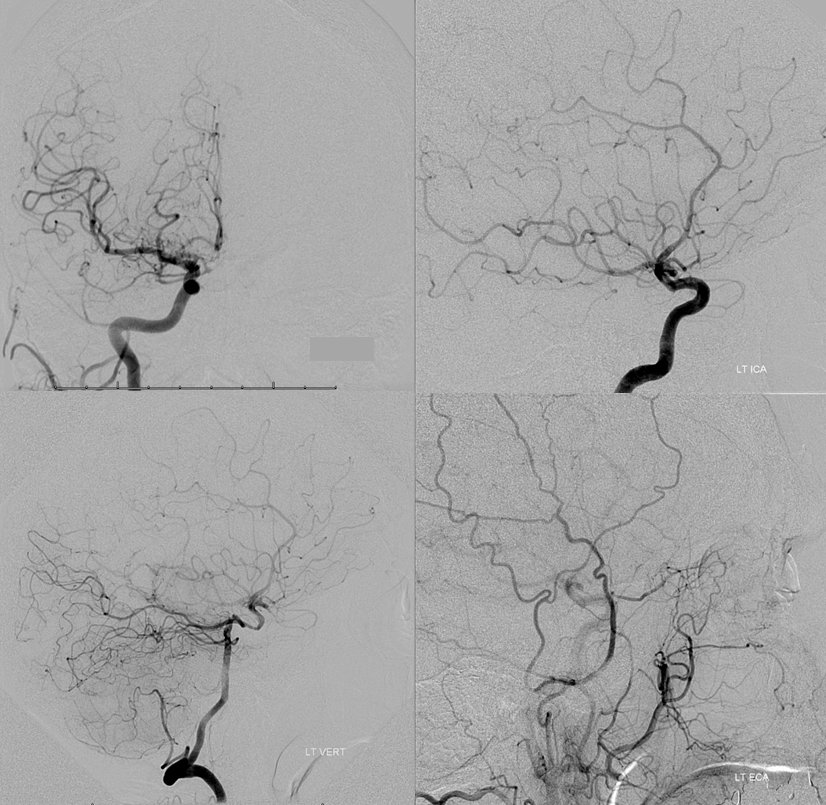
What is the risk to left hemisphere, should left ICA close? What is the status of collateral circulation, in other words. This is where angiography is indispensable. Right ICA injection (top left) shows a hypoplastic ACOM. Left ICA lateral projection injection (top right) shows absence of ophthalmic artery, and overall good filling of the left hemisphere vascular territory thru the dissection. Lateral view left vert injection (bottom left) shows retrograde PCOM flow into the left ACA/MCA territory, as required in setting of left ICA compromise. Notice foci of radiolucency within the left ICA distal to the ophthalmic segment, due to unopacified blood retrograde inflow from the ophthalmic. Left external carotid injection shows retrograde flow into the ophthalmic artery via the anterior deep temporal artery. For in-depth discussion of collateral circulation, see Collateral Circulation section.
Functional significance of neoplastic blood supply
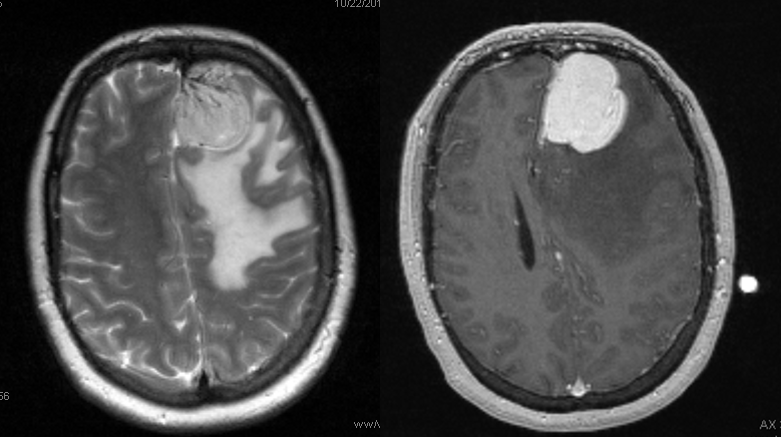
A meningioma, no doubt. Much surrounding edema, which may or may not imply invasion of the brain, according to radiology sources. What it does imply, I think pretty definitively, is pial invasion.
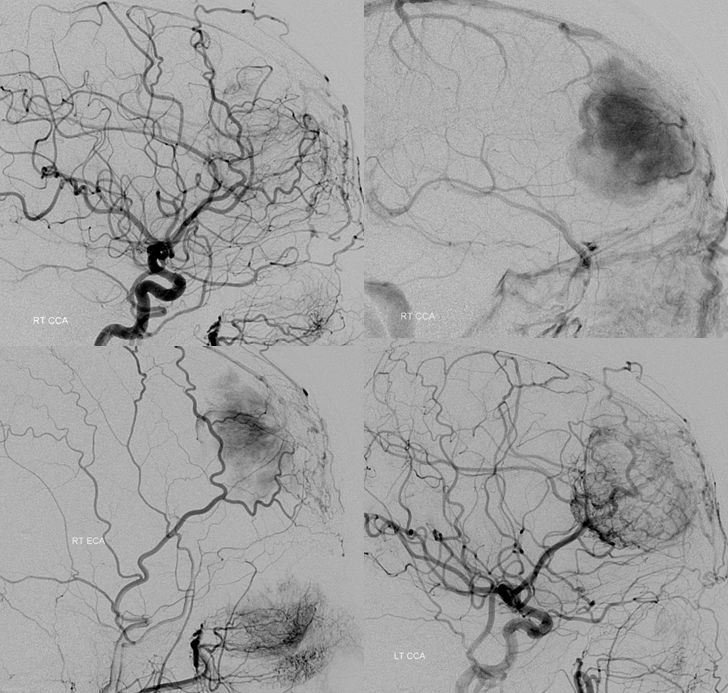
What is the blood supply to this mass? The surgeon will need to know, and angiography is the best way to answer this specifically. Regional supply can be anticipated well based on location, but extent of internal carotid (ACA in particular in this case) contribution can only be guessed, but needs to be known definitively.
