Anterior Inferior Cerebellar Artery (AICA)
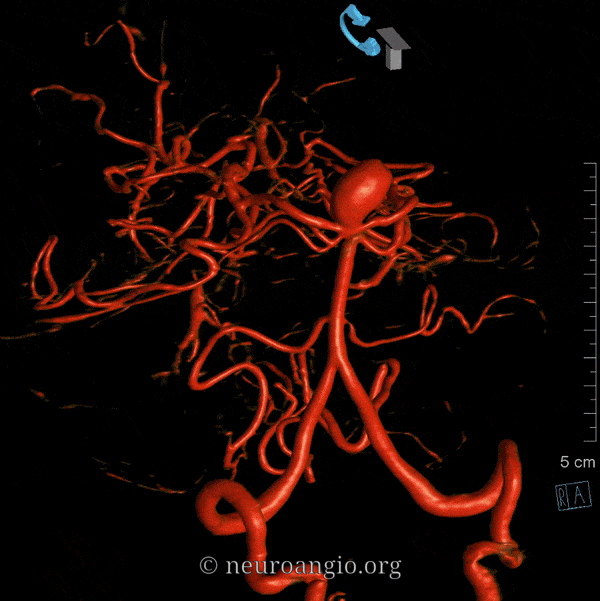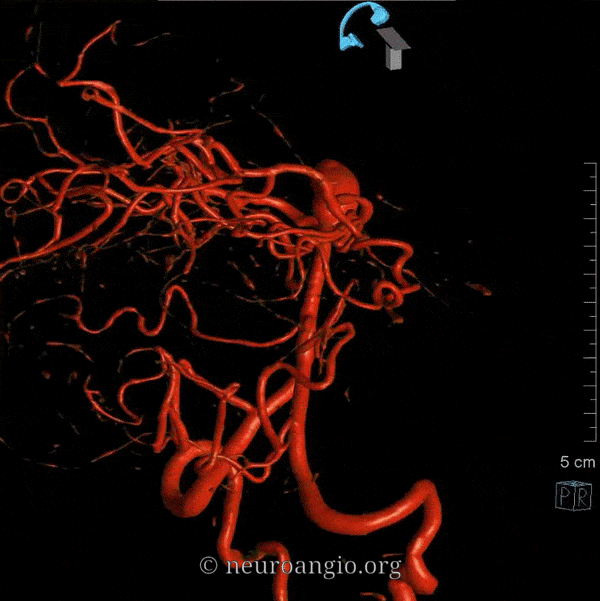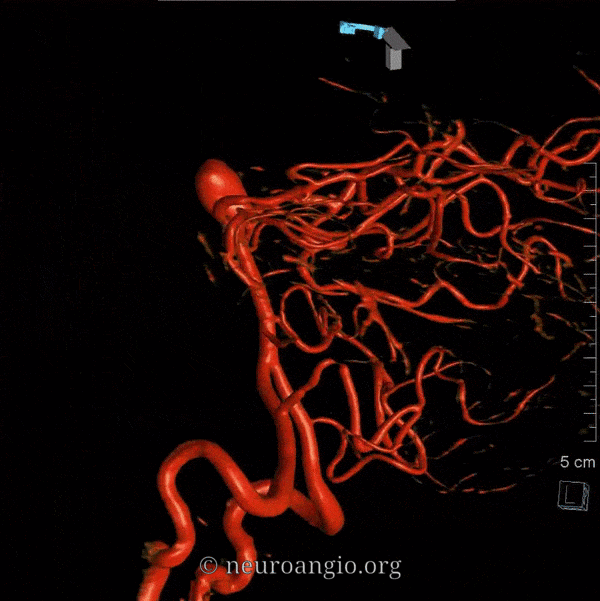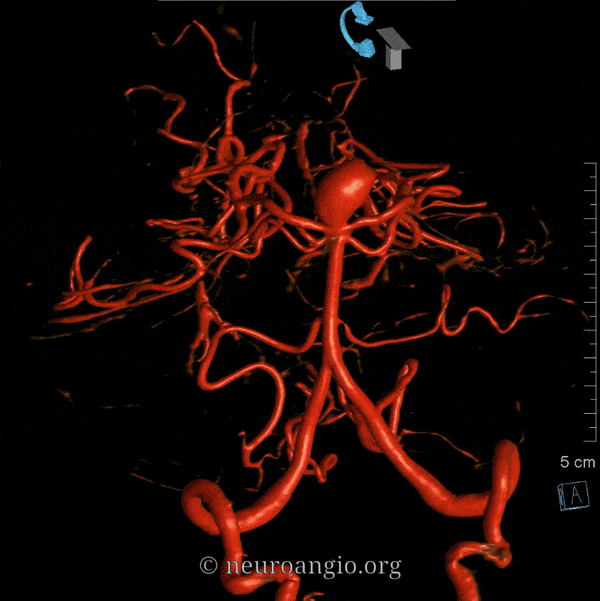
Balance: AICA is in well-known balance with the PICA, but also with the SCA.
Evolution and Embryology: Unlike PICA , which can be conceptualized as a cervical artery impressed into posterior fossa service by expanding needs of the cerebellum, the AICA is a true cerebellar and brainstem artery. It develops as a branch of the longitudinal neural system, a forerunner of the basilar artery. One can think of the early vertebrobasilar system as a longitudinal vessel (basilar) with a myriad transverse vessels, each having the potential to become SCA or AICA by capturing the cortical territory of the developing cerebellum. It seems that hemodynamics favor selection of a dominant vessel (or two) for this role, and so most transverse vessels remain confined to supply of the brainstem (perforators). The dominant vessels which emerge are thus named AICA, SCA, and PICA . This concept helps explain most variations seen within the arrangement — a duplicated AICA, for example, represents co-persistence of two adjacent trasverse vessels with cortical territory. The same goes for the cortical territory as well, where hemodynamic balance exists between SCA, AICA, and PICA in extent of cortical supply. Any variation is possible, depending on which trunk dominates the cortex. Dominance of AICA produces the well-known AICA-PICA variant. The reverse (PICA-AICA) is just as legitimate, with a small AICA and large PICA cortical supply. SCA can dominate as well. An “otic” artery — segmental anteroposterior anastomotic artery similar to persistent trigeminal and hypoglossal — has been postulated to traverse the inner ear to connect with the carotid — but has never been conclusively demonstrated to exist.
Origin and course: The AICA is a highly variable artery in its position along the basilar axis, and extent of cortical territory. Classically, it arises from the mid-basilar, and sweeps posterolaterally, covering ventral to pons in the prepontine cistern, to head posterolaterally within the cerebellopontine angle along the anterior cerebellar surface. Its supply, therefore, includes portions of pons and the anterior cerebellum. It gives a very important branch to the inner ear — the Labyrinthine artery — perhaps the most consistent branch of the entire AICA territory. An inconstant but important Subarcuate branch to the petromastoid (aka subarcuate) canal and adjacent dura / bone is an important component of many petrous apex and other regional dural fistulas.
Depending on hemodynamic balance, a more prominent AICA will continue inferiorly to capture the inferior cerebellar hemisphere and, potentially, the inferior vermis, due to relative hypoplasia of the PICA, corresponding to the aforementioned AICA-PICA variant. It does not, to my knowledge, ever extent its inferior cortical supply to the posterolateral medulla. Thus, even in extreme dominance of AICA, a small and, perhaps unrecognizable as such, “PICA” exists, limited to its medullary territory. This has important clinical implications, in that even dominant AICA strokes do not produce lateral medullary infarcts (Wallenberg and other similar syndromes), whereas vert occlusions at the level of small PICAs typically do. The inferior vermian territory can also be supplied by the SCA, which typically vascularises the superior vermis.
Conceptual homology of vertebrobasilar and spinal arterial anatomy
The basilar artery is formed by fusion of the longitudinal neural system, which in its most primitive form consists of loosely connected channels running along the undersurface of the brainstem. Lasjaunias and his colleagues view arterial system of the brainstem and cerebellum as a natural extension of the segmental arrangement found in the spinal cord. The conceptual brilliance of this view allows one to understand all the myriad variations to which the basilar artery and its daughter vessels are subjected. In other words, if you consider the basilar artery to be a continuation of the anterior spinal artery, and its named branches and perforators as homologs of the coronary and sulco-comissural arteries (see Spinal Vascular Anatomy section), then the overall arrangement and its possible variations make perfect sense.
The following diagrams serve to illustrate this concept. On a personal note, I generally find anatomical diagrams to be somewhat wanting; when applied to the living body, they too often suffer from both rigidity and inconsistency, and almost universally fall short of the predictive potential for which their creation was originally intended. In this case, however, I believe that the genius of Lasjaunias (and supporting giants), may prove an exception. It is not, by any stretch, The Periodic Table, but some time investment into a bit of theoretical discussion is likely to produce major dividends.
Below is a diagram of cervical spinal vasculature (left), and brainstem vasculature (right), without the cerebellum.
