How to select optimal Pipeline cases, maximize good outcomes, and avoid re-treatment.
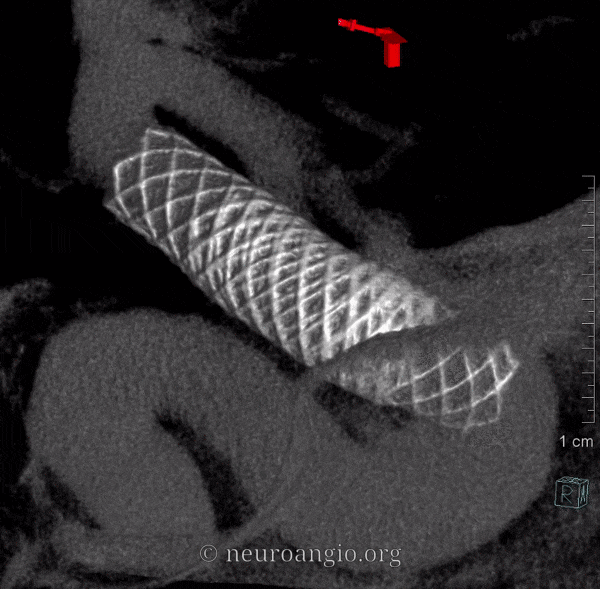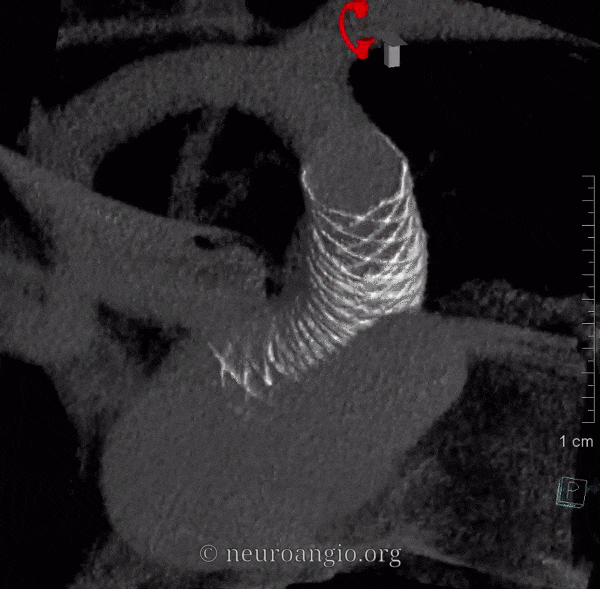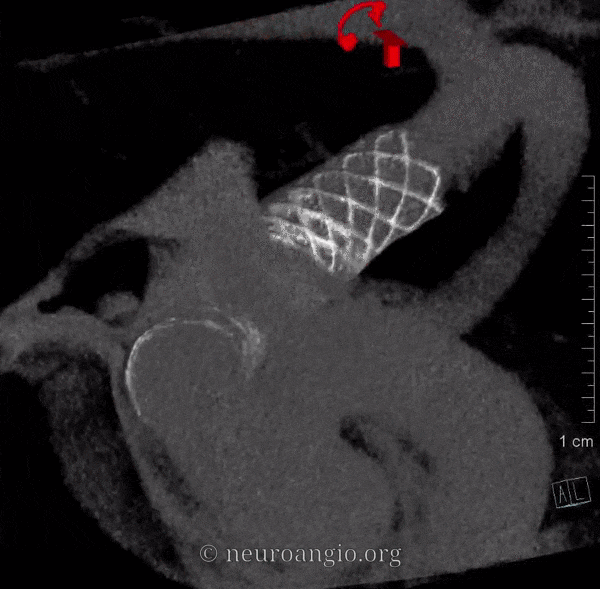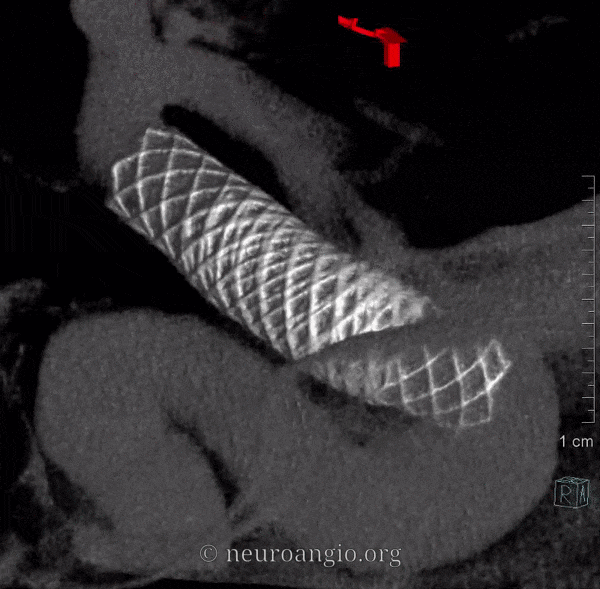
The short version of this page is this: sufficient metal coverage across the neck, with no alternative source for aneurysmal inflow or outflow, maximizes treatment efficacy. Below is the long version:
No device has made more impact on aneurysm treatment since introduction of the GDC in 1990. It is by far the most effective and versatile endovascular aneurysm treatment method today — anything from the smallest blister, to the typical small/medium saccular, to the largest fusiform aneurysm is a legitimate, high-probability target. The mechanism by which it works — a scaffold across the diseased aneurysmal segment, followed by endothelialization to effectively re-establish a healthy endothelial segment — allows for durable aneurysm occlusion without recurrence — the Achilles heel of all methods which target the aneurysm sac rather than the underlying dysplasia of the vessel from which the aneurysm arises. In the PUFS trial, 86% of all aneurysms — mostly complex, fusiform large and giant aneurysms were completely occluded at 12 months, progressing to 93% after 3 years, notably with re-treatment for some aneurysms (3 year PUFS follow up study) Out of tens of thousands aneurysms treated to date, no recurrence has ever been demonstrated, not even once. This is a truly incredible achievement, not just for brain aneurysms, but for medicine in general, far surpassing any other aneurysm treatment method with exception of the generally unappealing parent vessel sacrifice (including surgery, which is often touted as being permanently curative, but in reality is considerably less so — not to mention the fact that many aneurysms curable by Pipeline are not amenable to any reconstructive surgical approach).
It is, however, key to recognize that failure is an excellent teacher — focusing on the 15% of aneurysms which do not close by 12 months, as much as on the 85% that do, leads to very important insights. We did just that, by analyzing individually the first 100 aneurysms treated at NYU / Bellevue with the Pipeline, and reviewing the features of the 19 cases that did not progress to complete occlusion by the 1 year mark. There is no “near-complete” here — the aneurysm is either closed or it is not — and there are good reasons to think this way. Results of this analysis were published in JNS (link here) The following is a summary of our observations.
Fundamentally, understanding how Pipeline and other “flow diverters” work allows one to predict when they may not work so well. Because the Pipeline works by aneurysm exclusion via endthelialization, any factor which interferes with it will result in reduced efficacy. Broadly, these can be divided into two categories:
1. Insufficient metal coverage — due to oversizing or use of too few devices
2. Alternative source of aneurysmal inflow or outflow — this category includes vessels incorporated into the aneurysmal compartment and endoleaks due to poor device-to-wall apposition (endoleak is a source of inflow or outflow, just like a branch).
More specifically, four kinds of aneurysms are at increased risk of non-occlusion:
1. Aneurysms incorporating branch vessels, such as the ophthalmic, posterior communicating, or anterior choroidal artery. Persistent flow into the associated branch may result in treatment failure — the branch vessel remains patent and therefore the aneurysm is not fully excluded by endothelial overgrowth. Success in such cases may be dependent on closure of the culprit artery, with typically effective and clinically asymptomatic collateral reconstitution — for example by the ECA of the ophthalmic artery, and by the P1 segment of the posterior communicating branch.
2. Previously stent-coiled aneurysms — the stents such as Neuroform and Enterprise typically do not appose the vessel wall completely, thus preventing effective Pipeline implantation, with poor contact with the parent vessel, endoleak between the Pipeline and the vessel wall, etc. In practice, many of these implanted stents are much longer than they needed to have been, and many have been suboptimally deployed, in a stretched manner. Often, it is not possible to find the “true lumen” of these stents. Extending the pipeline beyond the proximal and distal landing zones of these oversized stents results in very long constructs that take a long time to endothelialize. In light of these limitations, it is really important to think long and hard before stent-coiling cases where superiority of the Pipeline has been conclusively demonstrated.
3. Aneurysms with insufficient metal coverage — the question of whether a single Pipeline, or any other flow diverter, is enough or not enough to cure an aneurysm is no more intelligent than asking whether one coil or one clip or one vial of Onyx 500 is always sufficient for the same purpose. It is a tool that one needs to understand and know how to use best. This begins with appropriate device sizing — maximizing metal coverage over the aneurysm neck to promote endothelialization is key to success. Oversizing the device (choosing devices of substantially higher diameter than recipient vessel) leads to metal coverage values far below the colloquial 30-35% range (the real-life single device coverage values are in fact 20-25% — see Pipeline Device Properties Page for more info). For large and giant, fusiform aneurysms, such as those of PUFS trial — where 3 devices were used on average per case — metal coverage represents the limiting factor in occlusion. In our experience, insufficient coverage leads to predictably suboptimal results, frequently requiring re-treatment, exposing patients to continued risks of rupture and mass effect, and uncertainty over the antiplatelet regimen in “nearly occluded” cases. The emerging consensus on necessity of cross-sectional evidence for aneurysm occlusion stems in large part from a realization that aneurysms which appear to be “nearly occluded” in many instances continue to enlarge and thus constitute a clear and present threat.
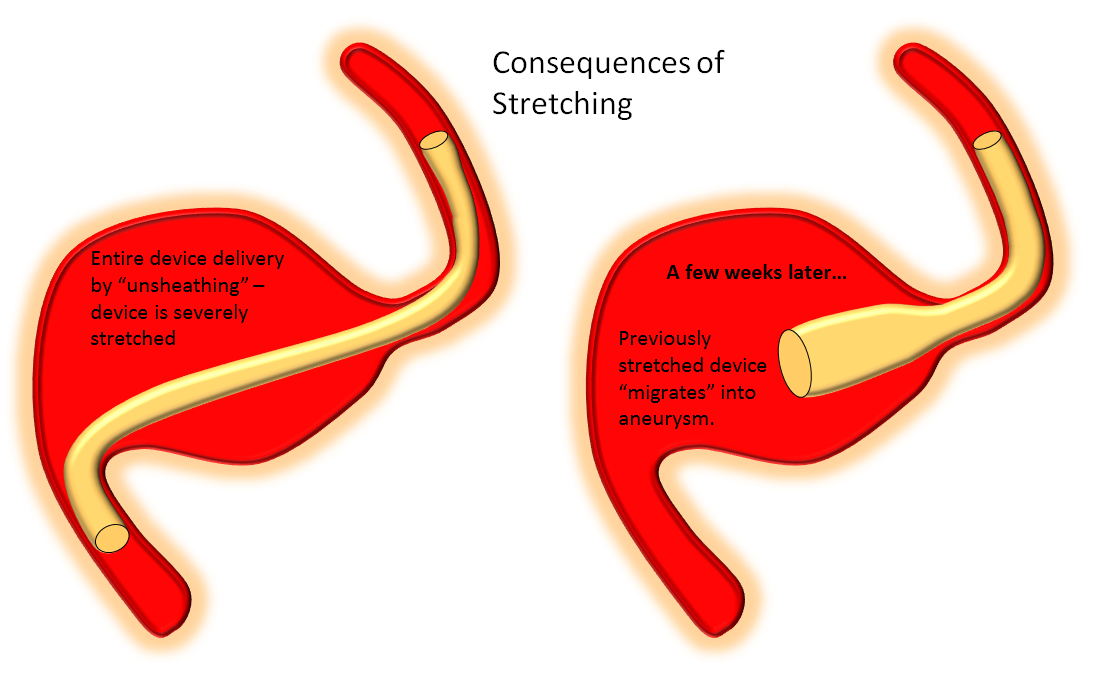
4. Suboptimal Device Implantation — poor apposition of device to vessel wall, because of inappropriate sizing or insufficient length of the landing zone, deploying the device in a stretched manner, either because of subotimal technique (too much unsheathing) or inflexible desire to bridge a large neck with a single device, leads to formation of endoleak or “delayed device migration” when a stretched device eventually foreshortens to its natural length — not as a result of some intrinsic device defect but rather because it behaves according to its natural physical properties. This last category is becoming relatively rare, with better understanding of device properties by the operators and support personnel. An endoleak, however, is no different from a vessel incorporated into the aneurysm — it is an alternative source of outflow.