Right common carotid injection demonstrating extensive supply via the ethmoid branches of the right ophthalmic artery. Right CCA injection (top right), later phase, showing a dense, persistent and late meningioma blush. Right ECA injection showing superficial temporal transosseous supply of the mass. Left CCA injection (bottom right) demonstrating extensive ACA (anterior frontal branch) supply to the posteroinferior aspect of the mass, via pial capillaries. This alerts surgeon to necessity of controlling the ACA feeder during resection, and anticipates need to separate the mass and adherent pia from brain tissue.
Spatial Resolution — a few times a year we end up doing an angiogram to prove that an infundibulum is an infundibulum, indeed.
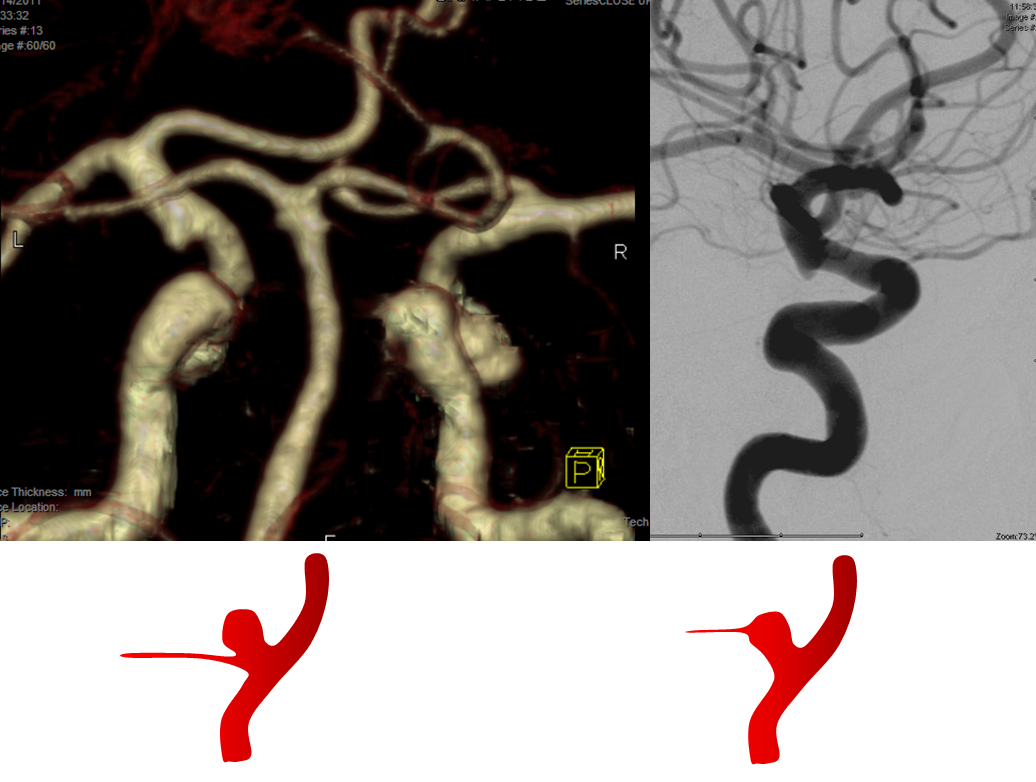
CTA and Angio images of a PCOM infundibulum. An infundibulum is a sacular or cone-like dilatation of a vessel ostium. A normal vessel, by definition, arises from the apex of an infundibulum (right-sided image). If the vessel arises separately from the apex (left image), then its an aneurysm. CTA spatial resolution will often be insufficient to detect the vessel, but visualizes an infundibulum which may be mis-interpreted as an aneurysm. Angio image on right shows a tiny PCOM arising from the apex of the infundibulum.
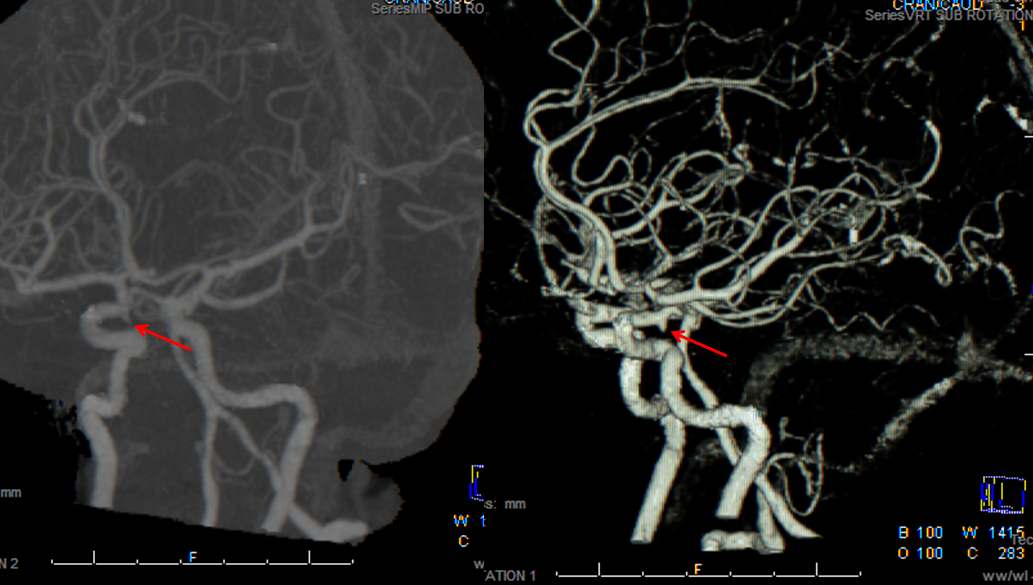
CT angiogram of patient with headache and SAH on CT, interpreted as a PCOM aneurysm.