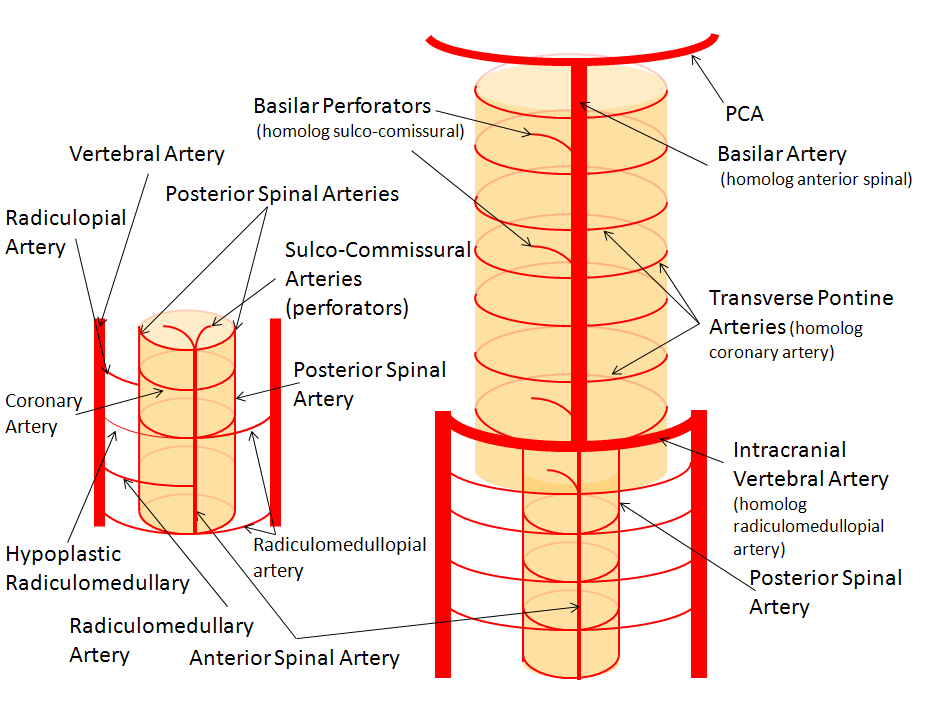
The image on the LEFT represents cervical spinal cord arterial supply, which consists of the anterior spinal artery and a paired, loose network of posterolateral vessels known as the posterior spinal arteries, and which are conceptually represented here as contiguous vessels (which is at least mostly true in the cervical spine). The anterior and posterior spinal systems are connected by anastomoses running along the circumference of the cord, although known as “coronary” arteries, are conceptually quite clear. A number of perforating arteries into the substance of the cord exist; when arising from the anterior spinal artery and penetrating through the ventral cord sulcus, they are named “sulco-comissural” arteries. The entire spinal cord system is supplied via segmental radiculomedullary arteries, which connect the vertebral artery to the anterior spinal artery. In practice, as you know, the radiculomedullary and radiculopial arteries are fewer, and may arise from longitudinal vessels other than the vert. Radiculopial arteries are those which supply the posterior spinal system. Radiculomedullopial arteries are those which happen to supply both anterior and posterior spinal systems simultaneously, sometimes via a coronary artery or via separate connections. For a more complete discussion of spinal vasculature (essential, I believe), see Spinal Vascular Anatomy section, particularly Spinal Arterial Anatomy.
Now, lets add the brainstem to the spinal cord, and use existing arterial vascular networks to furnish its supply. Think of the brainstem as just a somewhat larger diameter biomass than the spinal cord, and things start to make sense. The unapaired basilar artery is a homolog of the equally unpaired anterior spinal artery. The intracranial vertebral arteries, although obliquely oriented, are essentially homologs of the radiculo-medullary arteries, inasmuch as they serve as transverse connections between the extraspinal vertebral system and the anterior spinal axis. The transverse pontine arteries are homologs of the coronary arteries. The basilar perforators are homologs of the sulco-comissural arteries. The posterior spinal arteries, in the superior cervical spine, are sometimes termed “Lateral spinal arteries”. This creates much unnecessary confusion, but the posterior spinal system and lateral spinal system are one and the same longitudinal arrangement.
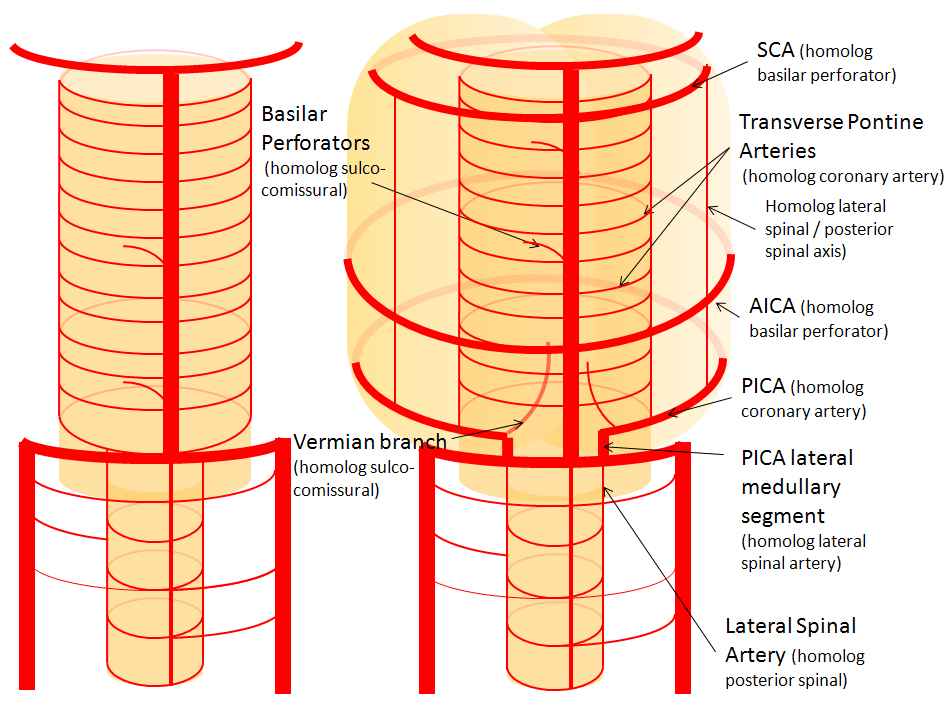
Now, add a cerebellum to the back of the brainstem, — again, simply more biomass — and use existing arteries to supply it. As the cerebellum develops, some of the transverse pontine perforators are recruited to capture the cerebellar hemispheric territory. Superiorly, this happens relatively consistently, and produces what is known as the Superior Cerebellar Artery. At the mid to lower basilar segment, a homologous enlarging channel is the AICA. At the bottom, the Posterior Inferior Cerebellar Artery (PICA) is the latest addition to cerebellar supply, Unlike AICA and SCA, it seems to arise from the lateral spinal system (yet nevertheless also a coronary artery homolog). The vermian arteries (of which only inferior is shown here) may be regarded as homologs of the sulco-comissural vessels.
There are many advantages to viewing the vertebrobasilar system in this way. All kinds of variants become quite predictable. For example, duplicated and triplicated SCAs and AICAs are simply persistence of adjacent transverse pontine (or midbrain) arteries in supply of the cerebellar hemisphere. AICAs arising higher or lower along the basilar are either results of dominance of higher or lower transverse arteries, or consequent to a relatively “short” basilar artery fusion. C1 origin of PICA reflects dominance of the C1 radiculopial artery, which via the C1 segment of the lateral medullary artery, gives rise to the PICA. The AICA-PICA balance in extent of cerebellar territory capture is a consequence of either anterior spinal (AICA) or lateral spinal (PICA) dominance.
In conclusion to this long introduction, we state one of neuroangio’s principles — individual variability is best understood when seen as part of a whole. The second key principle — understanding vessels not by site origin but by territory of supply — is a fundamental anatomical axiom. All of these cases are given angiographic illustrations below.
Basilar artery perforators

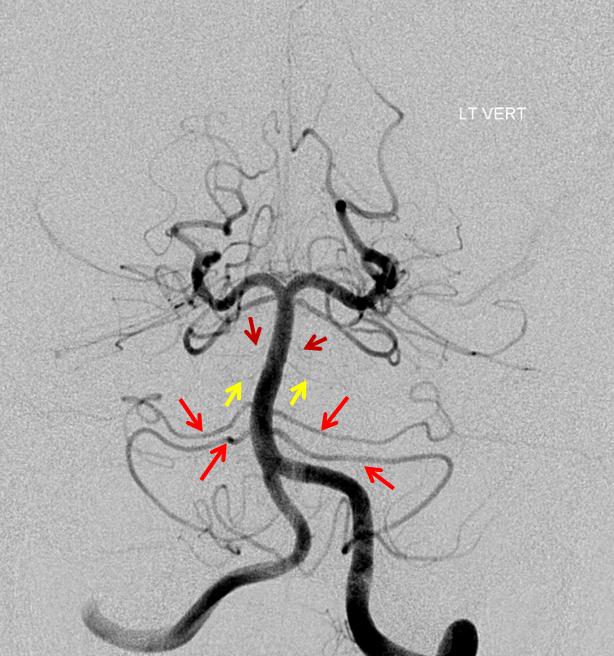
Duplicated AICAs: Bilateral AICA duplications (red arrows), in setting of AICAs dominance (AICA-PICA). Notice the low position of the AICAs with respect to vertebrobasilar confluence. Two groups of “ziggii” perforators are seen superior to the AICAs — yellow and brown arrows — yellow ones being located in position where “classical” AICA origin might be expected. This disposition illustrates the above concept of AICAs arising as a dominant perforator which captures the cortical territory of the cerebellum (similar to how MCA develops from ACA perforators, see Vascular Neuroembryology). In this case, it so happened that two of of the more inferior (with respect to basilar artery) perforators developed into “AICAs”, whereby the more superior (yellow and brown) perforators are slightly larger than usual due to hemodynamic need for supply of the brainstem and, probably, some anterior cerebellum, typically addressed by branches of the more rostrally located “classical” AICA. In other words, the yellow perforators might be what typically becomes AICA, but in this patient the lower ones did, also capturing PICA territory.