Below are examples of the above four categories
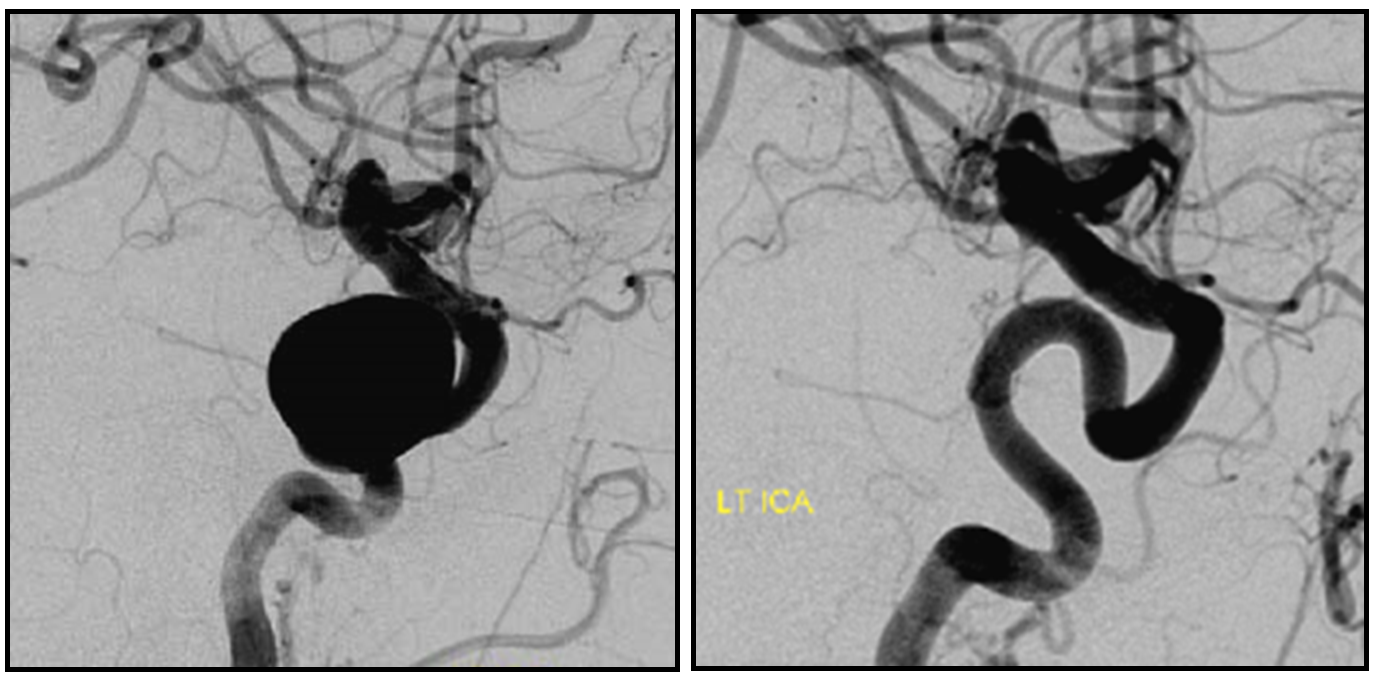
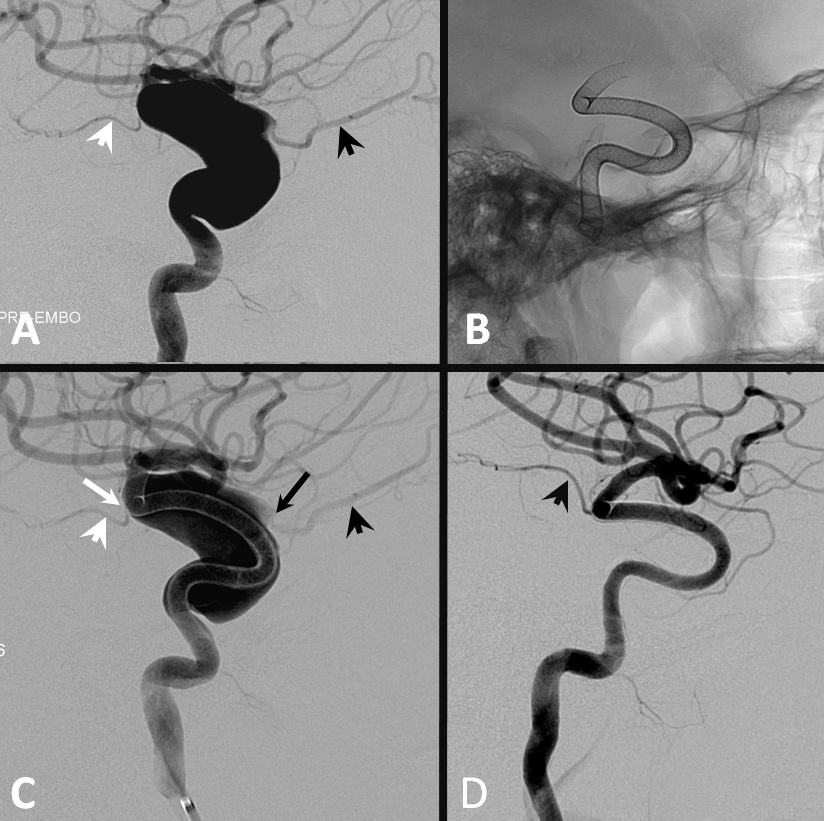
1. Aneurysms Incorporating a vessel — the most common scenario is vessel origin from neck or dome of aneurysm, typically in case of ophthalmic, posterior communicating, or anterior choroidal arteries.
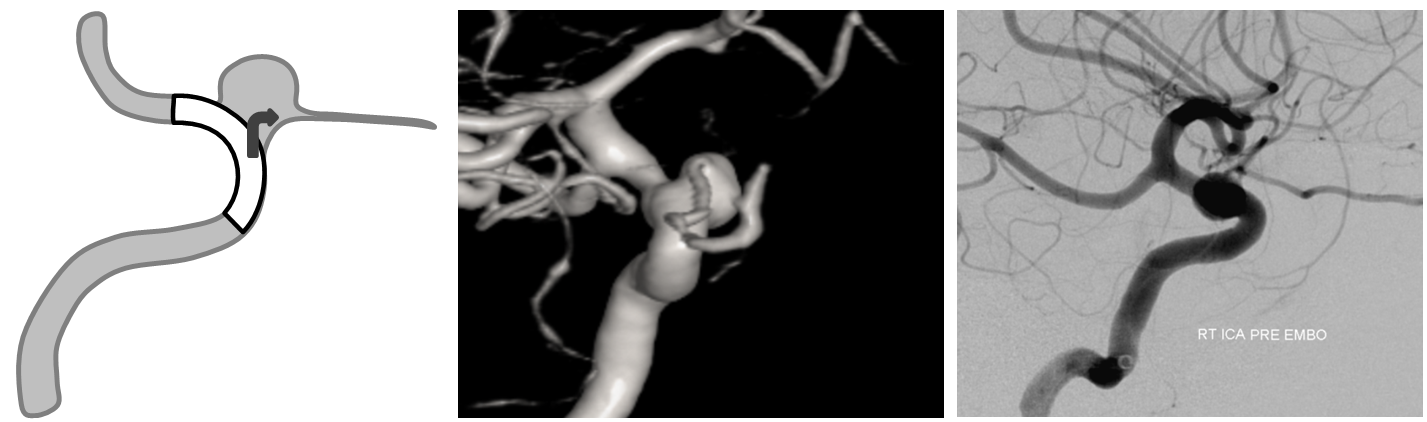
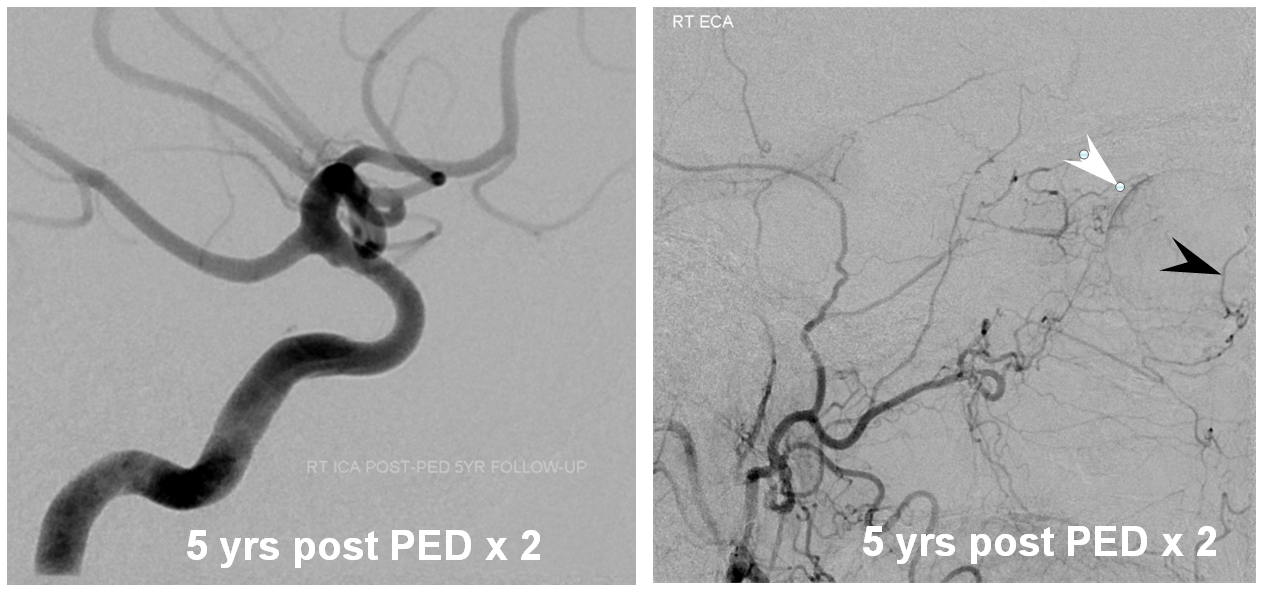
Three years later, the aneurysm remains unoccluded due to runoff into the ophthalmic artery — typical of runoff cases, lack of occlusion tends to be persistent (if not closed by 12 months, it is unlikely to close at all)
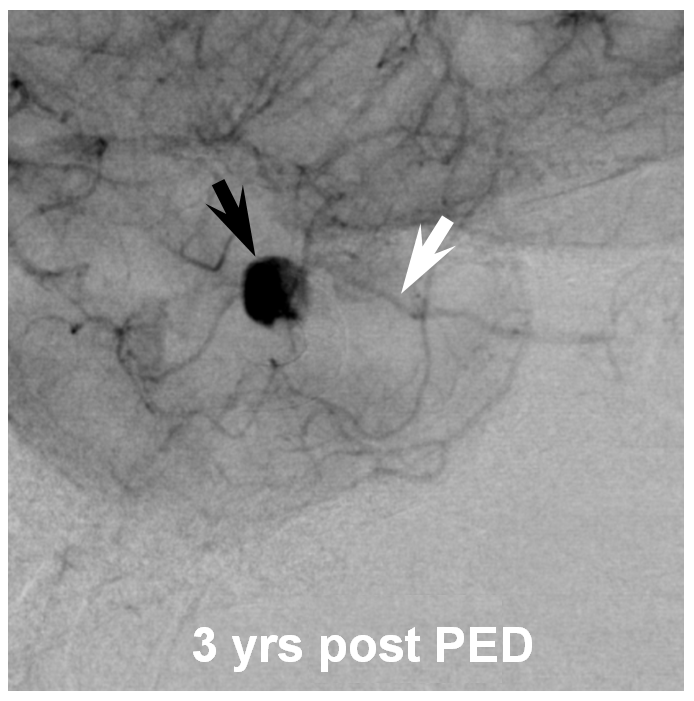
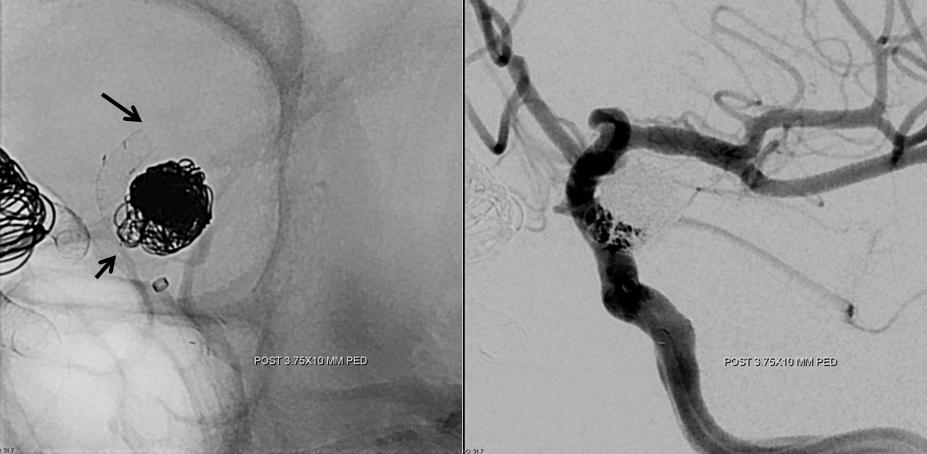
The solution is to place additional device or devices across the aneurysm neck, with the expectation that the ophthalmic artery will need to close. This has been, in our experience, a clinically asymptomatic event in every instance — as you can see in image below both the ophthalmic artery origin and