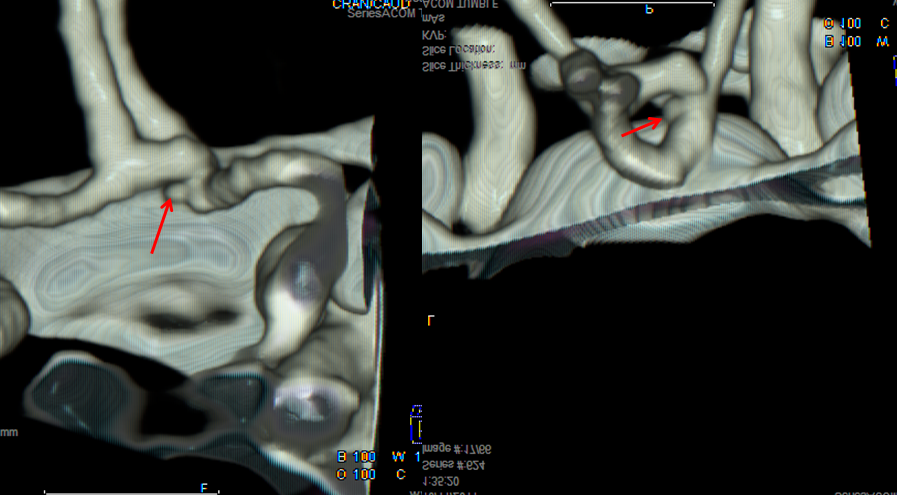
Same patient, a small ACOM aneurysm is reported.
The patient was surgically explored; no aneurysm was found at either location. Diagnosis of aneurysm < 3 mm in size on CTA is going to be somewhat tenuous. A catheter angiogram may be needed.
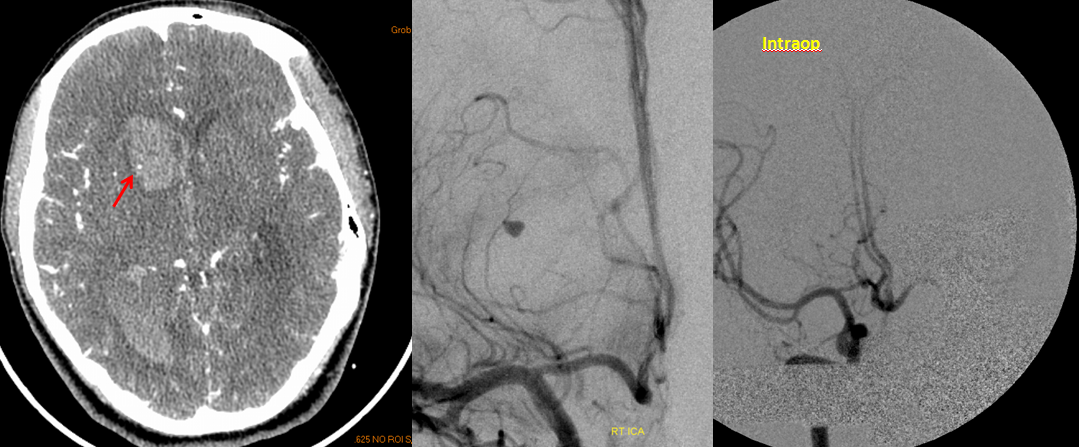
RAH pseudoaneurysm — a case of parenchymal hemorrhage from a RAH. The CTA demonstrates a “spot sign” (red) which, within a hematoma, is strongly suggestive of source of bleeding. (The spot is dye in the middle of hematoma. The only way dye can get there is through some vessel, which is smaller in caliber than the dot, since only the dot is visible. Therefore, the dot represents a pseudoaneurysm related to rupture of the feeding vessel). No delayed images were acquired. Angio (middle) demonstrates the pseudoaneurysm. Intraoperative angiogram (right) following aneurysm surgery.
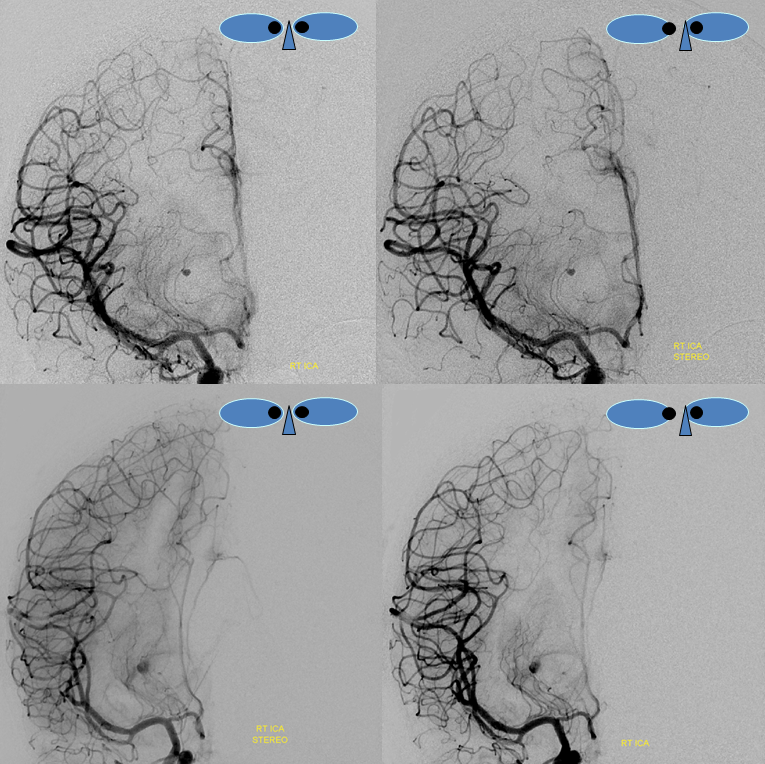
Stereo pairs of same case 1 week (top) and two weeks (bottom) following hemorrhage, demonstrating interval aneurysm growth — in fact showing an enlarging pseudoaneurysm in face of resolving hemorrhage. The patient was operated on after the second angiogram.