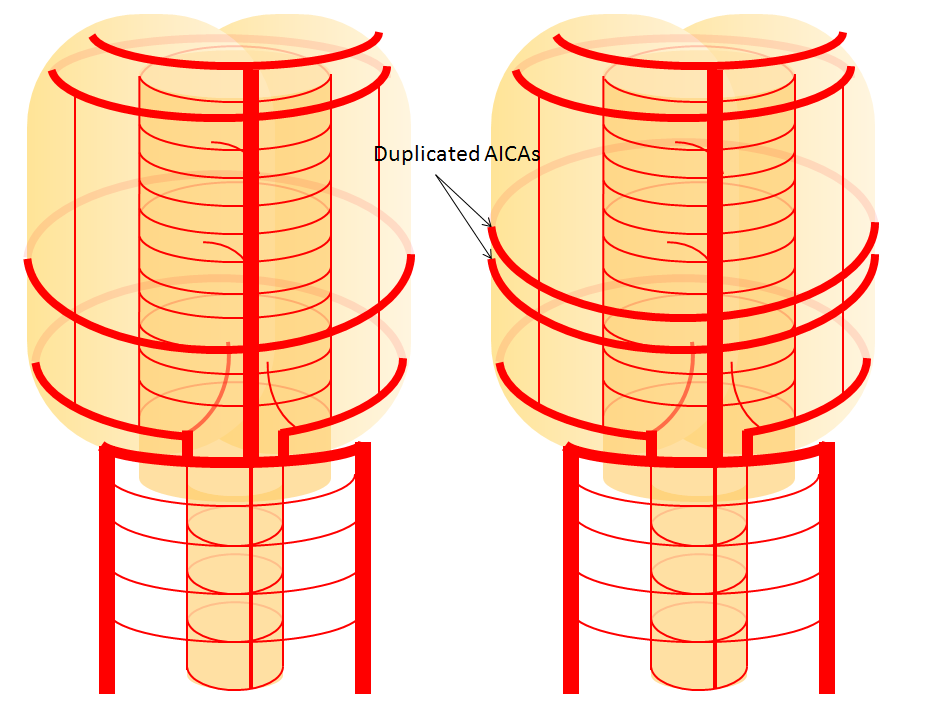
Schematic of AICA duplication (right), as co-dominance of adjacent perforators in capture cerebellar territory. Classical dispostion is on the left.
The perforators between AICA and SCA are of great functional significance, typically supplying the ventral pons. Occlusion of these often manifests as a hemiparesis.
Duplicated, dominant AICA
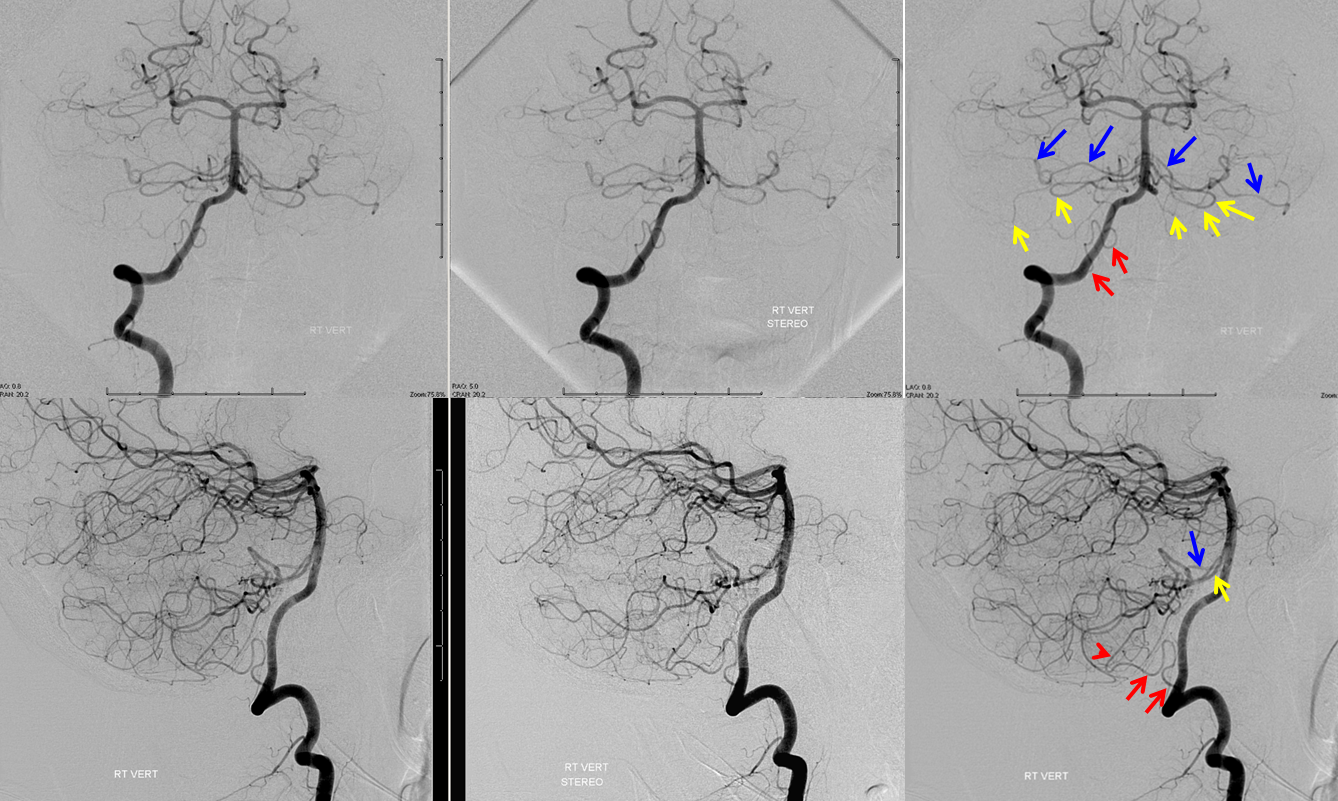
Bilateral duplicated AICAs. The superior ones (blue) supply the anterolateral cerebellar hemispheres, while the inferior ones (yellow) have a more medial territory, in this case. A relatively small, C1 origin right PICA (red) is limited to medullary and inferior vermian territory.
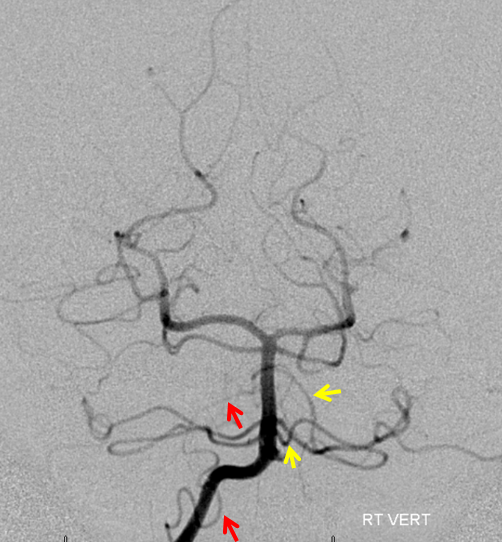
Earlier phase of the same patient, showing AICA duplications somewhat better. Notice how left lower AICA (yellow) curves medially to balance the vermian territory of the right C1 origin PICA (red).
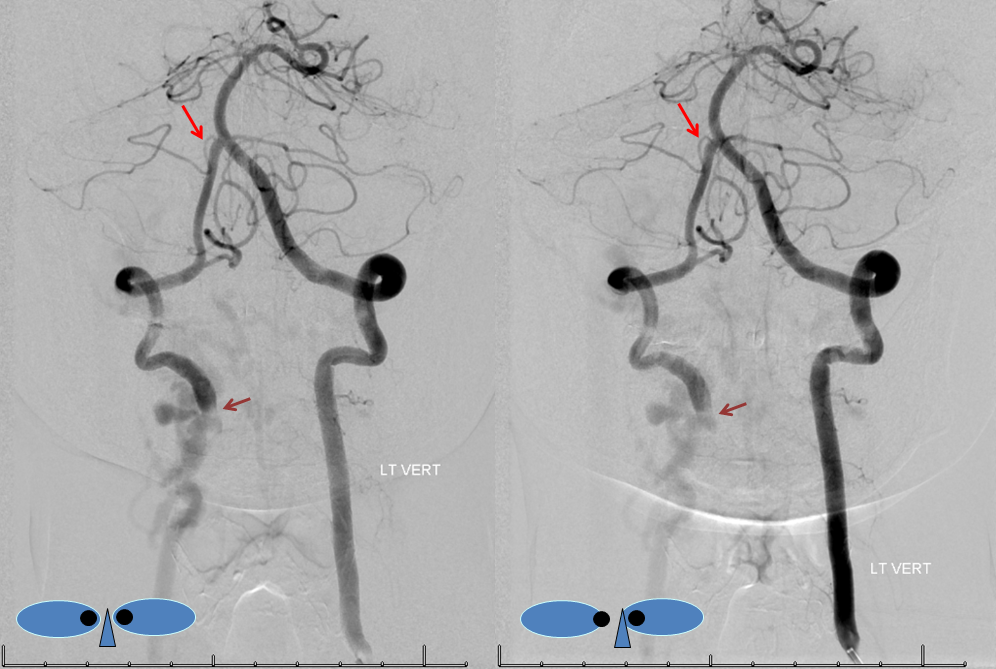
Submental view of the basilar artery, showing different positions of left left and right AICA (red arrow) origins — again supporting the notion that AICAs develop from a number of possible choices — representing basilar artery perforators. Notice also the perforators themselves (yellow arrows), of which there are two visible ones on the right (likely because of larger distance between SCA and AICA) and one on the left (shorter SCA/AICA distance). Both AICAs are about equal in size, vis-a-vis AICA/PICA balance. The “Post Rx” refers to stenting of right superior cervical vert dissection/pseudoaneurysm, below the field of view.