aneurysm are closed, with reconstitution of the distal ophthalmic territory, including the choroid blush (white arrow) and ring of the iris (black arrow) via external branches
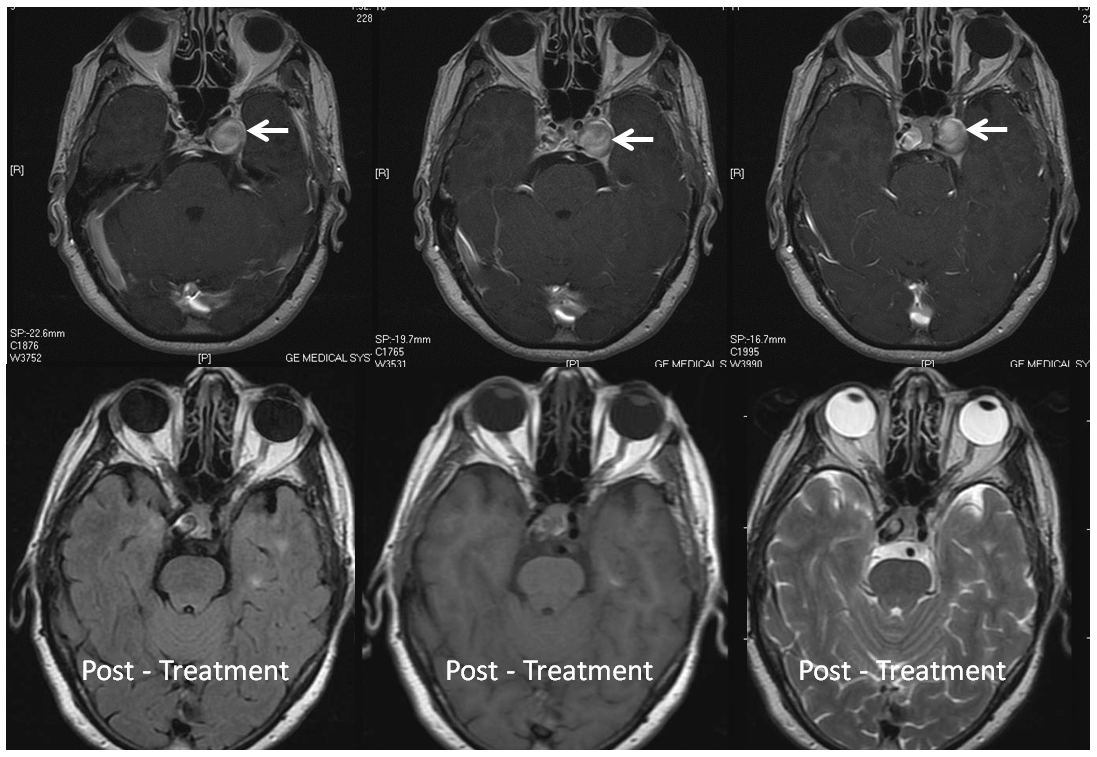
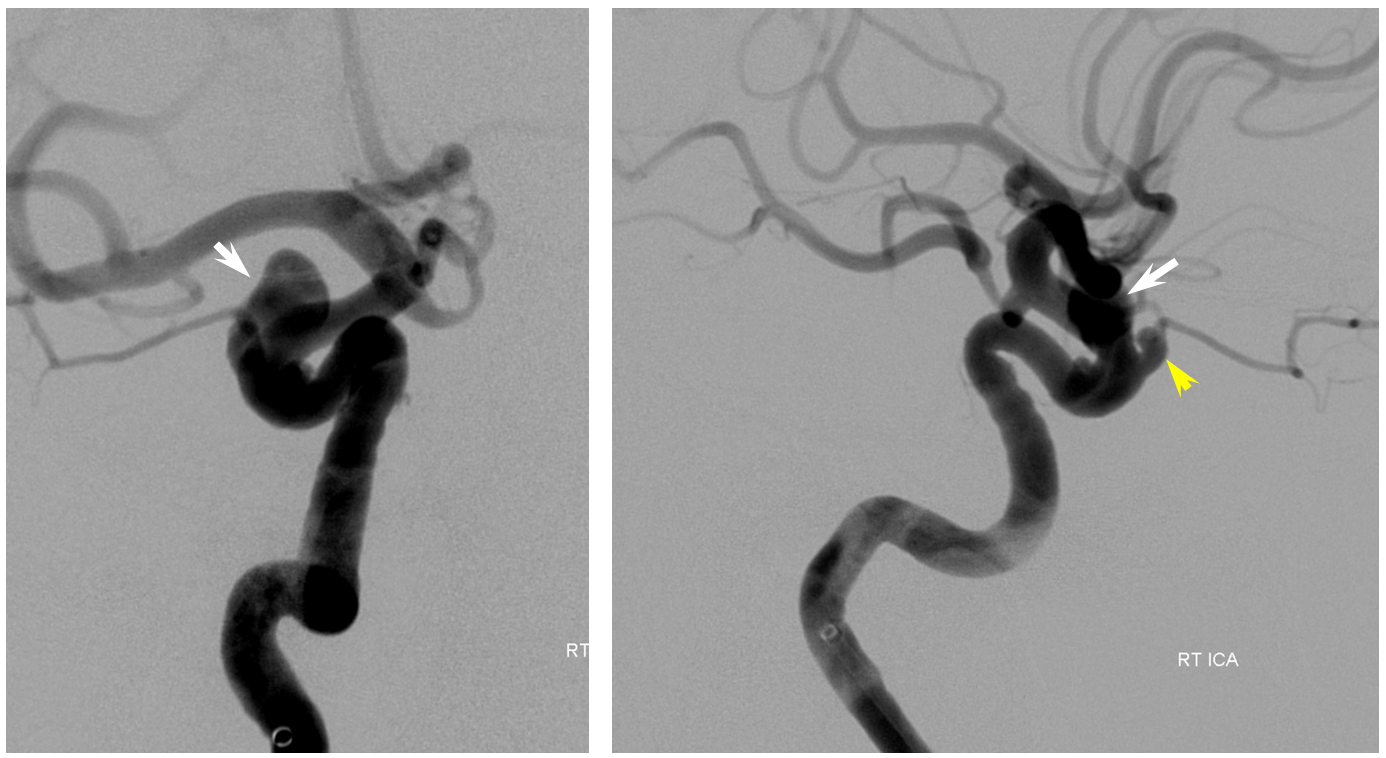
Here is a nice example of the same phenomenon in a patient with a larger paraophthalmic aneurysm (white) and a smaller ophthalmic aneurysm (yellow) incorporating the ophthalmic artery. A third unlabeled anterior genu anuerysm is also present lower down
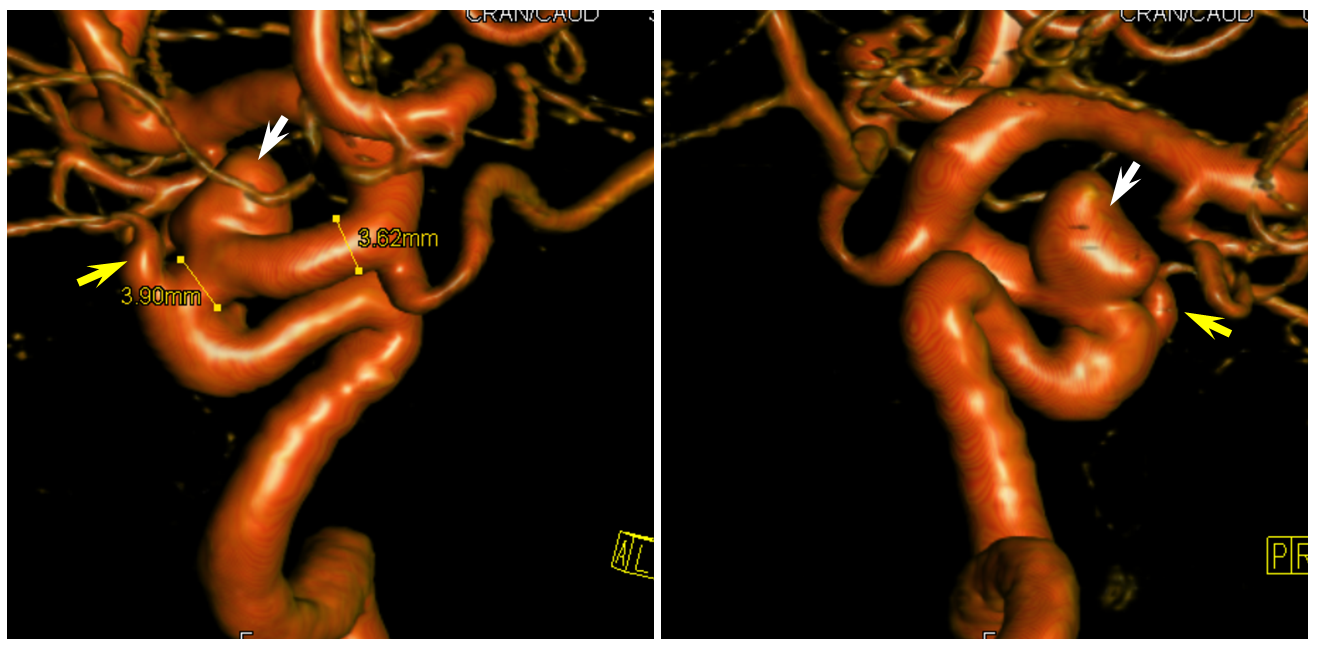
3D images of the same
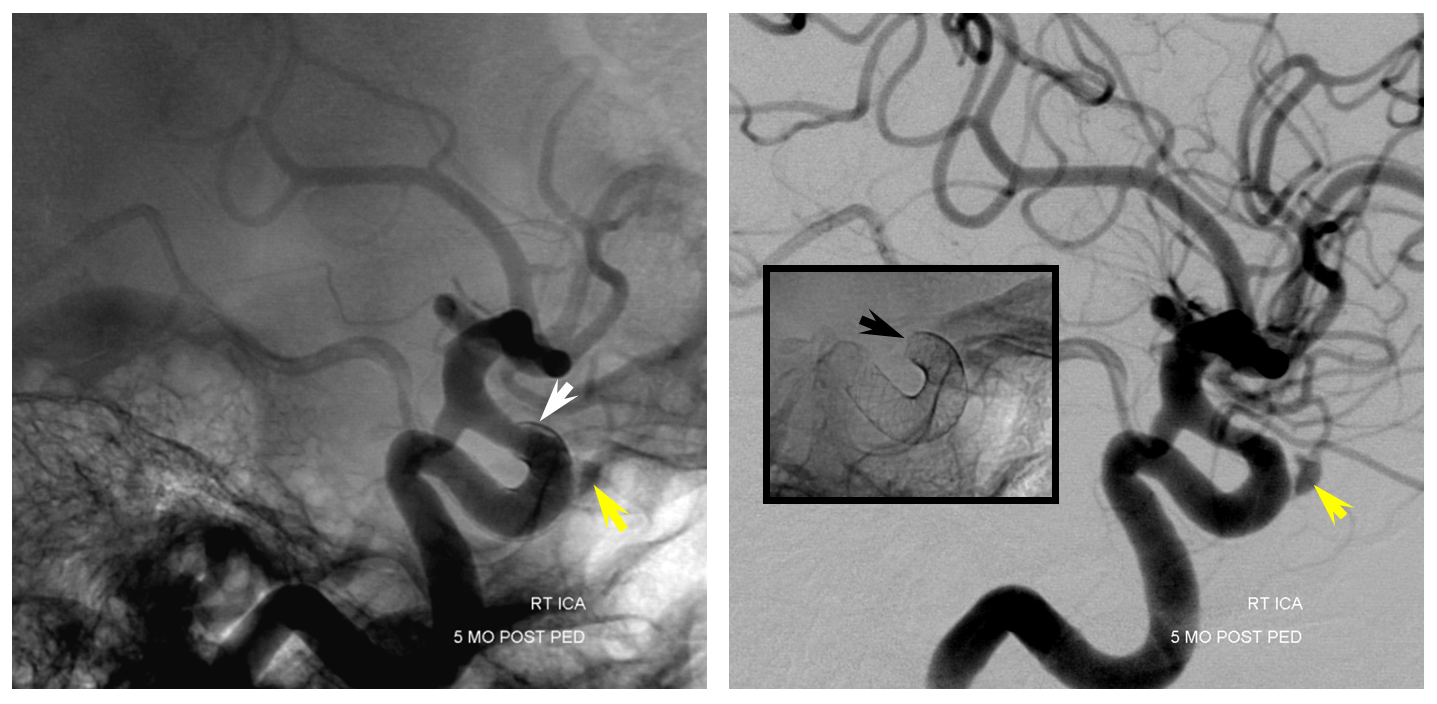
Post-Pipeline, 5 months later The larger paraophthalmic and smaller anterior genu aneurysms are gone. A rind of endothelial hyper-proliferation at the distal end of the device (white) produces a sometime-seen constriction of the leading edge (black). The ophthalmic aneurysm however is still patent, as is the antegrade flow within the ophthalmic artery.