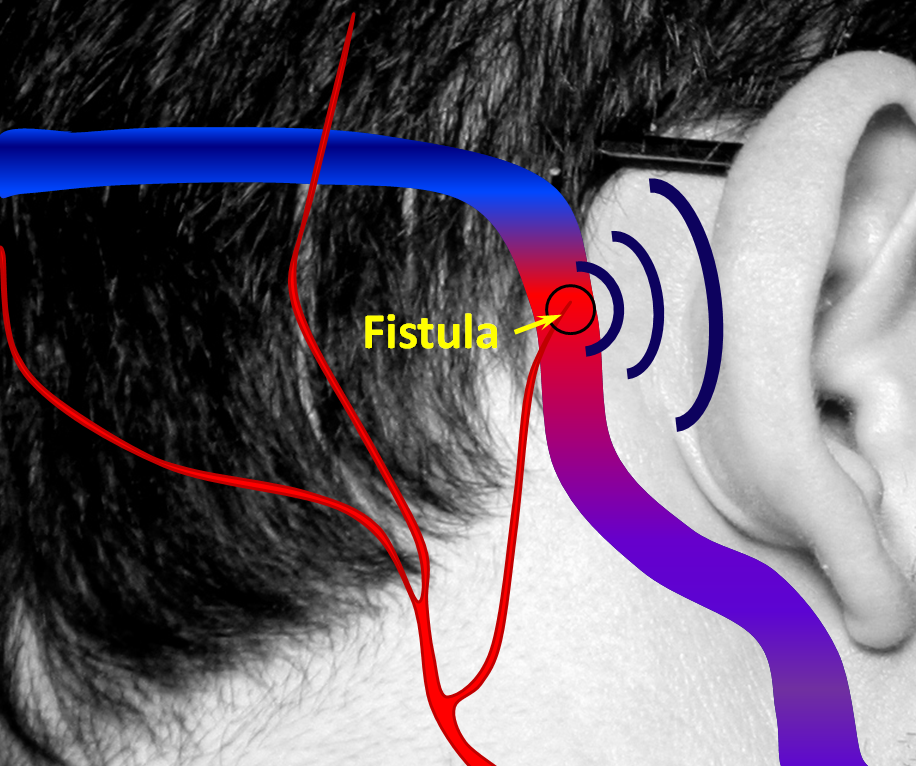
Arteriovenous Fistula — the gold standard for diagnosis and evaluation of arteriovenous fistulas, such as Brain Dural Arteriovenous Fisulas, and Brain Arteriovenous Malformations (AVM) is a catheter cerebral angiogram. Many dural fistulas present with pulsatile tinnitus — a whooshing sound that is synchronous with the heartbeat. Whenever the patient reports such sounds, the physician MUST put auscultate with a stethoscope over the ipsilateral mastoid bone and retro-auricular region to see if the fistula can be heard (objective tinnitus is defined as one that is heard both by patient and another human being). A harsh pulsatile sound is almost guaranteed to be a dural arteriovenous fistula, and requires an angiogram.
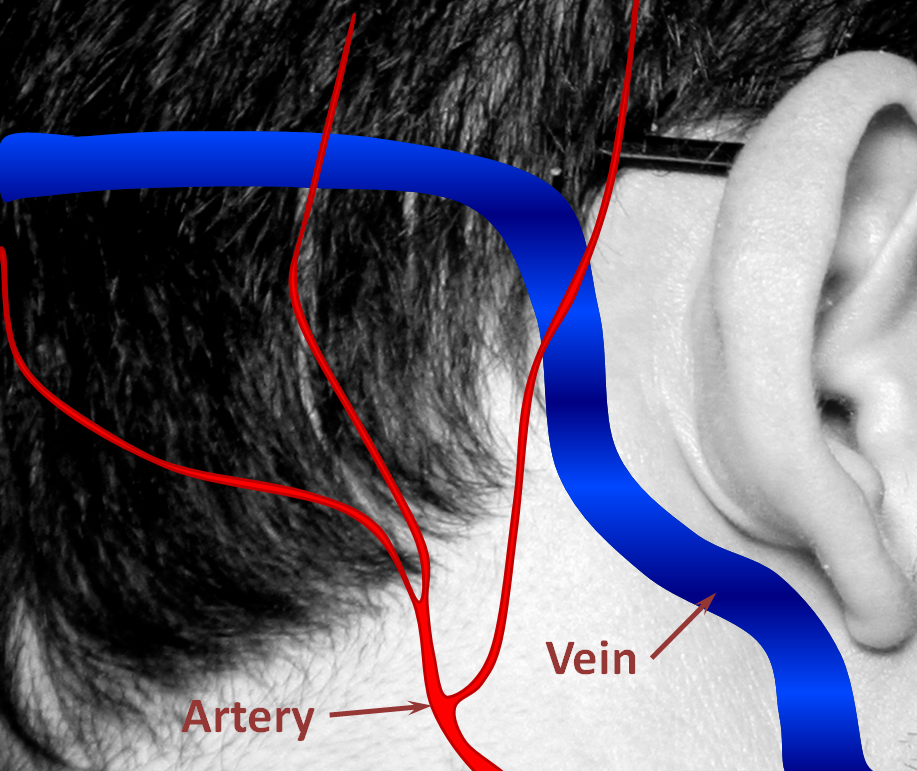
Below are pictorial diagrams of what a fistula is like and how it can be treated. The actual anatomy is much more complicated, but the pictures provide a frame of reference.