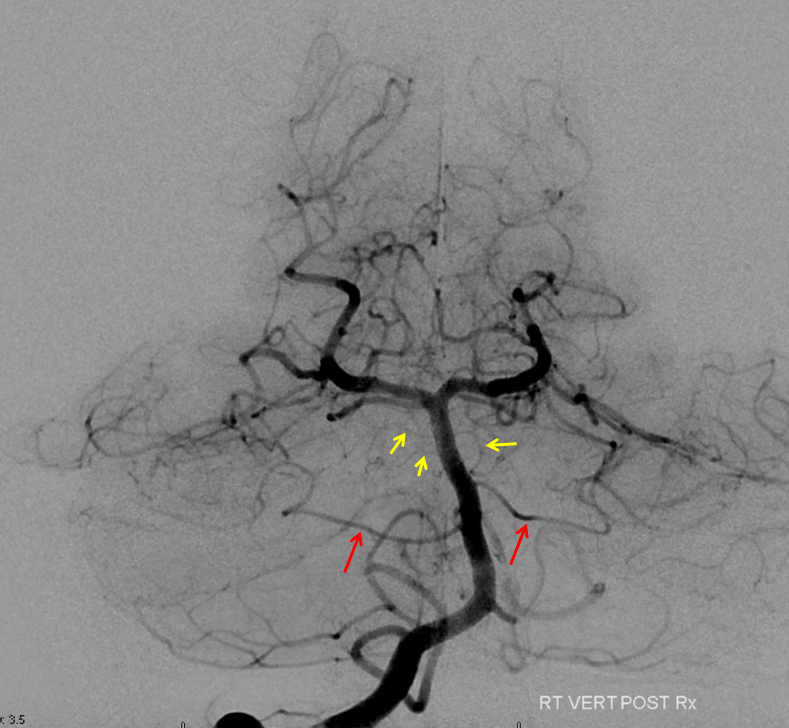
High vertebrobasilar junction (meaning short basilar, in contrast with the one immediately above), such that right AICA comes off the distal vert, in a patient with right vert spinal fistula (brown). Again, its just a matter of which perforator gets selected for capture of cerebellar territory. Short basilar means a VB junction somewhere around mid-pons (classically, it is at the pontomedullary junction), and so the AICA comes off where it does in relation to the cerebellum.

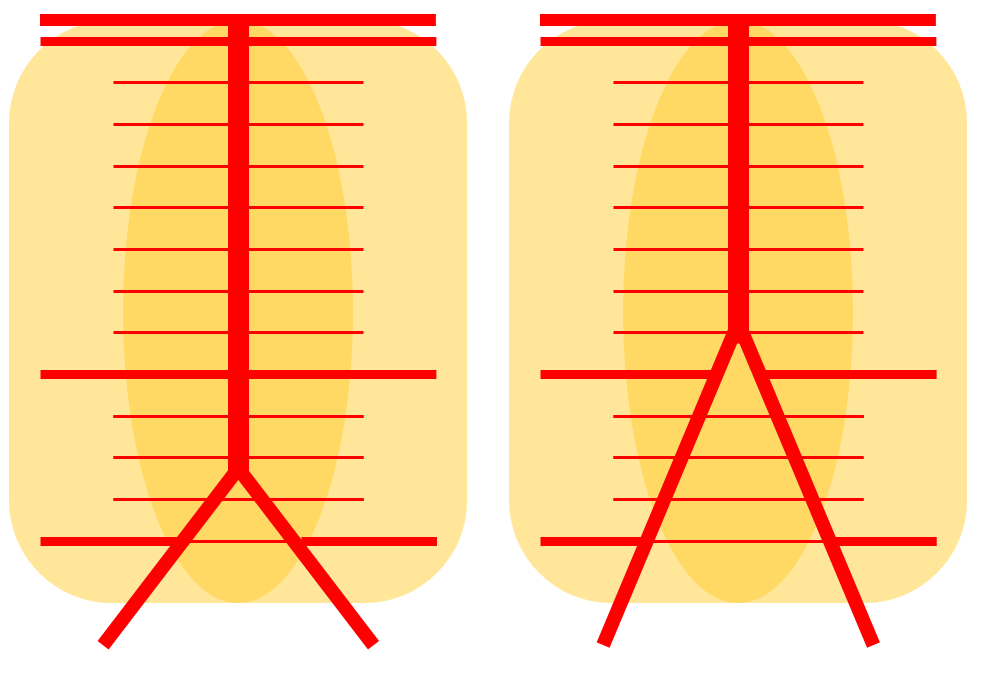
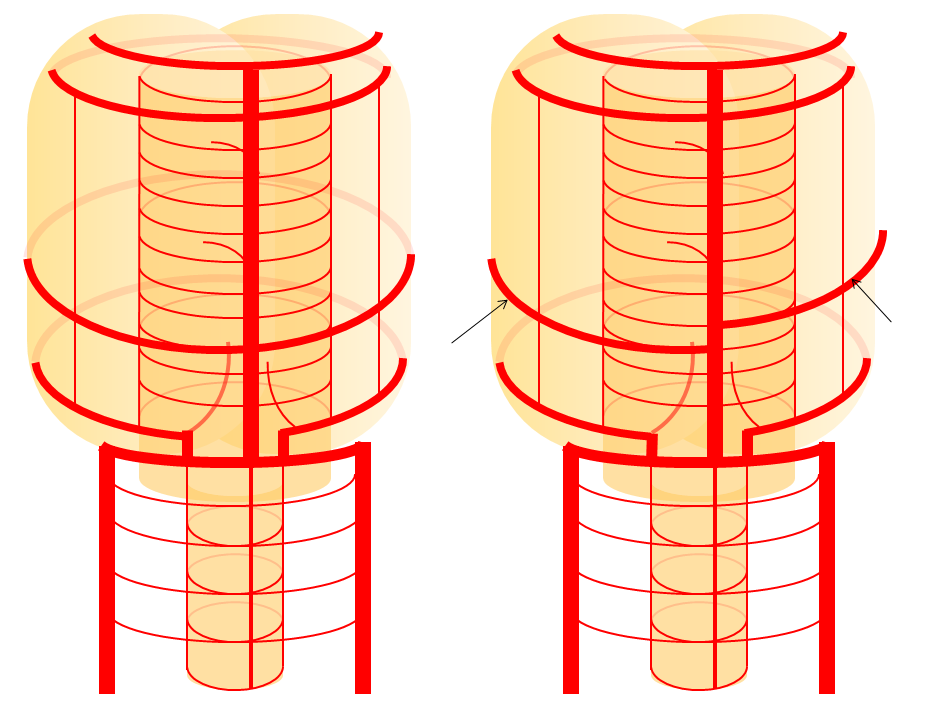
Diagrammatic representations of this disposition (right), with foreshortened basilar artery leading to origin of the AICAs from distal vertebral arteries, as compared with the classical arrangement (left)
Bihemispheric AICA-PICA
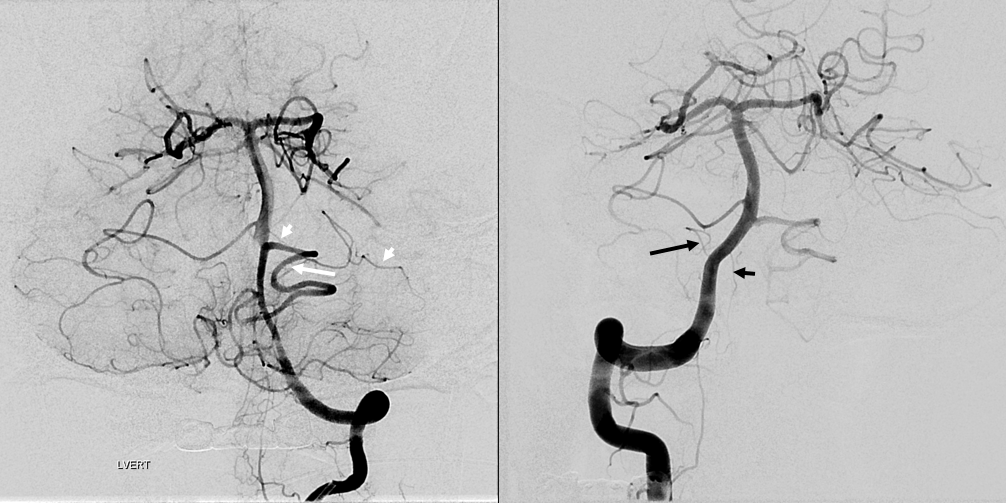
Occasionally, either left or right PICA will supply bilateral PICA territories (see PICA page). So, why cant an AICA-PICA do that? Of course it can, just very rare. Here is a left AICA-PICA so big (white arrow) it also takes care of the right PICA cerebellar hemisphere. Notice a small additional AICA (white arrowheads) as well. Also note prominent anterior spinal (black arrowhead) and lateral spinal (black arrow) pedicles — as would be expected (see PICA page)
SCA-AICA-PICA balance
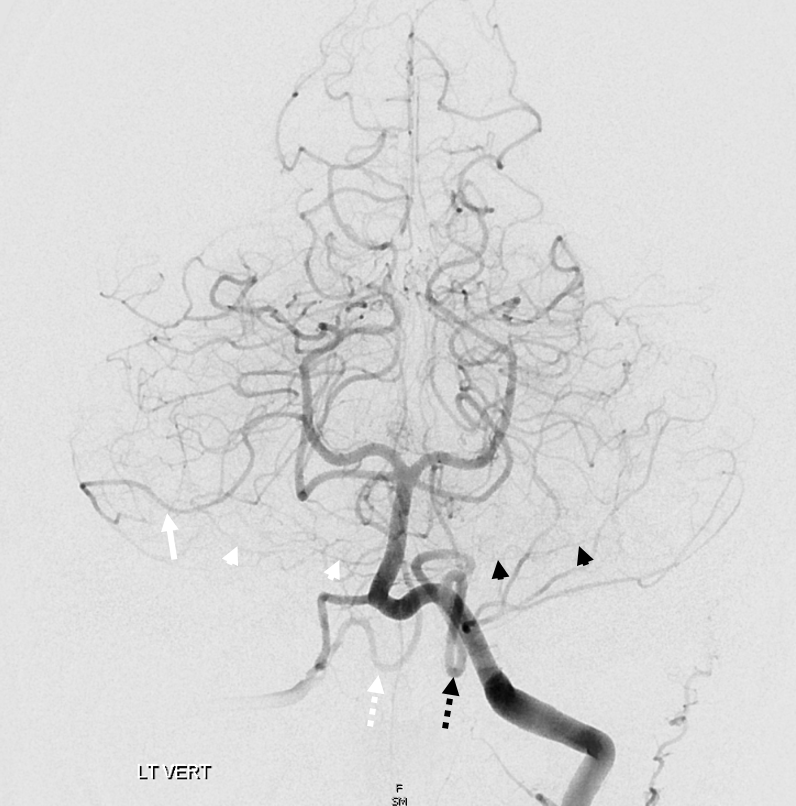
The overarching notion of “spectrum” in on display here. The important thing is terrritory of supply, not site of origin. How to put it all together? The right AICA (arrowheads) is small. Right PICA (dashed white arrow) supply is limited to vermis. What then supplies most of right cerebellar hemishere — in this case, the SCA (white arrow) — note larger caliber of right SCA compared to left. On the left, the AICA is also hypoplastic (black arrowheads). The PICA (dashed black arrows) is dominant, and controls lateral hemisphere. Right SCA holds usual superior surface.