One year angiogram shows the predictable resolution of hyper-endothealial response. The ophthalmic aneurysm continues to be open, and is very unlikely to close now. The case shows how different aneurysms in the same patient — one with a very much intact endothelial response — behave differently after pipeline embolization.
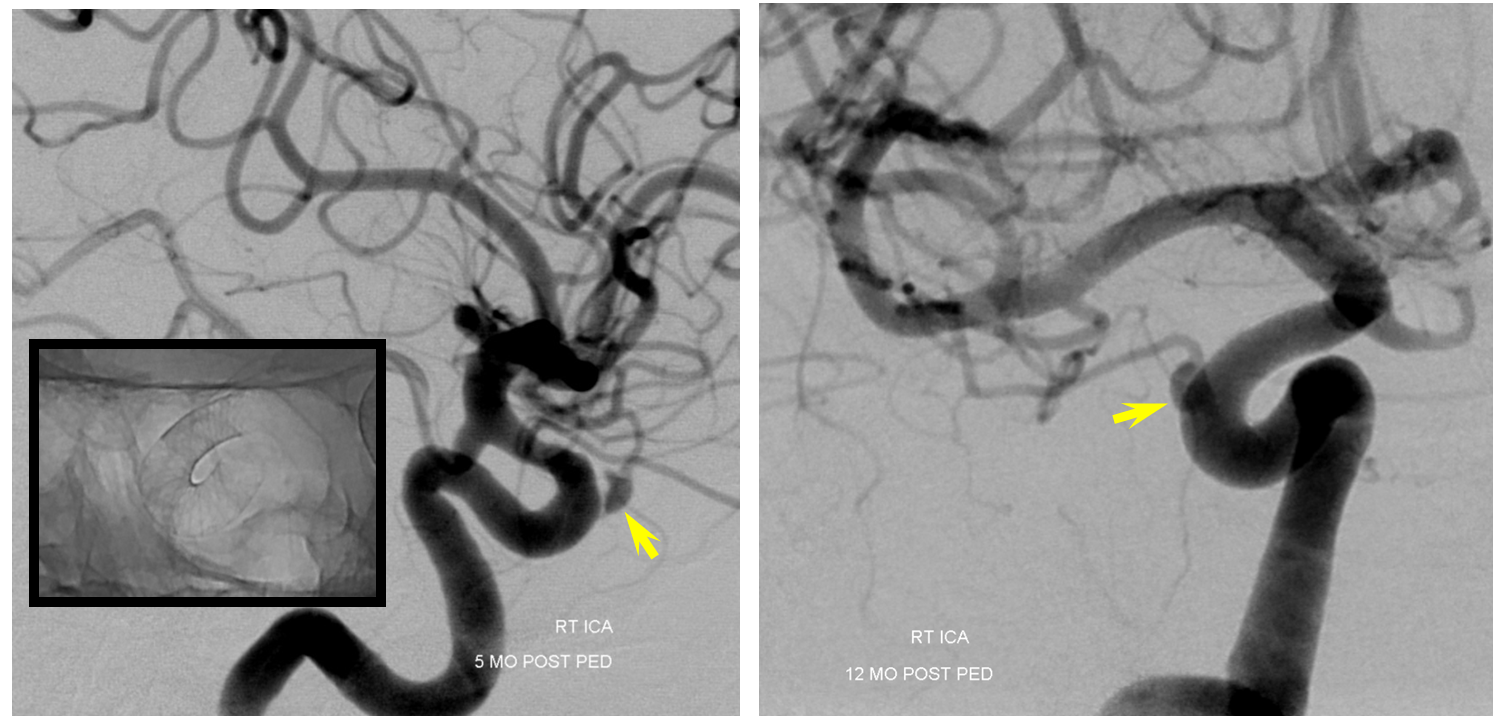
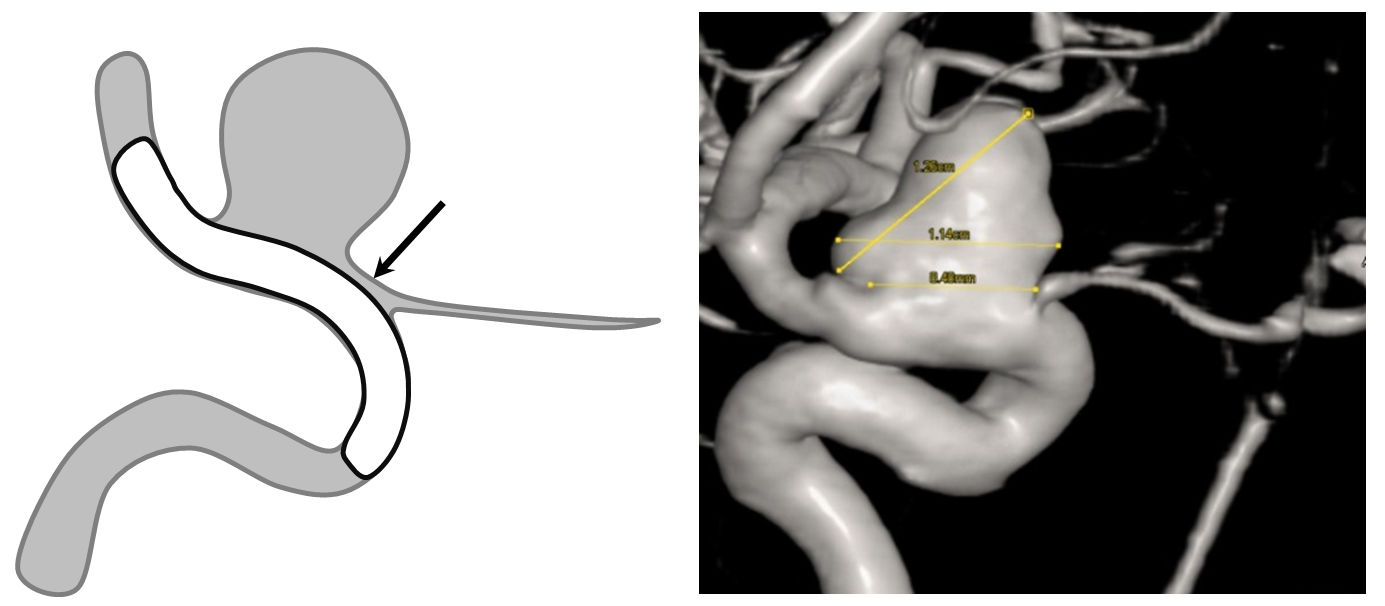
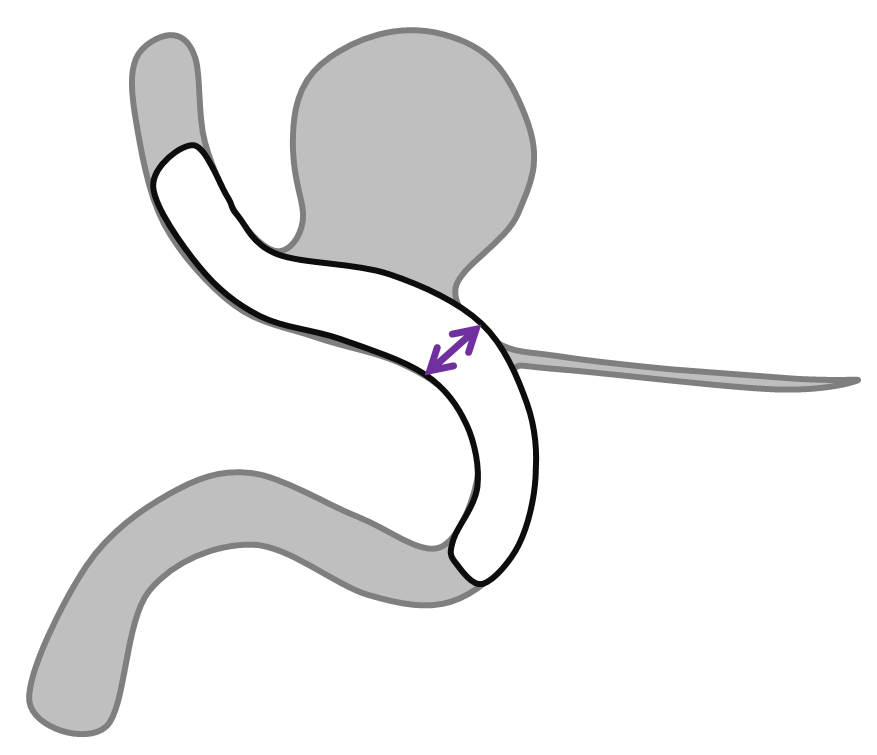
An important variation of the same phenomenon occurs when an artery which is separate from the aneurysm becomes secondarily incorporated into the aneurysmal compartment following pipeline placement. A schematic illustration of how this happens is shown below, with nonapposition of the pipeline to the vessel segment between the aneurysm and the artery (black arrow) effectively creating a larger aneurysm which now incorporates the ophthalmic artery. The cartoon and a representative aneurysm with ophthalmic artery arising close to the aneurysm base show the importance of properly sizing and deploying the device.