1. Normal Situation — a big vein (colored in blue, actually dural sinus vein) runs in the back of the head next, with part of it close to the ear. All kinds of arteries (red) are also present in that area. Normally, arteries and veins do not communicate directly.
2. A fistula forms when an artery becomes directly connected to a vein. It so happens that a common place for these fistulas to form is behind the ear. The flow of blood through the fistula is heard by the ear. Because blood flow in the arteries is not uniform but changes speed with each heartbeat, the noise made by the fistula follows the heartbeat, or pulse, hence “pulsatile tinnitus.” The stethoscope (bell, if you still have one) is applied to site of suspected fistula.
Angiographic views of a typical sigmoid sinus fistula. This patient presented with pulsatile tinnitus.
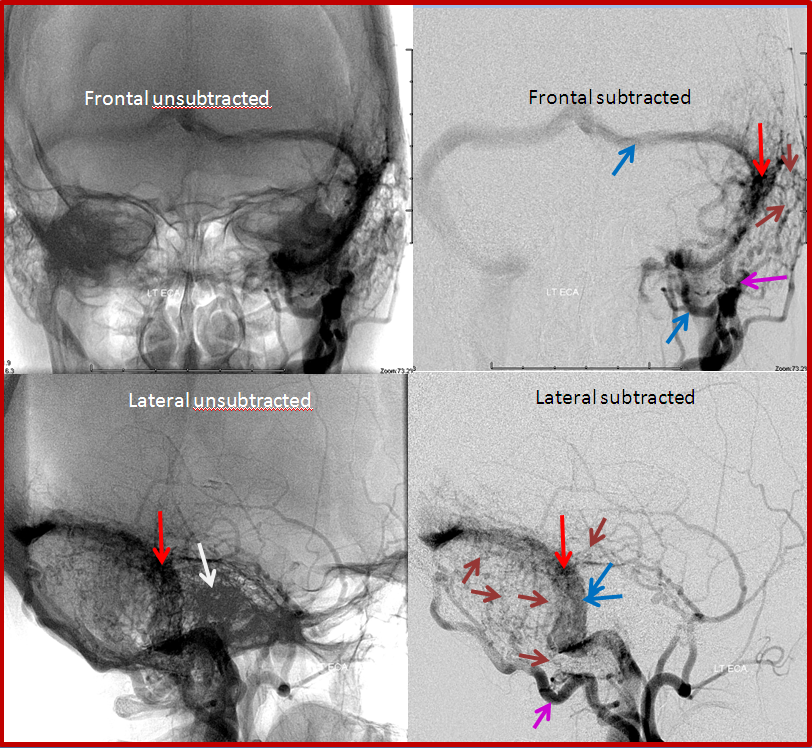
Left external carotid injection, showing innumerable occipital feeders, and several middle meningeal ones. This might be construed as a multiple hole fistula, but it is not. The single fistula point is marked by red arrow, onto which middle meningeal branches converge.
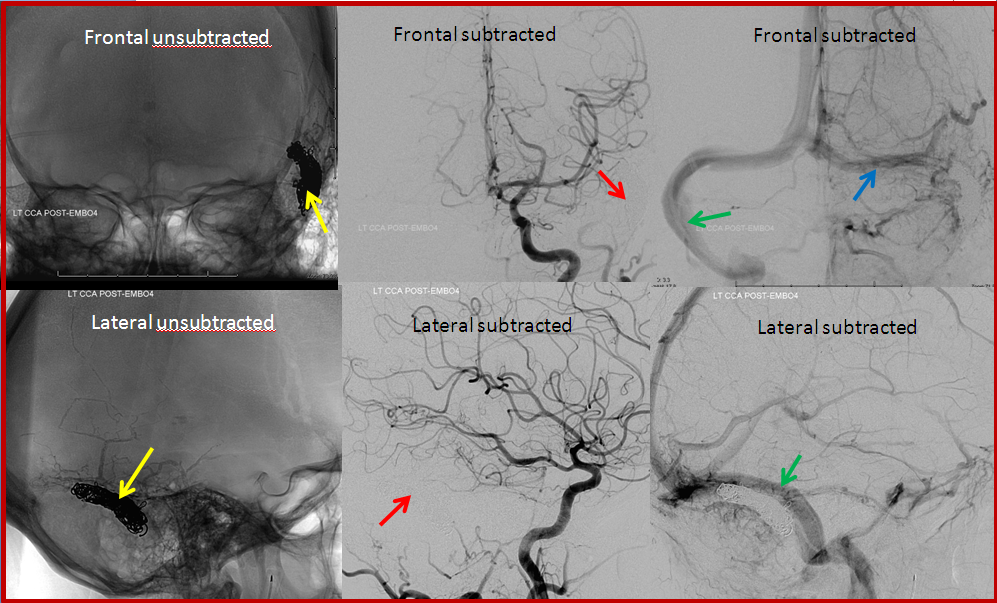
Transvenous coil embolization of the sigmoid sinus. Notice that coil mass is tightest near the fistula, which is completely occluded. A posterior temporal vein drains into the left transverse sinus and retrograde to the right.
This patient developed a fistula on the dural covering that separates the back part of the brain, called cerebellum, from the overlying occipital lobe. The fistula is far away from the middle ear cavity, and there was no tinnitus.