Another “global” analysis — pre (left) and post (right) Pipeline embolization of right vert aneurysm. On the right, AICA (white arrow) is dominant. The right PICA (unalabeled) supplies inferior vermis. On the left, there is a “duplicated AICA”. However, this perception is only true insofar as the “lower AICA” (dashed black arrow) originates from the proximal basilar artery. In reality, this vessel is in every other respect a PICA — supplying classical PICA inferior hemispheric territory. The “true” AICA is the upper vessel (black arrow). If vertebrobasilar fusion had been a bit higher, the dashed black arrow vessel would be called a PICA instead of a duplicated AICA. In short — territory of supply is key. Site of origin is not.
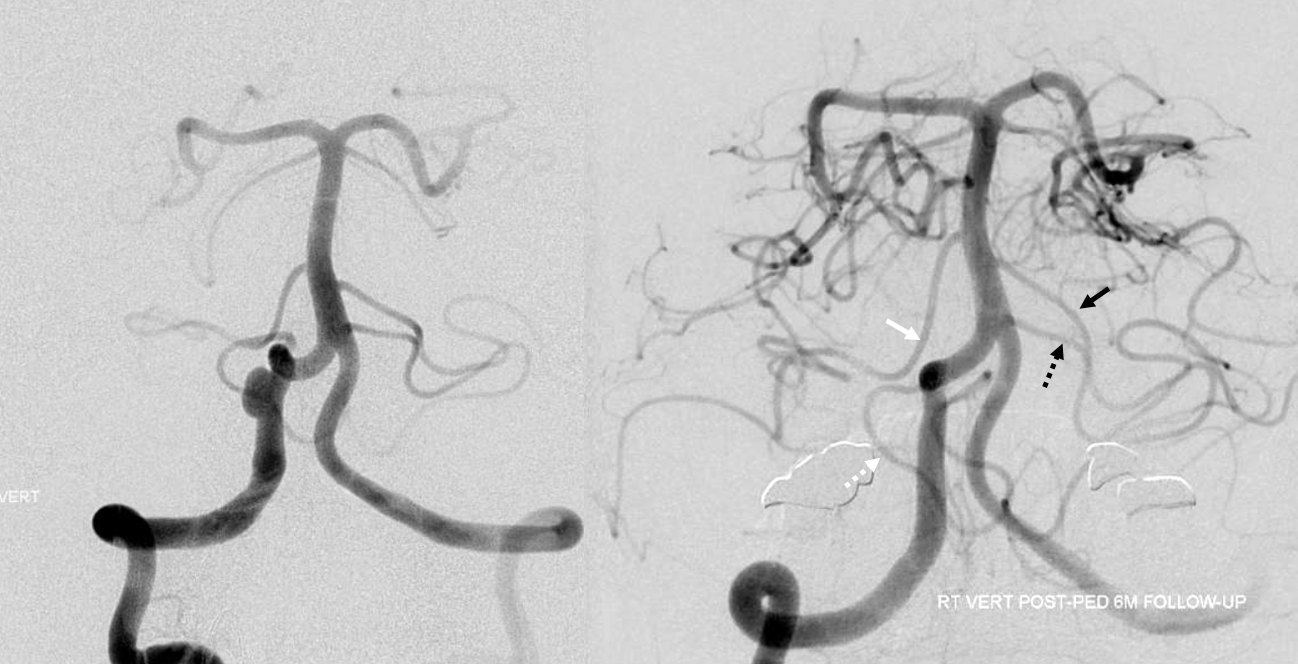
Another illustration of this concept — the seemingly bilateral duplicated AICAs below. Notice how long the basilar trunk is. The classic “AICA” vessels are marked by arrowheads. The lower (arrow) vessels are in every respect PICAs, except for their site of origin from this extra-long basilar trunk. The hypoplastic right vert is marked by white ball arrow. Because of the relatively low origin of the “true” AICAs, multiple prominent basilar perforators are seen between the AICAs and the SCAs (unmarked).
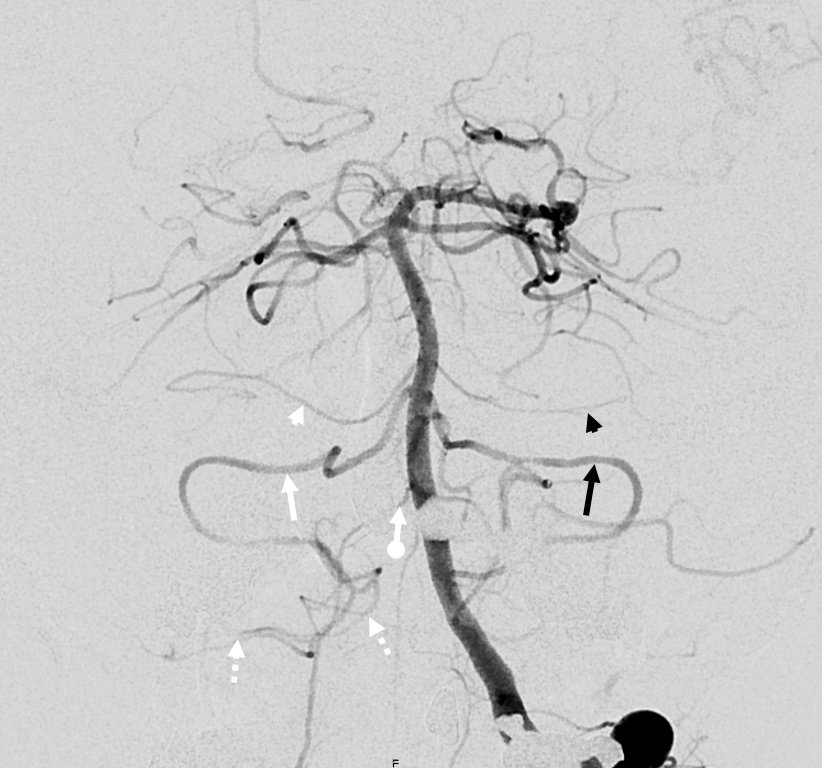
Want more? How about this — see below — the left AICA is hypoplastic. Left PICA (white arrows) supply is limited to vermis. The left SCA is small. How come? There are two possibilities — either the tissue is missing (infarcted, resected, etc), or there is yet another source of supply. The answer is Trigeminal Artery — (black arrows). It often supplies “AICA territory”. However, insofar as there is really no such thing as “AICA territory” — it being in balance as we keep saying — the extent of trigeminal cerebellar supply is as variable as anything else. Here, it is responsible for a big chunk of it (white oval in frontal view), self-evident in lower right lateral view — taking care of both lateral and inferior right hemisphere.