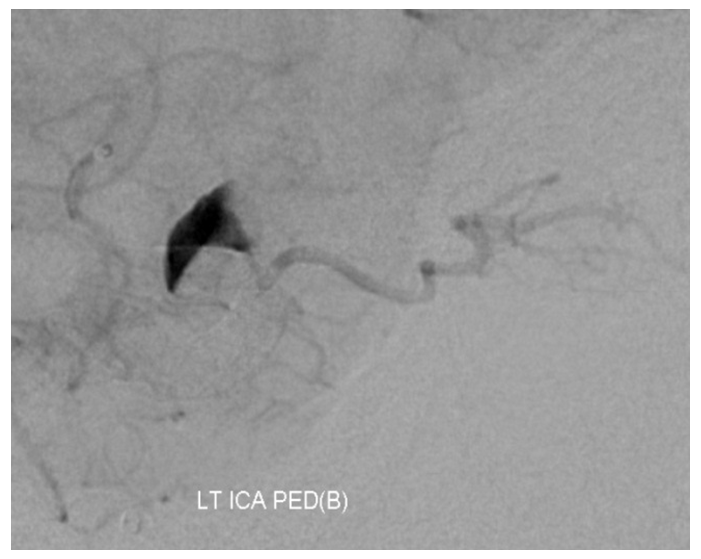
After embolization, the aneurysm remains patent, with runoff into the ophthalmic artery.
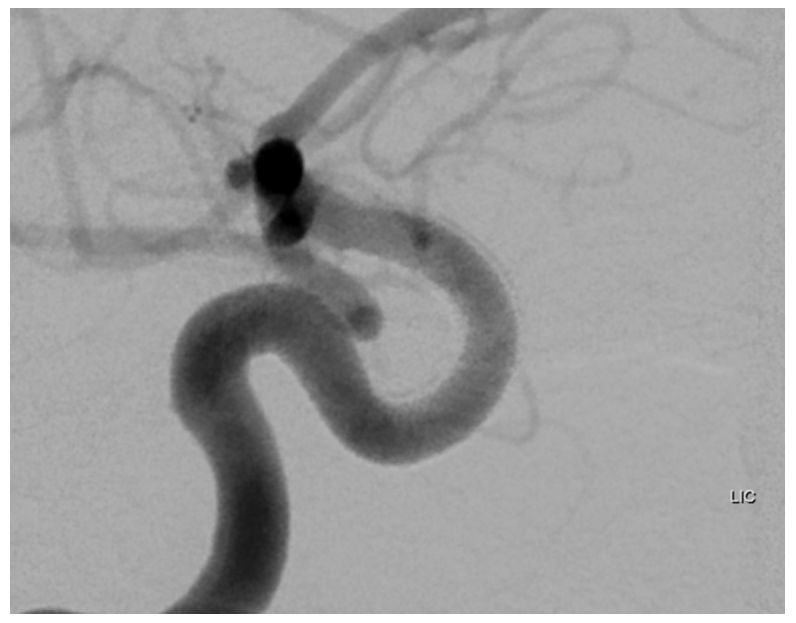
Following placement of additional device, both aneurysm and ophthalmic artery are closed
The solution to avoid this problem is to size the device to the segment of potential non-opposition. This is not the typical area where people measure vessels, and it tends to be wider than at least the distal landing zone, but that’s what you have to do. Also important in such a case is to make sure the Pipeline is well-deployed and not stretched, opposing the walls fully at the segment of concern.
2. Previously Stent-Coiled Aneurysms
To be perfectly frank, there is very little reason out there to continue using stents in instances where a “flow diverter” has proven to work better. Stent-coiling aneurysms which are amenable to flow diversion not only exposes the patient to a procedure of demonstrably lesser efficacy, — it also limits possibilities to rectify the consequences of initial treatment choices.
Devices such as pipeline work by endothelialiation, which requires contact with the vessel wall and absence of endoleak around the device or device construct. Indwelling stents nearly always prevent this contact and promote endoleak formation. To work around this, the Pipeline needs to be implanted across the entire length of the stent, with both distal and proximal landing zones extending beyond the stent. This usually leads to very long constructs, as the indwelling stents are typically quite long — very often much longer than was necessary. Just like some Pipelines, these stents also are often deployed in a stretched manner, which further reduces optimal stent-to-vessel apposition. Certainly, it is possible to cure a stent-coiled aneurysm, but the efficacy is significantly reduced.
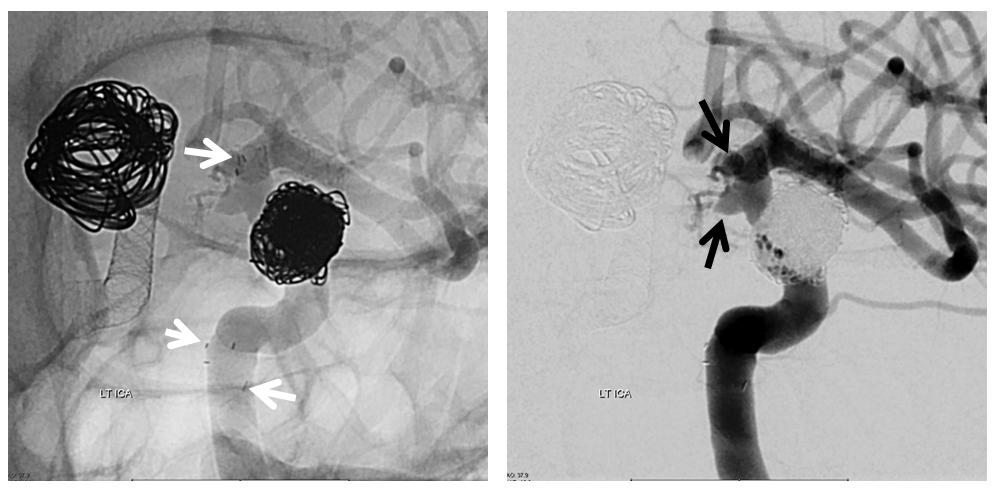
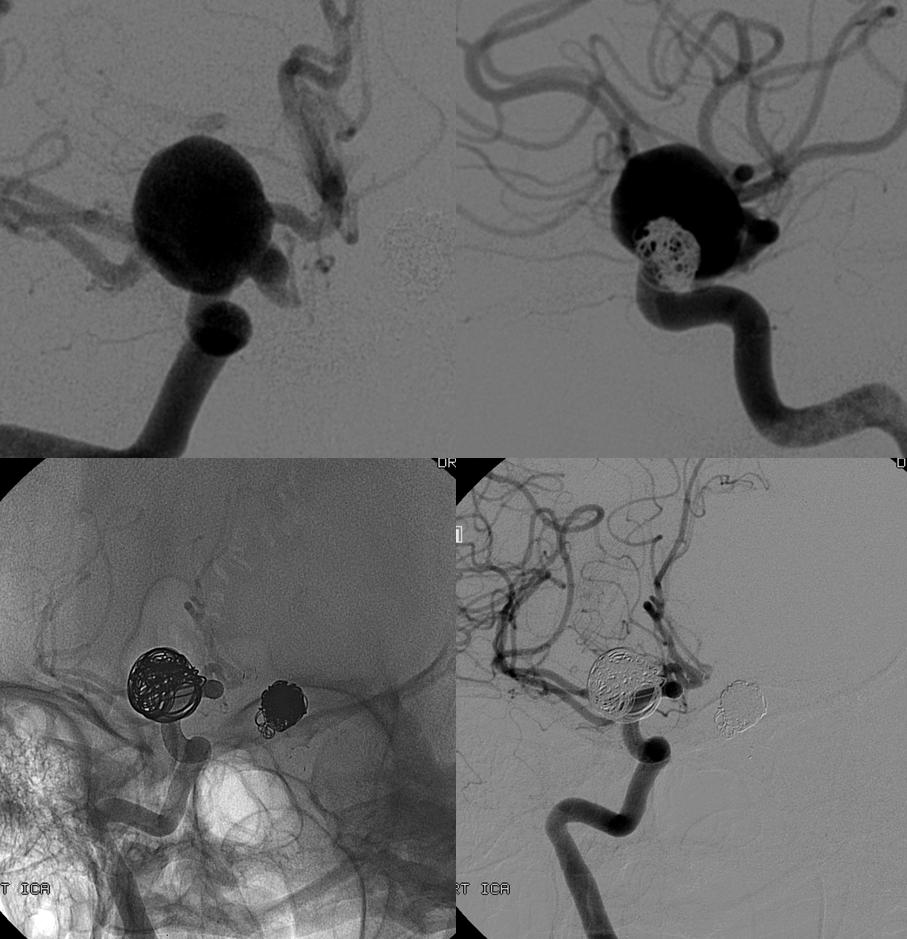
Here is a previously neuroform-coiled case. Notice how long this stent is — from the choroidal segment (black arrows point to choroidal and PCOM aneurysms) to the vertical cavernous (lower white arrows)
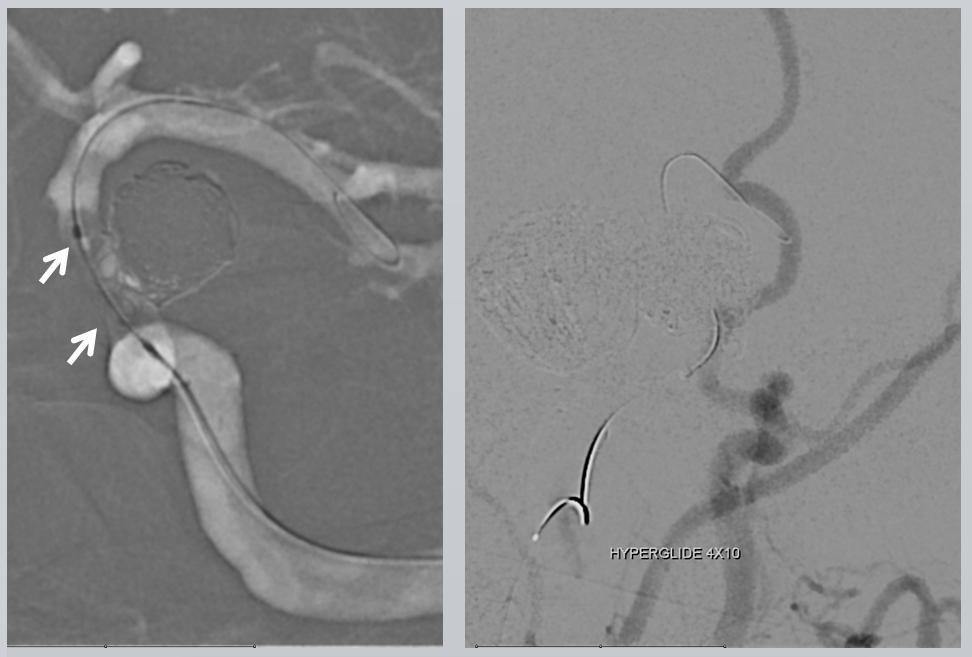
One way to know how the stent is sitting in a vessel is to inflate a balloon in it. Here, a Hyperglide 4×10 mm compliant balloon is inflated in the PCOM segment, demonstrating good wall apposition. Injection of the guidecatheter below the balloon (right image) shows runoff into the external vasculature only, confirming that the balloon is completely occlusive