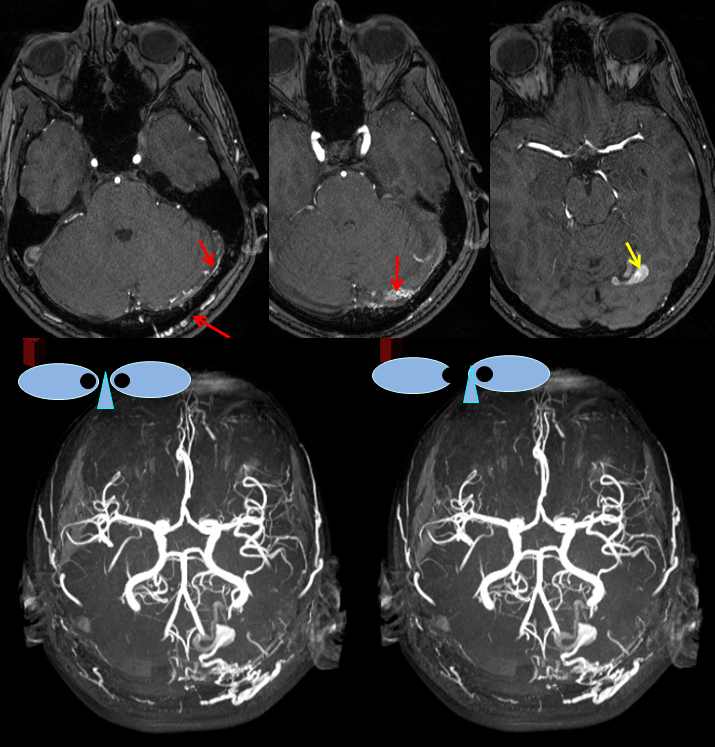
MRA images (top) and stereo MRA composite (MIP) images on the bottom, demonstrating multiple intracranial and extracranial vessels (red arrows) feeding the fistula. An aneurysm near the fistula is suspected (yellow arrows)
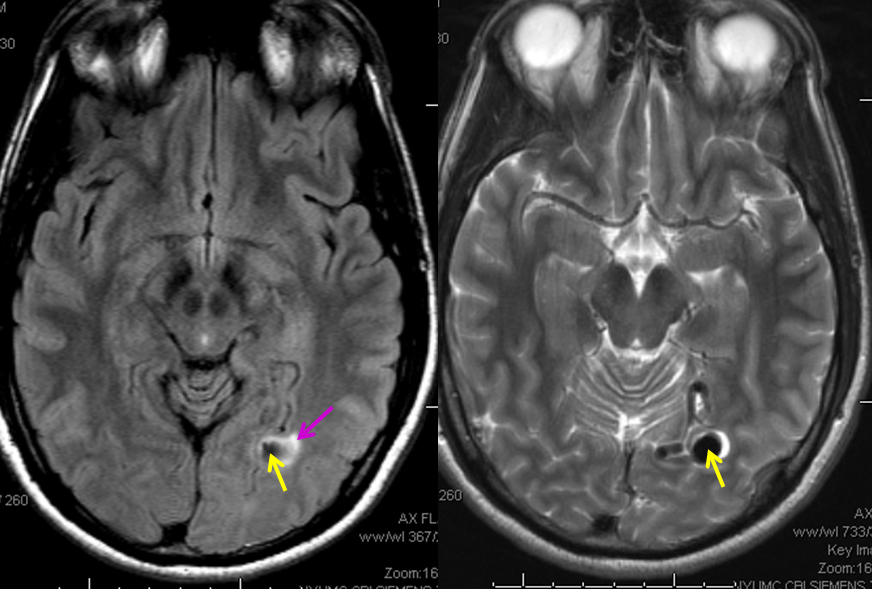
MRI images of the brain, showing the aneurysm (yellow arrows) associated with the fistula (because of high flow, vessels around the fistula may form these kinds of aneurysms). There is edema (swelling) present in the surrounding brain (pink arrow). The patient came to medical attention after developing a seizure which was preceded by a visual disturbance. The location of the aneurysm, in the occipital lobe of the brain (which is responsible for processing of visual information) strongly suggests that seizure was caused by presence of the aneurysm and secondary local brain swelling.
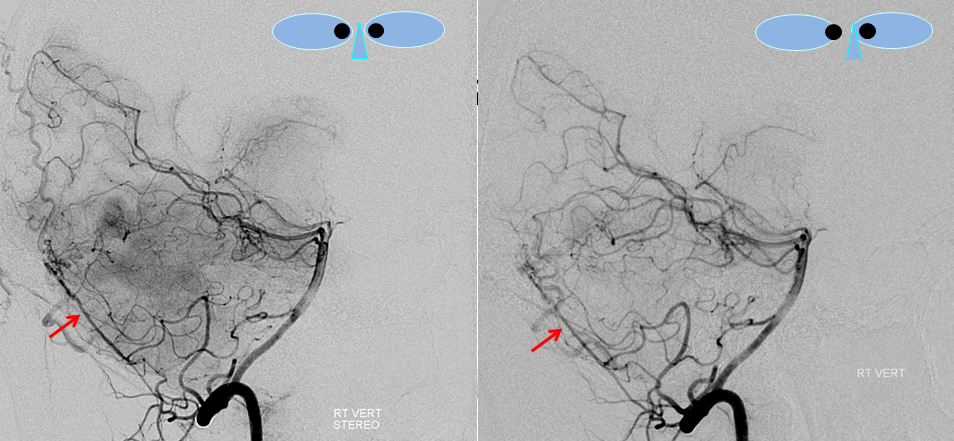
Stereo views of right vertebral artery injection, showing an enlarged artery of the tentorium cerebelli (red arrow), with a hint of the aneurysm (not labeled)
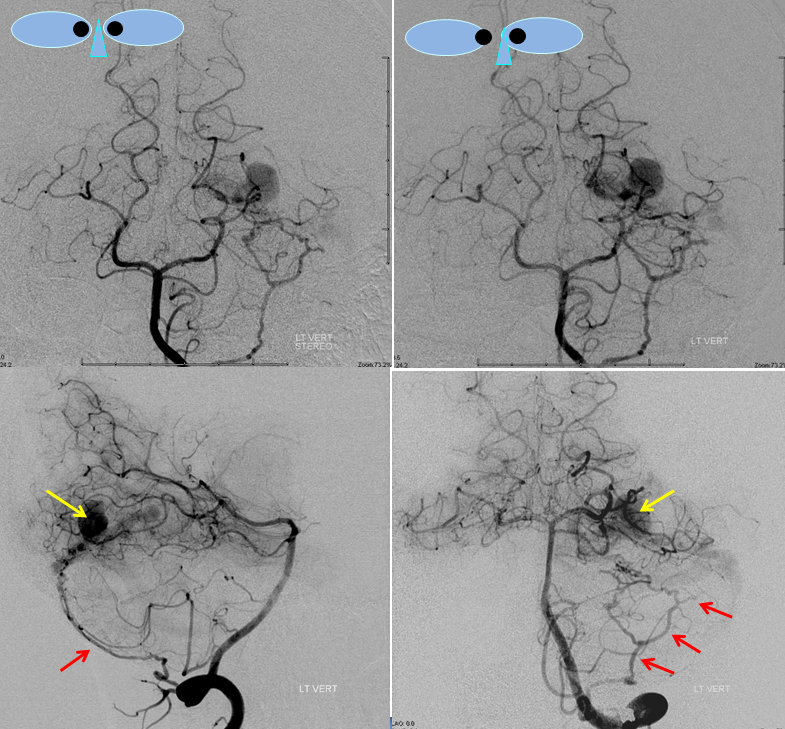
Injections of the LEFT vertebral artery now clearly show the fistula and the associated venous aneurysm (yellow arrows); the fistula is supplied, in this case, by the posterior meningeal artery (red arrows)
Dynamic CT angiography has recently become available. Similar to CT angiography, dye is injected a high rate into the vein and the head scanned. Unlike conventional CTA, where only one, or perhaps two scans are done in coordination with the injection, dynamic CTA acquires a series of scans (between 2-20), at about 1 scan/second, to capture all phases of contrast flow, from arterial to venous. The study is designed to detect arteriovenous shunting, visualized as an early draining vein of some lesion such