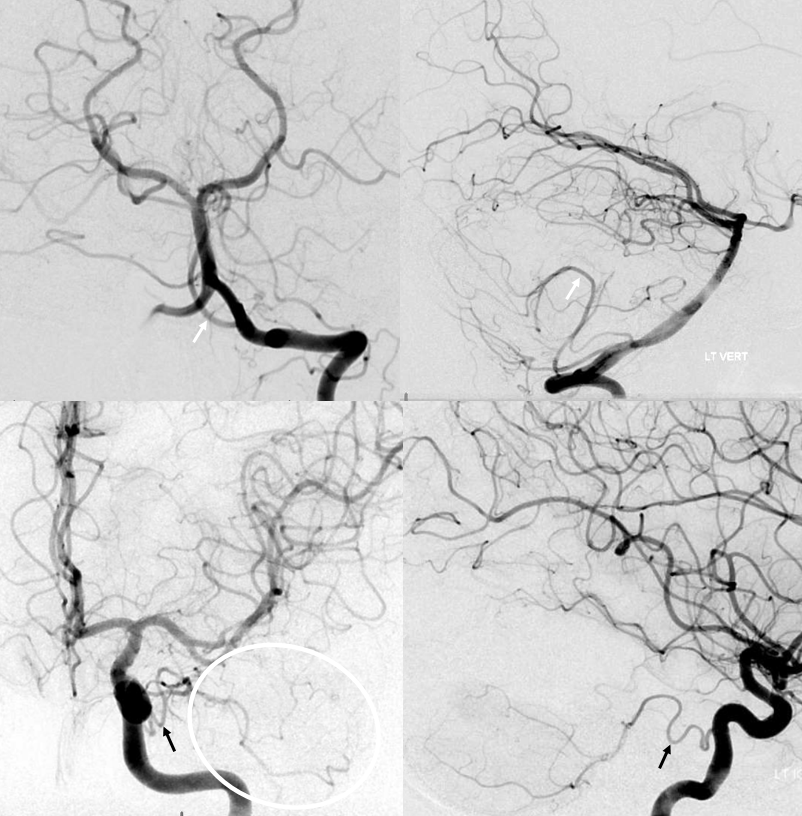
Ascending Pharyngeal Supply of the AICA
Very very rare. Here is an MRA example — via pars nervosa of jugular foramen. Outlined by solid white circle. SCA is dashed white circle. By Eytan Raz MD PhD
Without circles
Had enough? Next will come some clinical examples…
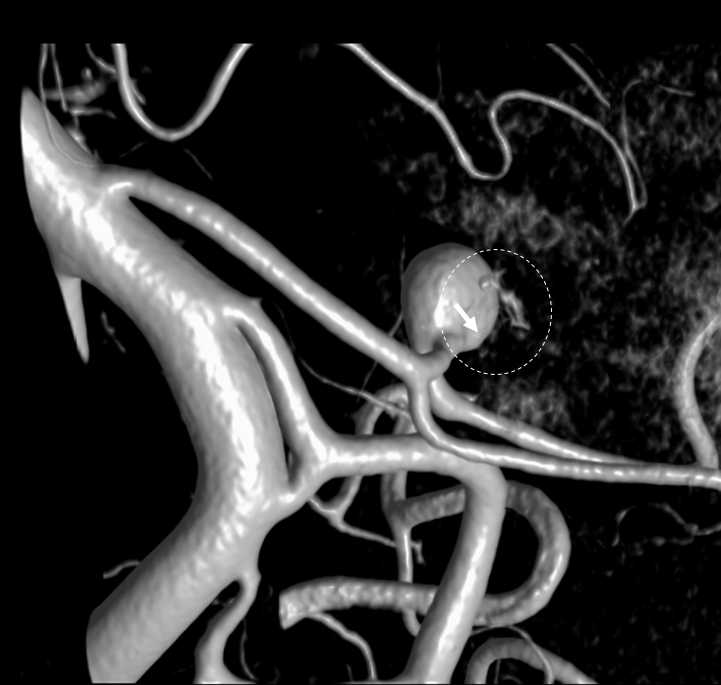
Check out this great case by Dr. Raz — tying together the embryology and skill to treat a fusiform AICA aneurysm
Cochlear Supply — Labyrinthine Artery
Classically, there are two AICA-origin arteries supplying the vestibulochochlear region. The labyrinthine artery is responsible for CNVIII and cochlea / inner ear structures. Presumably in balance with inferior tympanic artery from the ascending pharyngeal. Not sure about that though — pretty much everyone with an occlusion at the AICA loop goes deaf — so where are the collaterals in that? Probably more like the central retinal artery. The labyrinthine artery usually originates from the AICA (maybe 90% of time?) most often at the level of the IAC AICA loop.
The other AICA origin artery, less constant, is the “subarcuate” artery. It is a piodural vessel — like the Davidoff-Schechter or the Wolfschlaegger and Wolfschlaegger. Contained in the petromastoid (aka subarcuate) canal. Supplies the adjacent bone and dura. It is this artery, presumably, which participates in the piodural anastomoses of dural fistulas — not the labyrinthine one.
In practical terms, embolization of AICA, distal to brainstem, carries risk of ipsilateral deafness. The labyrinthine artery is tiny, essentially never seen on DSA. Can be well seen on high-resolution CBCT. Its origin from AICA is one of early embryologic events — even in cases of dominant PICA, there is often a very small “AICA” vessel which, beyond the brainstem, essentially supplies the labyrinthine branch only. Be careful!
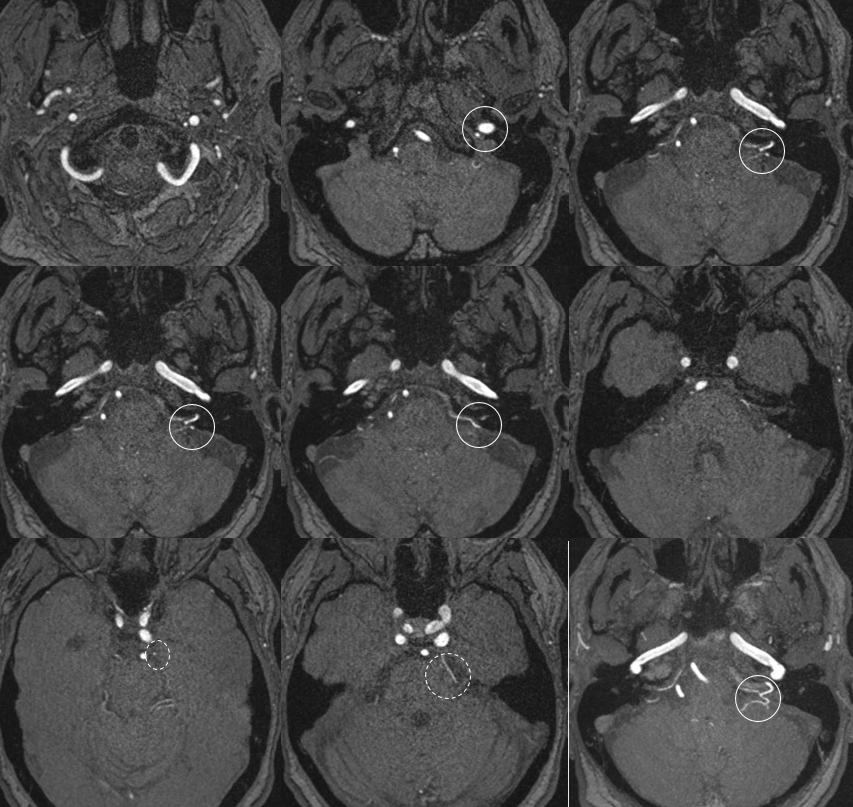
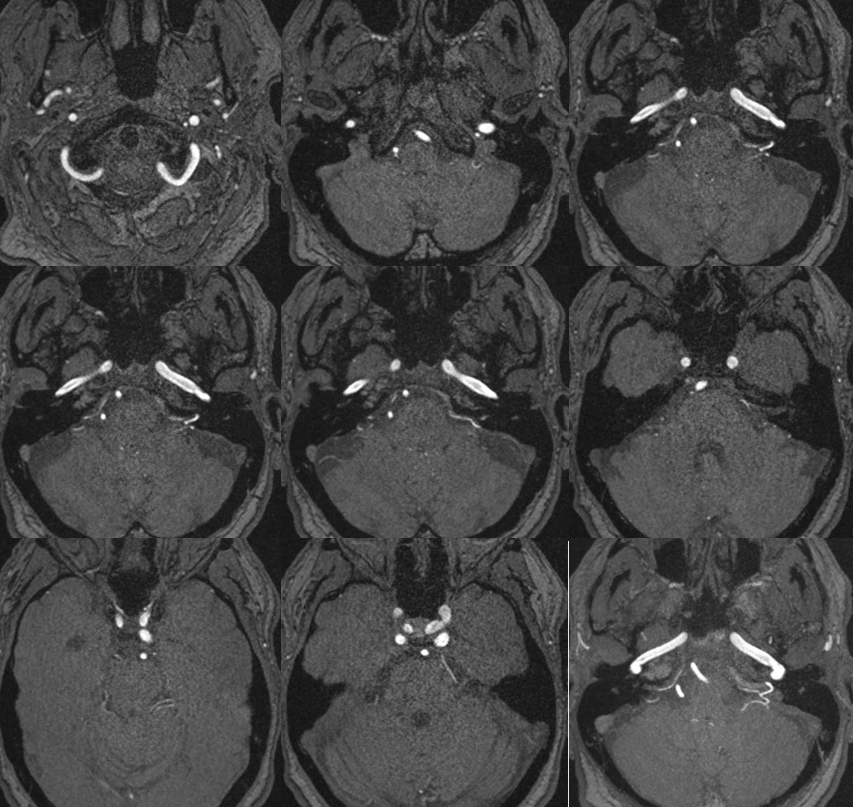
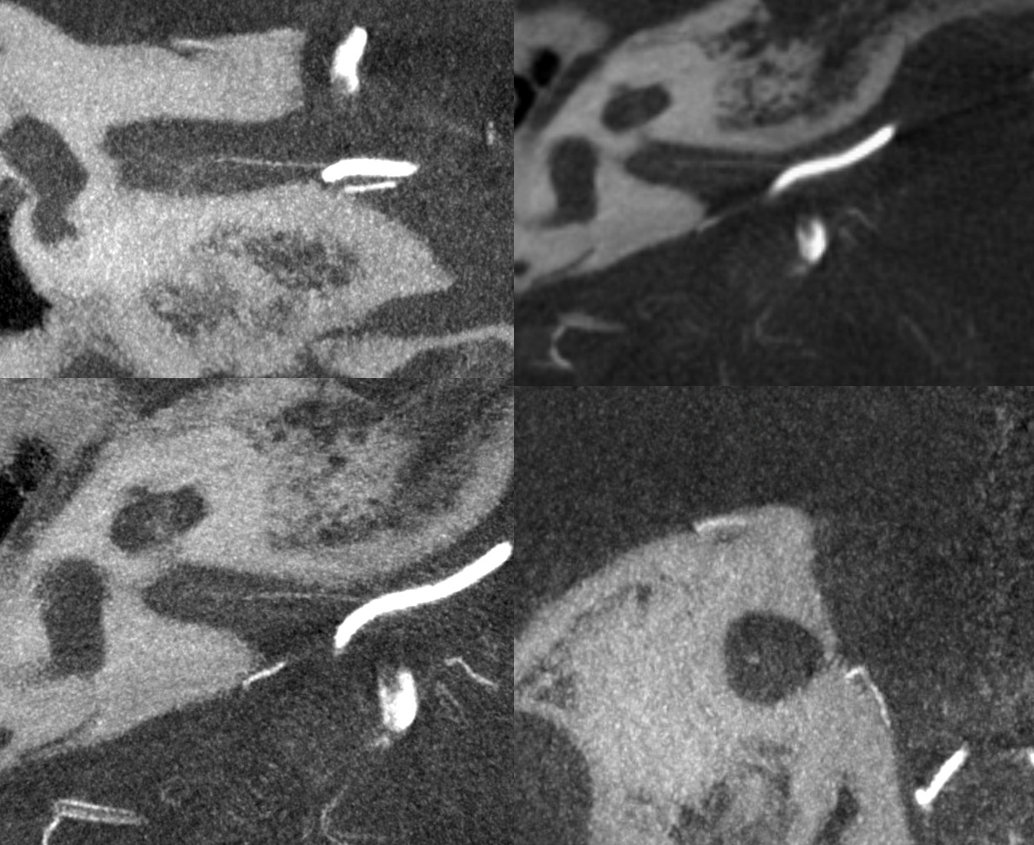
Below is a typical AICA “loop” within the IAC (top right, image the IAC…) The labyrinthine artery (several branches in fact) are well-seen.