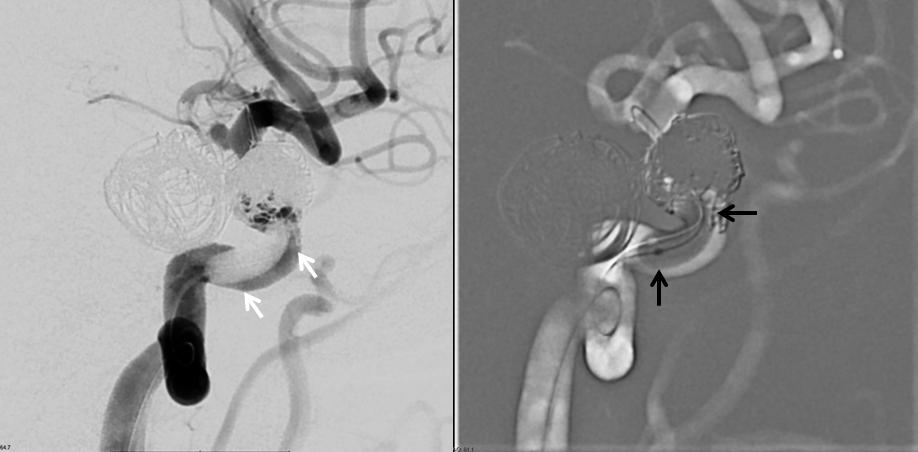
Now, we move the balloon proximally, into the cavernous segment — where the over-inflated balloon (black arrows) is nowhere near the vessel wall (white arrows) and there is plenty of flow getting around the balloon from a more proximal carotid injection (left image this time)
There is no point in placing the pipeline unless one wishes to put it all the way down into the vertical cavernous or horizontal petrous segment. The recurrence at this point is not worth it. We therefore pipeline the PCOM and choroidal segments in this patient with a ruptured aneurysm, as is our general approach. The short PED distal segment does not cover the A1, while the proximal end is within the Neuroform portion where good apposition to the vessel is possible based on balloon inflation test shown above
By contrast, the contralateral right paraophthalmic aneurysm was a priori treated by Pipeline embolization with adjunctive coiling, and is completely cured. A straighforward and effective 1-step procedure — how much better than unpleasant choices between endless follow up or complex re-treatment for the left side!
Below is another aneurysm, a ruptured PCOM, stent-coiled with subsequent recurrence
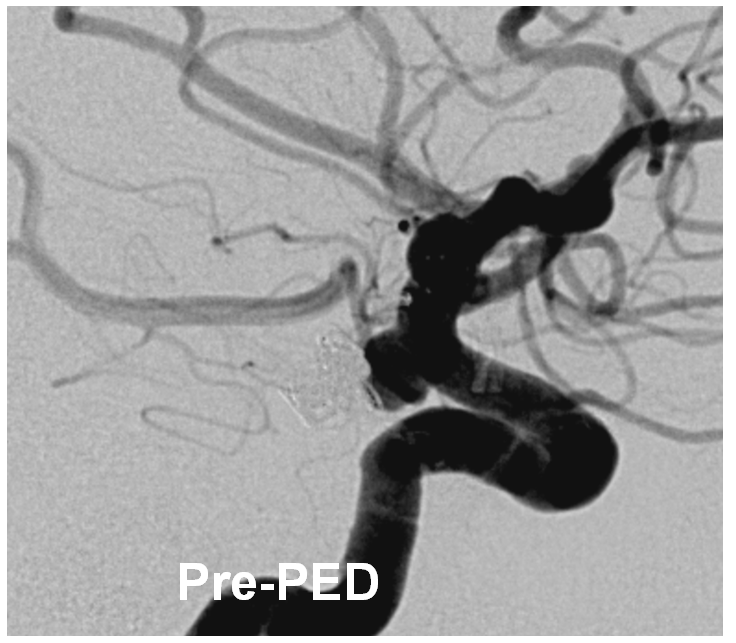
Post-PED, there are clear areas of non-apposition (yellow arrows) between the pipeline (white arrows) and the vessel wall. Neuroform markers are shown with black arrows. This Pipeline was not extended into the horizontal cavernous segment, as we would do now.
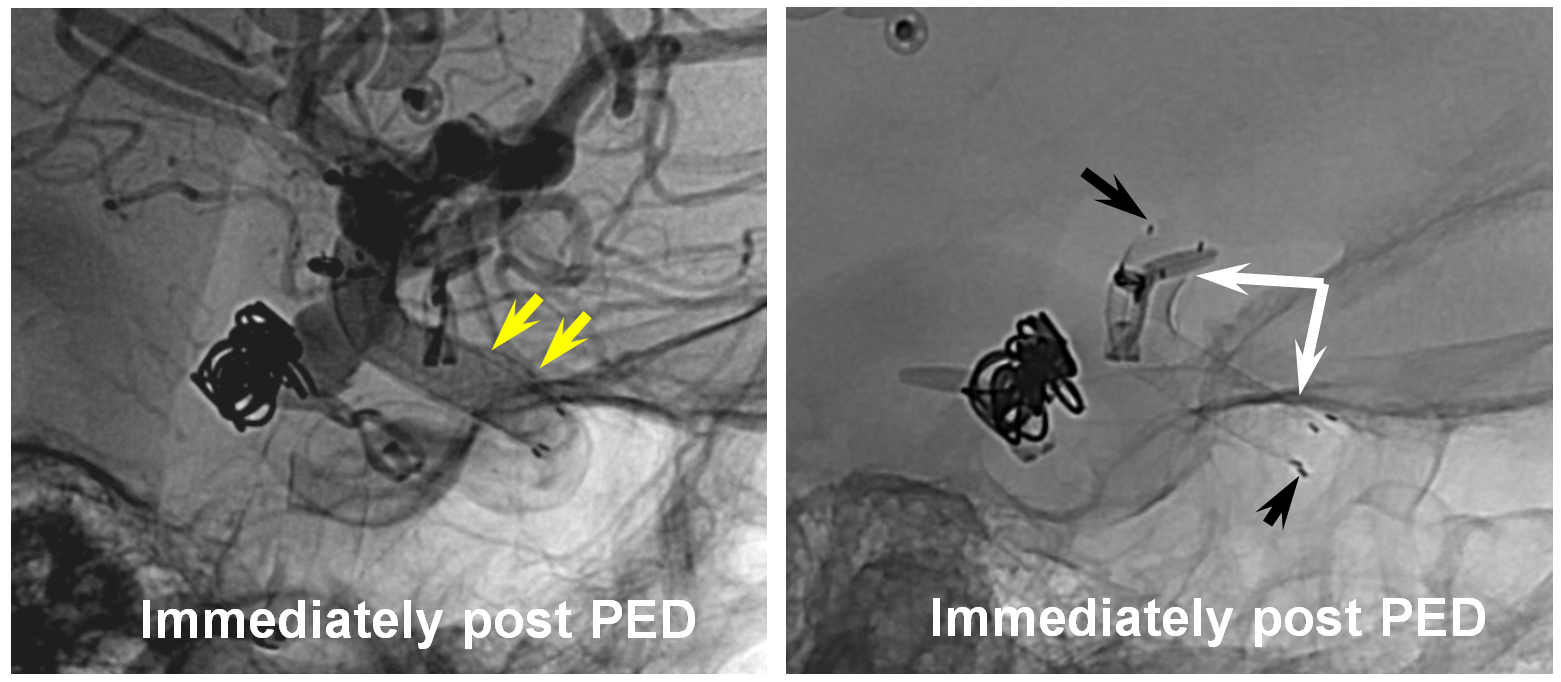
The result, 12 months later, is pretty much the same as before. All kinds of non-appositions and endoleaks are present (white arrows)
3. Aneurysms with insufficient metal coverage
The reason Pipeline works the way it does is because of metal coverage, and not lack of it. Unless there is a dense enough scaffold for endothelial overgrowth, there will not be a cure, which exposes the patient continued risk of aneurysm expansion, rupture, and leaves open the still unanswered question of what to do with antiplatelet coverage in cases of incomplete occlusion — should the antiplatelets be dialed down to promote thrombosis in hopes that it will progress to cure, or continued at high levels because lack of cure implies possible continued non-endothelialization and risk of thromboembolism or parent vessel occlusion.