as AVM or fistula. Another benefit is in minimizing the possibility of poor scan timing and thus obtaining a sub-optimal CTA. The disadvantage is increased radiation exposure which, depending on the number of CTA data sets acquired and CT machine x-ray settings, can be quite significant. The reader is urged to evaluate the technique in their own locale and make an informed decision as to whether its imaging advantages justify increased radiation exposure. Below is a normal dynamic CTA movie.
Dynamic MR angiography, a radiation-free technique, is evolving. Most are based on various proprietary methods of extreme k-space undersampling. These methods aim to balance spatial and temporal resolution, and will hopefully become more widespread in the near future. Current commercial sequences do not yet offer the same spatiotemporal resolution capability as dynamic CT angiographic techniques. Nevertheless, a well-tuned sequence, evaluated by a highly experienced radiologist, can now serve as a reasonable screening tool for AV shunting in a patient who is unable, or unwilling, to undergo catheter angiography. Below is a movie of TWIST (Siemens) dynamic MR angiography of a patient with right carotid occlusion.
Advanced vascular problem solving
Sometimes, its good to get an angio when you are at a loss about something that might be vascular — bleeding, for example. Here is a young man with spontaneous left temporal lobe hemorrhage. MRI shows no evidence of AVM, aneurysm, or any other problem. In our book, this needs an angio
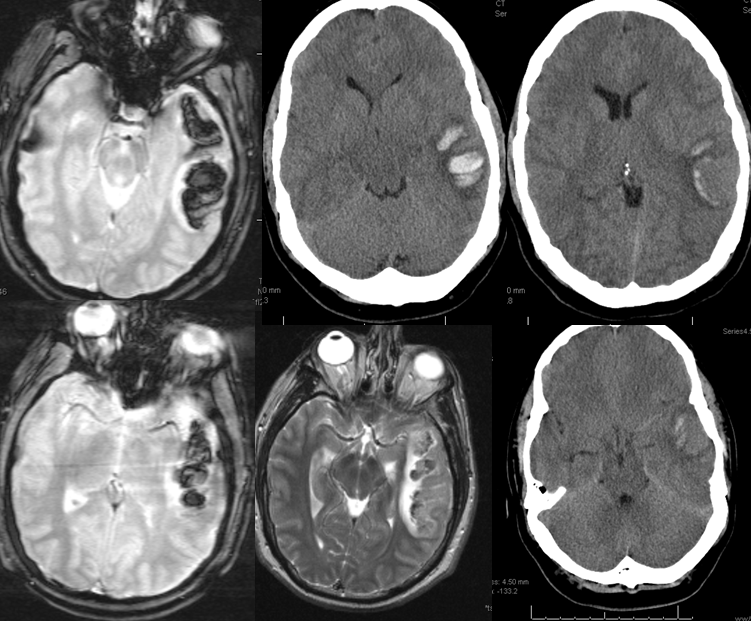
MRI (hemoflash and T2, and CT images of left temporal hemorrhage). Note to radiologists — please don’t ask to see the whole study — there is nothing suspicious there, believe me.
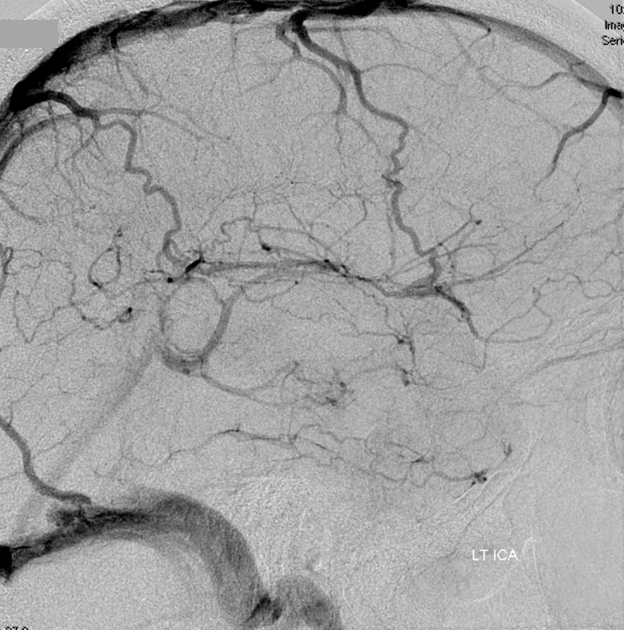
Angio of the same patient — what is the diagnosis?