Without labels
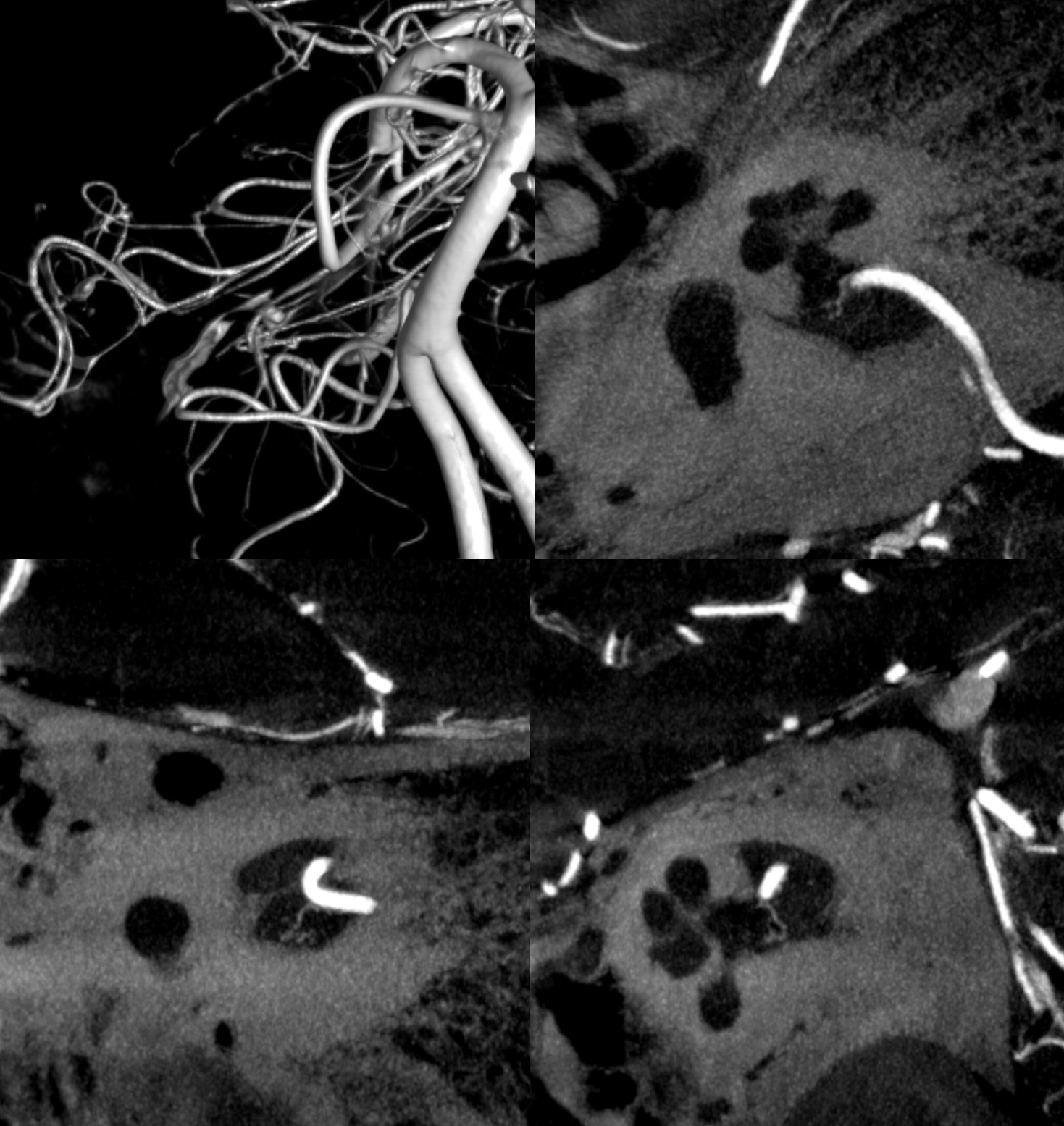
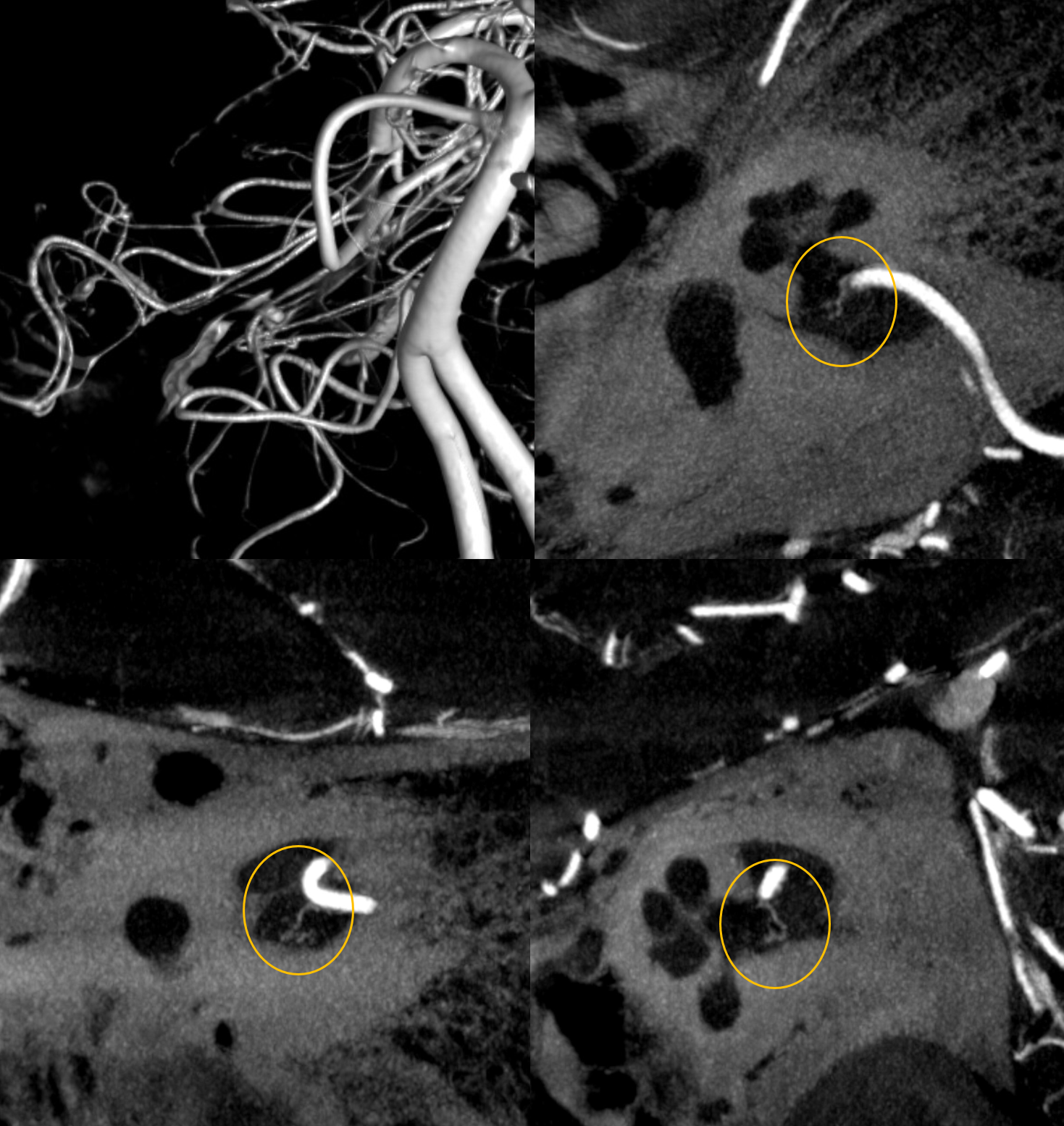
Below is CBCT visualization of the labyrinthine artery from a dedicated AICA injection by Eytan Raz. Not much can be seen on 2D-DSA…
CBCT of AICA injection
Presumably, the labyrinthine artery can be seen on 7T-MRI (reference here). The question of whether this vessel could represent a larger vein rather than artery is unclear. The size of the artery on MRI would suggest that it should be readily seen on CBCT, which it is not… However, veins are well-seen on CBCT too, so questions remain.