While it is possible to get away with unduly low coverage for small aneurysms that are at very small risk of rupture anyway, without obvious short-term harm, this does not work with large or giant aneurysms, which tend to produce all kinds of symptoms unless they are actually cured.
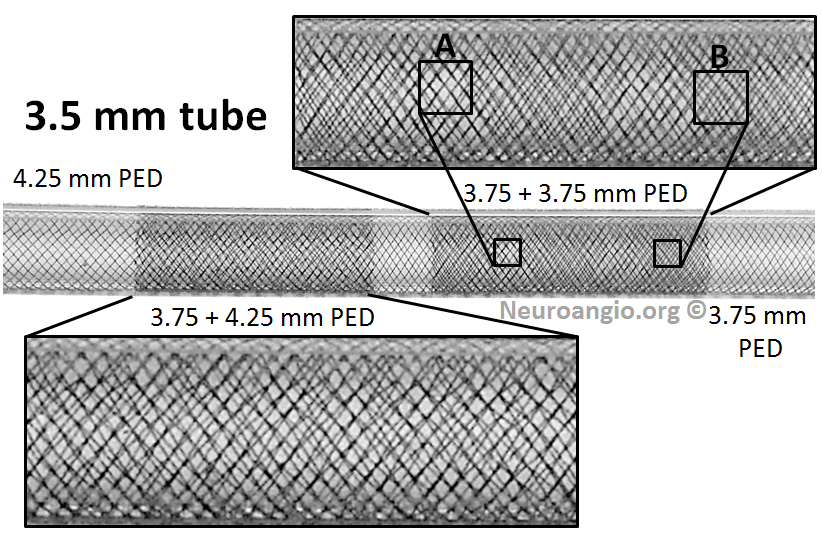
While it is not our practice to dogmatically treat always aneurysms with only one Pipeline, any more than to always coil an aneurysm with only one coil, or clip an aneurysm with no more than one clip, it is possible for aneurysms to remain un-occluded even with multicoverage. The reason for this has to do with variations in focal rather than overall metal coverage (see Pipeline Device Properties page for full discussion). Briefly, overlapping Pipelines of the same diameter produces areas of essentially no more coverage than for a single pipeline because of near-perfect overlap of the braids of the two devices — see box A for overlap of two 3.75 mm diameter PEDs below. For this reason, we typically overlap with progressively larger diameter devices to create a more even coverage surface — as you can see below from overlap of 3.75 and 4.25 mm devices.
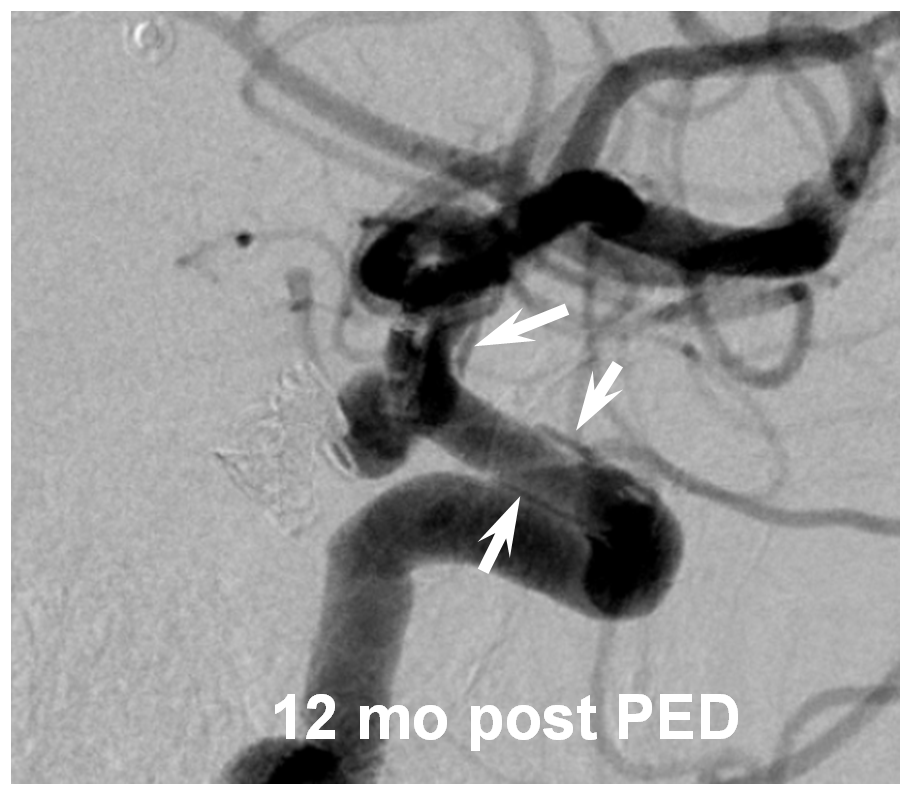
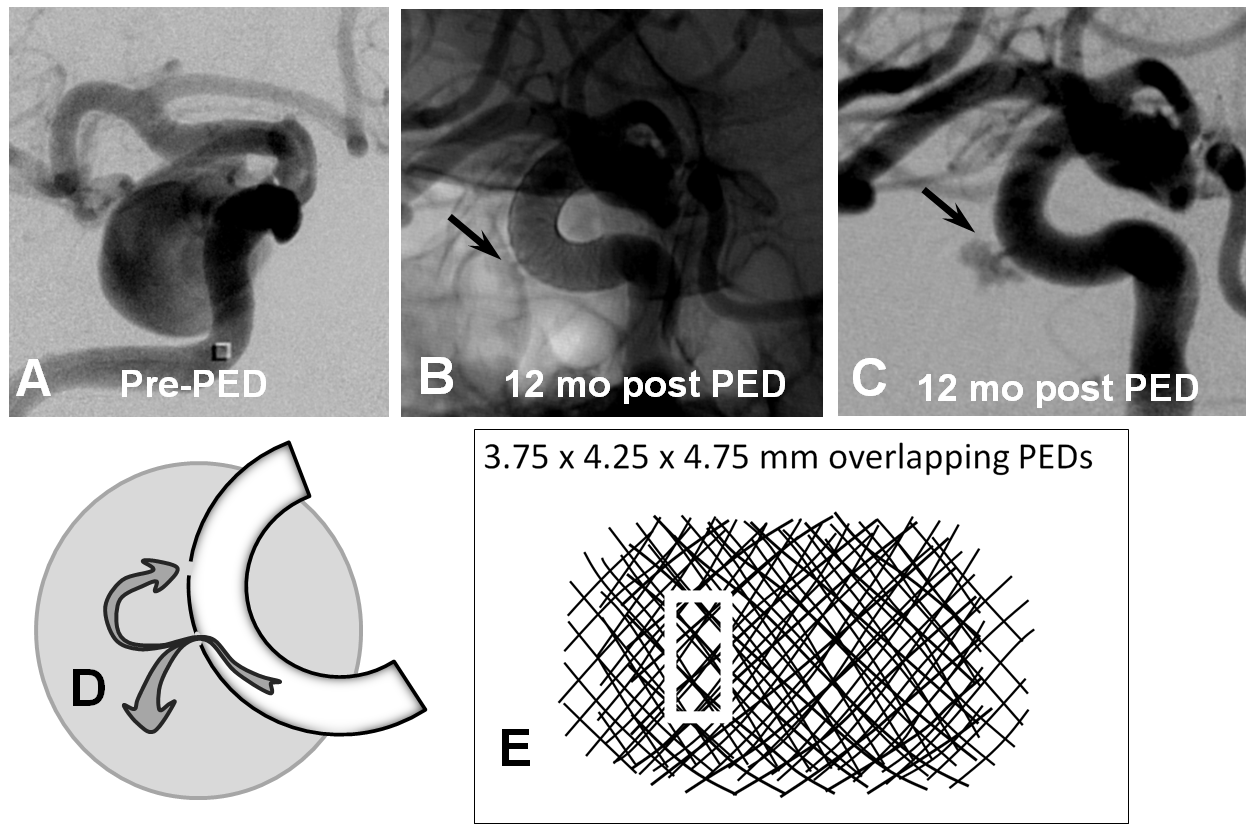
However, even in these instances, there may be foci where for quasi-random reasons the actual coverage is low. This is especially true on curvatures, where coverage is already lower. Here is our best example of this phenomenon. There is a focus of low coverage on the convex curvature of the Pipeline construct (black arrows in B, C). E shows how overlap of even 3 PEDs can lead to an area of relatively low coverage (white rectangular box)
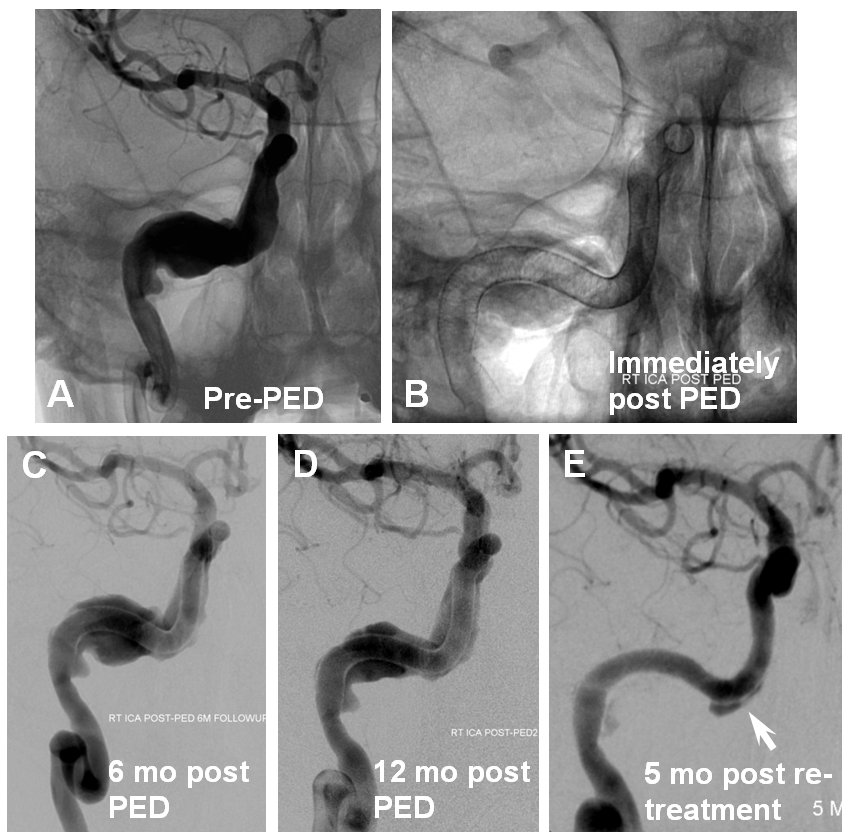
This kind of problem is even more prevalent in giant fusiform aneurysms where, in proportion to the length of Pipeline construct which requires endothelialization, there is higher probability of focal coverage deficiency. As one can see from images below, there is little progress between the 6 and 12 month angiograms in this case; with re-treatment, there still remains an areas of deficiency, particularly along convexity.