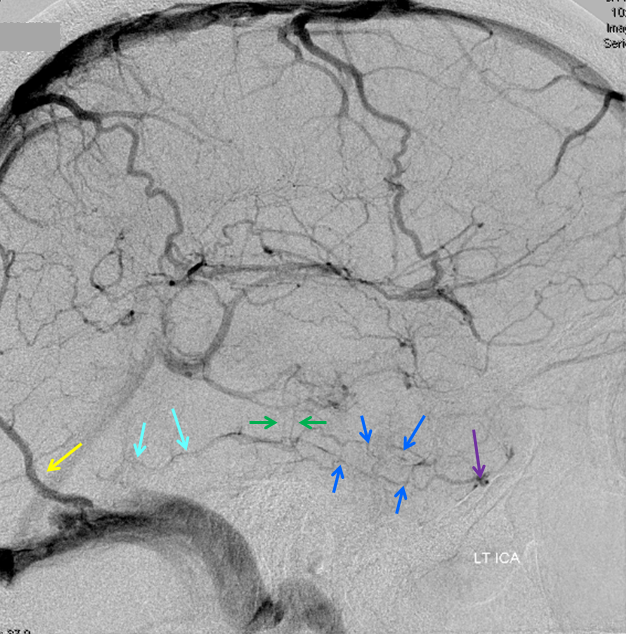
Cerebral venous thrombosis. Isolated thrombosis of the inferior temporal vein/vein of Labbe. Notice irregular and thinned appearance of inferior temporal gyrus vein (dark blue arrows), compared with the larger caliber of the same vein anteriorly (purple) and posteriorly (light blue), which is the vein of Labbe. Other signs are:
1) There are no normal veins seen draining the temporal convexity — this cannot possibly be normal. Deep Sylvian veins and striate veins are there (unlabeled, see venous system for clarification) If you do enough angio, you will recognize that this appearance is very abnormal. Every other lobe has juicy veins, for example.
2) There is evidence of temporal lobe congestion, which is somewhat subtle, but present — in the inferior and superior temporal regions. The middle “layer” of the temporal lobe is hypovascular — corresponding to location of the clot — its a hyperemia-clot-hyperemia sandwich.
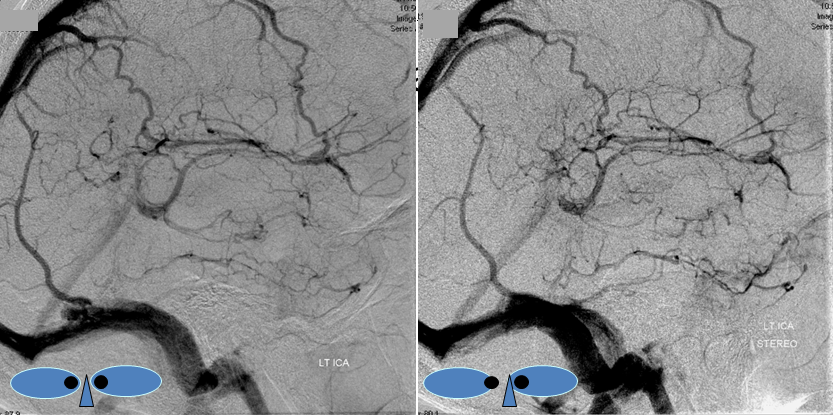
Stereo pair of the same patient, can’t resist having one. Notice hyperemia adjacent to congested inferior temporal lobe vein, and clot-related lucency above it.
Alternatives to diagnostic catheter angiography: Dynamic MR and CT techniques
Time-resolved MRA (TWIST, TRICKS) and CTA continue to evolve, and will play an increasing role in evaluation of arteriovenous shunts (such as AV fistulas and AVMs). Dynamic CTA became practical following development of 100+ slice scanners, and is capable of acquiring images of the head with ~1 second temporal resolution (1 whole head scan per second). A bolus of contrast is given, and dynamic imaging performed, allowing for separation of arterial and venous phases. The hallmark of a shunt, such as fistula or AVM, is premature visualization of the venous system. Dynamic CTA is more widely available, less complex, and produces images of higher spatial resolution compared with Dynamic MRA. Unfortunately, CT is an x-ray based technique, and multiple passes mean increasing total radiation dose, which can be very significant depending on how dynamic CTA is set up. Concerns over CT-related radiation doses are receiving increasing worldwide attention, and seem only likely to grow in the future. At present time, we strongly caution our patients with regard to radiation exposure of dynamic CTA; this will hopefully change as thechnique improves. MR imaging does not use radiation; however, most dynamic MR techniques of today lack sufficient temporal and spatial resolution combination to be reliably practical. Specialized centers do have this capability, but they are quite few. Nevertheless, I believe that the future of dynamic imaging belongs to MR.
An example of dynamic MRA scan from our NYULMC imaging center. Contrast bolus can be seen arriving via the subclavian vein, then seen in the great vessels, brain, and finally cerebral veins and venous sinuses, with a 1 second temporal resolution (see numbers below). As might be suspected, this is one of our best examples. Most are not so crisp (this older patient has diminished cardiac output, prolonging contrast transit time [notice pulsatile arrival of contrast into the subclavian vein]).
Below is a movie of normal dynamic CTA, in lateral projection. The patient presented with worst headache of life, and this evaluation was done to look for an aneurysm. Twenty 1-second CT passes were performed.