The unknown in all of these cases is almost certain variability in endothelial propensity among different patients. There is no currently available method to predict or influence this most essential biologic quality upon which treatment success depends.
Here is another example of persistent aneurysm due to lack of endothelial coverage at points of covexity, where coverage is expected to be lower
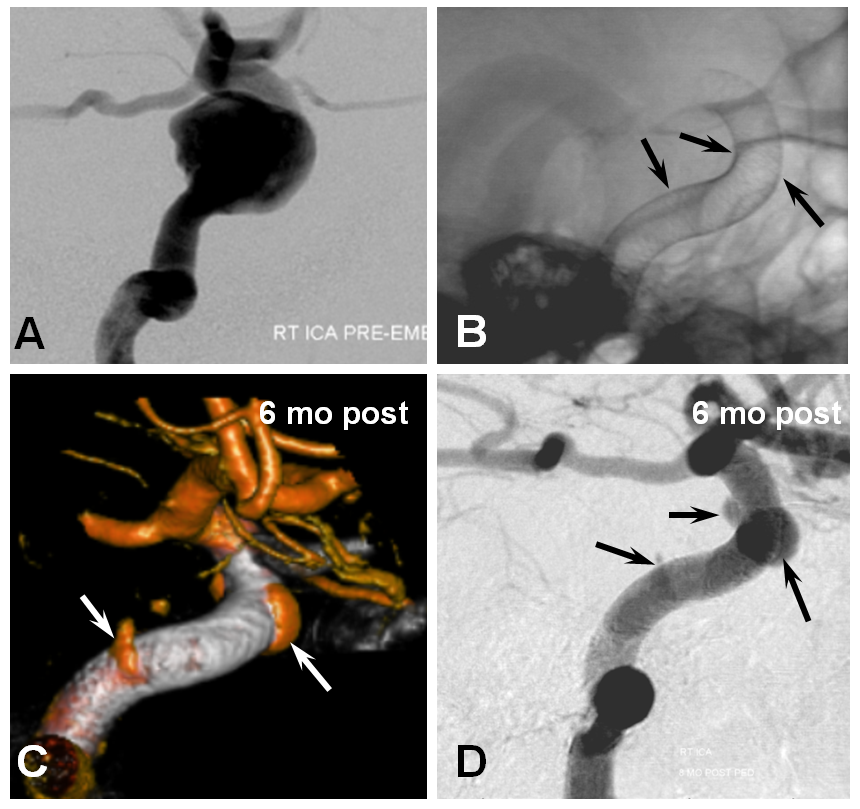
4. Poor Device Implantation — this one should be obvious. Stretched devices, or those which do not appose the vessel wall, are going to create problems. The stretched ones may “migrate” over time — foreshortening to their physically optimal state of length and diameter — it is important to understand that this is not a problem with the device, which simply behaves according to its properties, but with deployment. The number one reason for this problem is a desire on part of the operator to bridge a large neck with a single device and leave it, hoping for the best. Aside from being a problem a problem with stretching, it is also not the optimal approach because of coverage-related considerations already discussed above. We do not, fortunately, have examples of such problems in our collection — there are however more than a few examples in the literature. Here is a pictorial example:
Wall non-apposition is another issue — devices which are not circumferentially apposed to the wall will lead to endoleak formation. While this is an obvious problem on the proximal end, distal endoleak is equally bad. Here is an example of one, with a distal endoleak due to small device diameter selection highlighted between white and black arrows
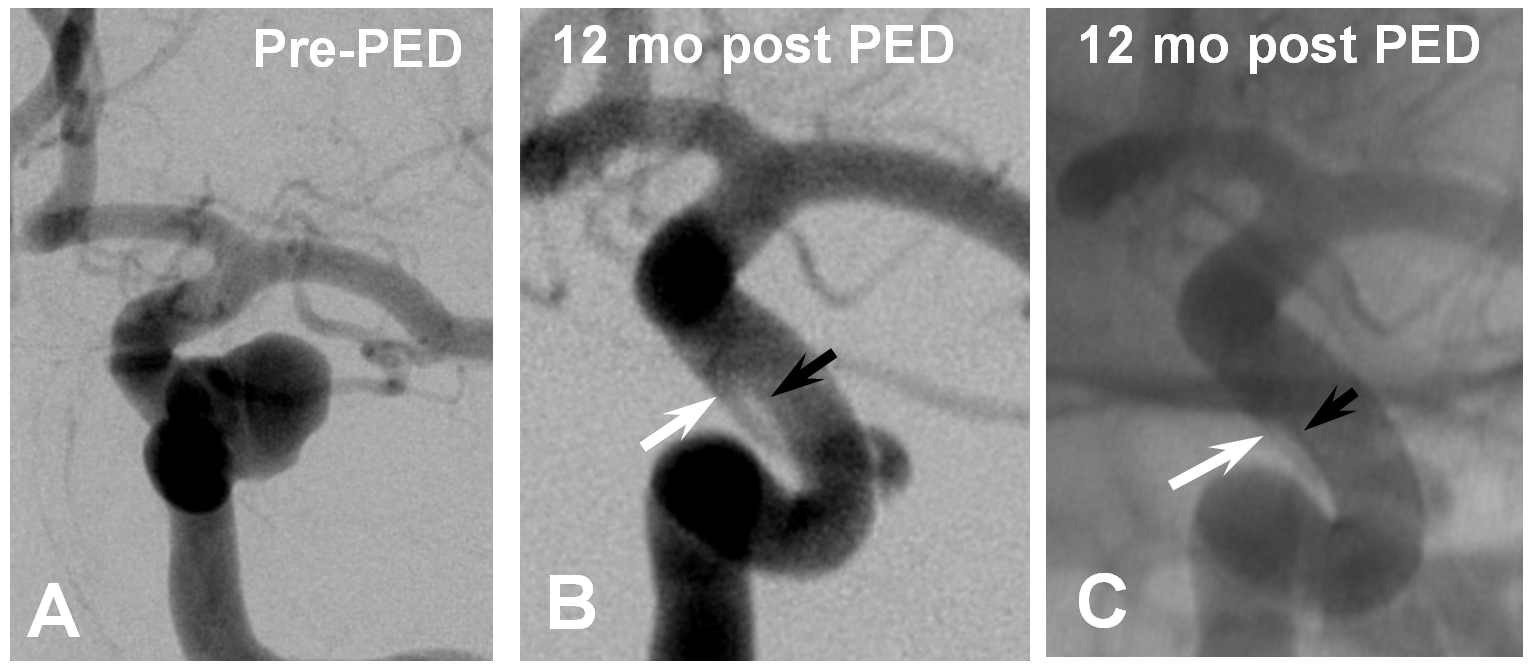
Late phase imaging showing how the endoleak serves as a source of aneurysm ouflow – the common theme between endoleaks and vessels incorporated into aneurysm compartment
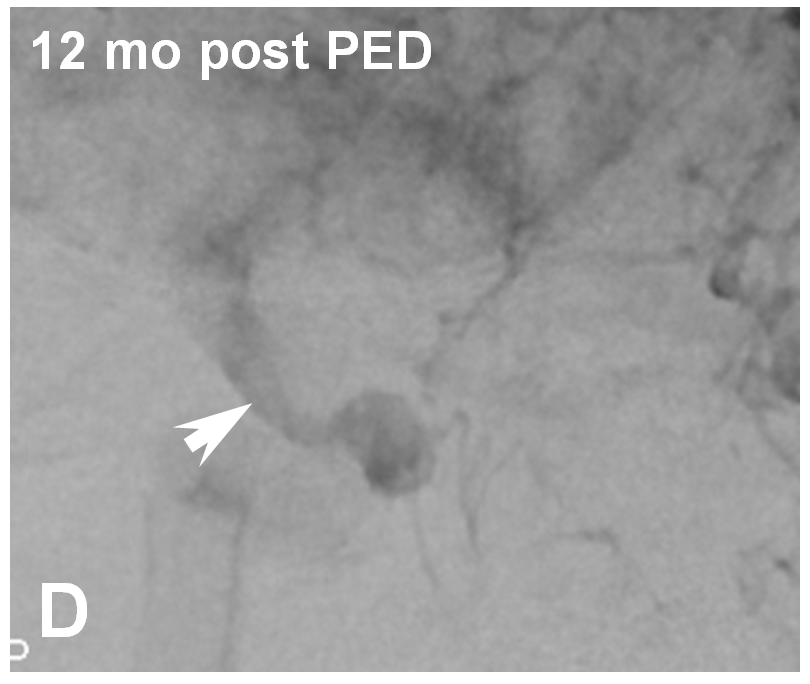
Re-treatment with a properly sized device at the distal end fixed the problem
Summary
Many aneurysms are amenable to Pipeline, including some of the types shown above. The key is understanding how Pipeline works, and thus identifying factors which may affect it.
As stated at the outset and shown later in detail, the mechanism of Pipeline success can be summarized very simply: Optimal aneurysm neck metal coverage, with no source for alternative aneurysmal inflow or outflow. The category of alternative flow sources consists of either branches primarily or secondarily incorporated into the aneurysm or endoleaks due to poor device implantation (too small a device not apposing the wall, pre-existing stents not allowing apposition)
In conclusion, here are some take-away points that will maximize the probability of success.
1. Carefully measure landing zone diameters. Many imaging systems, in my experience, systematically under- or over-measure distances. Spend time to understand your imaging system. Check by measuring length of catheter tip of known diameter (no need to formally “calibrate” to the catheter — just measure it and see if it is right. If not, adjust measurements proportionately.) The diameter of the 058 Navien, for example, is about 1.6 mm
2. Avoid secondarily incorporating branches such as ophthalmic artery into aneurysmal compartment by additional measurements, as shown above
3. Consider possibility of vessels incorporated into the aneurysm closing down as part of aneurysm closure. The clinical implications of this are minimal for ophthalmic and most PCOM vessels, for example. Choroidal and basilar branches are highly likely to be clinically symptomatic.
4. Deliberate oversizing (by about 1 mm) to minimize coverage of eloquent landing zone perforators can be part of a well-considered treatment strategy
5. Sufficient metal coverage is essential for cure, particularly with large and fusiform aneurysms; aneurysms which are not closed remain at risk for rupture and expansion. The practice of adjunctively coiling such aneurysms is a positive step that should be considered; however this is no reason to minimize neck metal coverage, especially since re-treatment with additional flow diverter devices is oftentimes more difficult after coiling due to visualization limitations
6. When creating multi-device constructs, avoid overlapping devices of the same diameter. Instead, typically choose slightly larger diameter devices to create more even metal scaffolds
7. Stretching devices to bridge long necks is a detrimental practice
8. Previously stent-coiled aneurysms may present access and efficacy challenges, for reasons shown above
9. Cross-sectional followup is essential to demonstrate treatment efficacy — large and giant aneurysms should either shrink or stay the same size after treatment — including coiled ones. Continued aneurysm expansion despite apparently good angiographic result is highly worriesome
Finally, nothing on this page concerns antiplatelet selection and monitoring — it continues to be an evolving topic with too much uncertainty and case-specific considerations to provide meaningful comments at this time
Questions, comments, complaints? — submit them right here.
Additional pages related to the Pipeline are