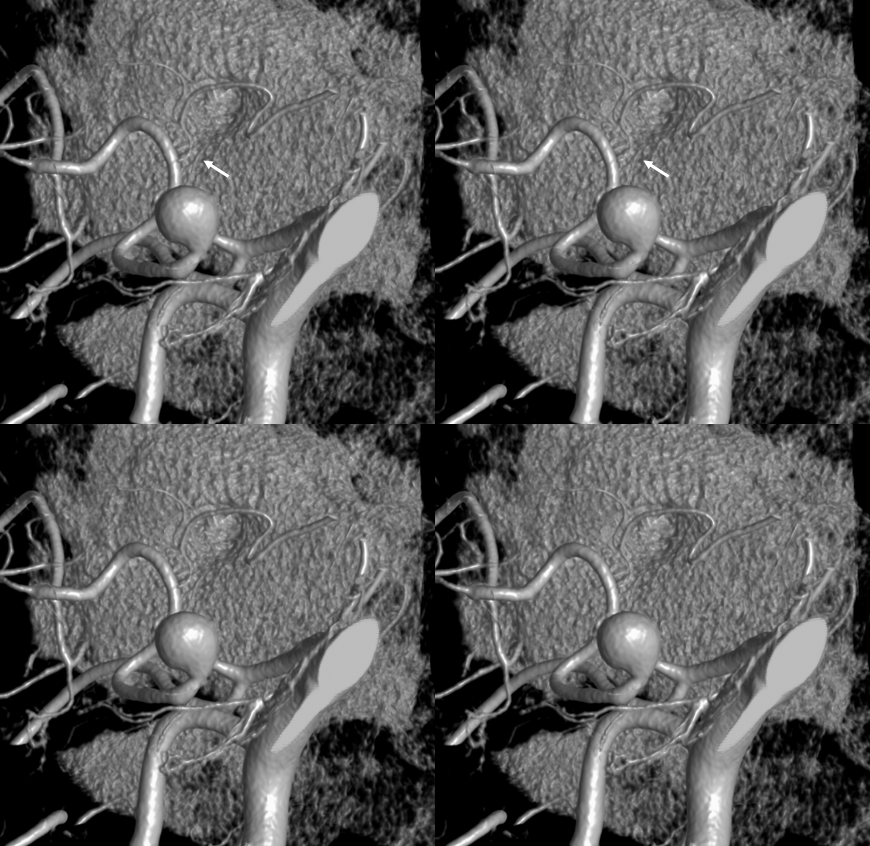
It is sometimes said that Labyrinthine artery “arises” either from AICA of directly from the basilar. Well, what does that mean? Is there a separate AICA then? Is direct origin seen in setting of an “AICA-PICA” — in which case the “AICA” is the labyrinthine branch. Can one take it one step further? Below is an example of “direct origin” labyrinthine from the basilar (arrows)
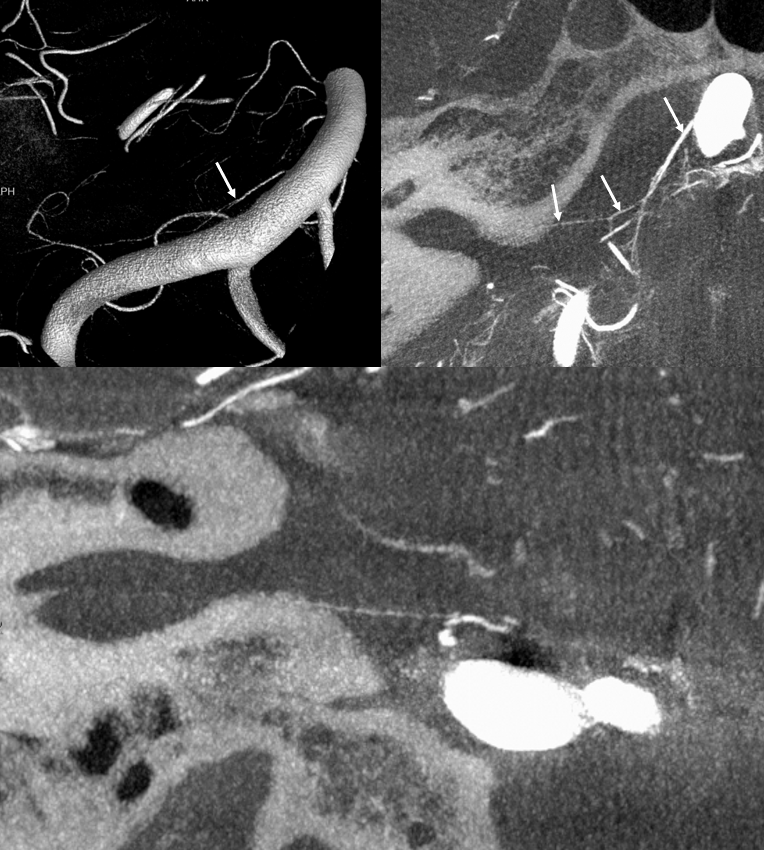
Labyrinthine Artery 2 — another excellent example — also with aneurysm
Stereo pairs below
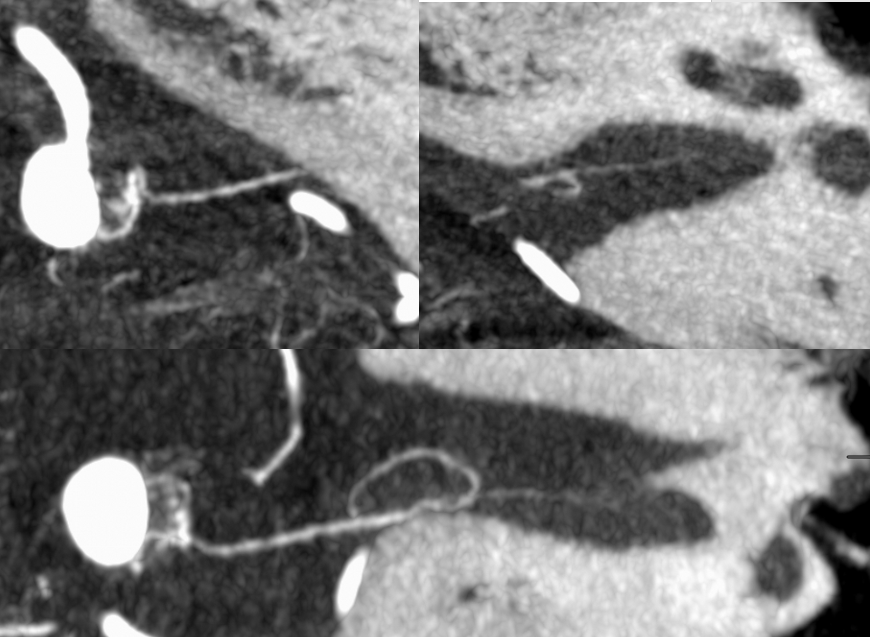
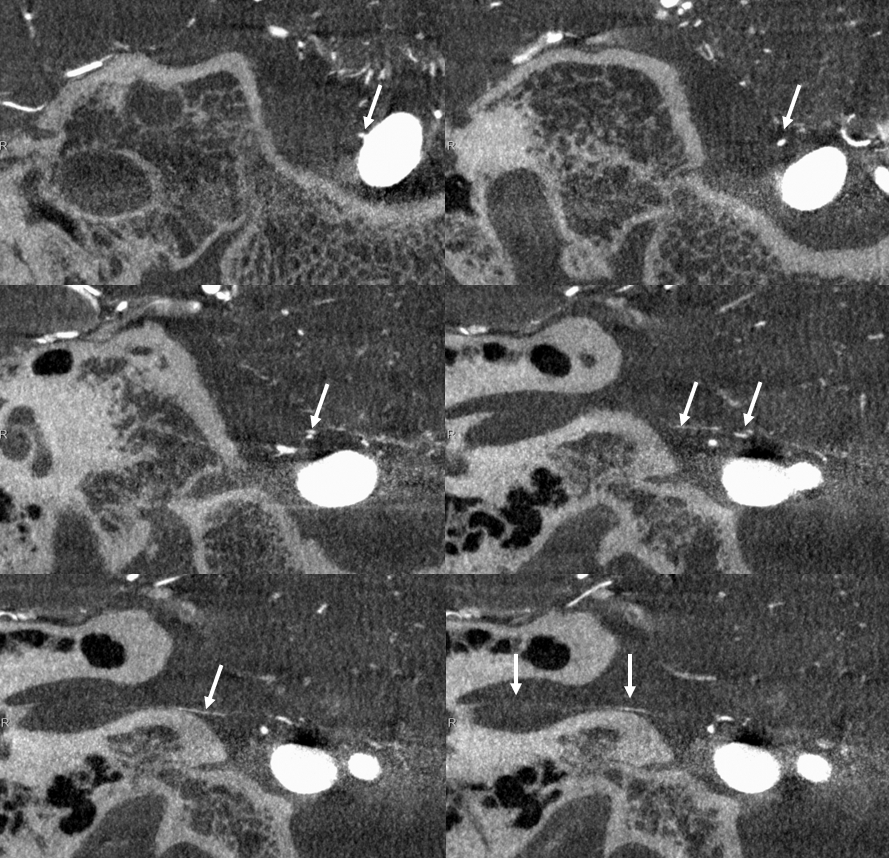
Here are bilatearl labyrinthine arteries — there are two branches in each IAC — in a patient with a basilar aneurysm. Top left (axial right), top right ( coronal right). Bottom left (axial left). Bottom right (axial left)
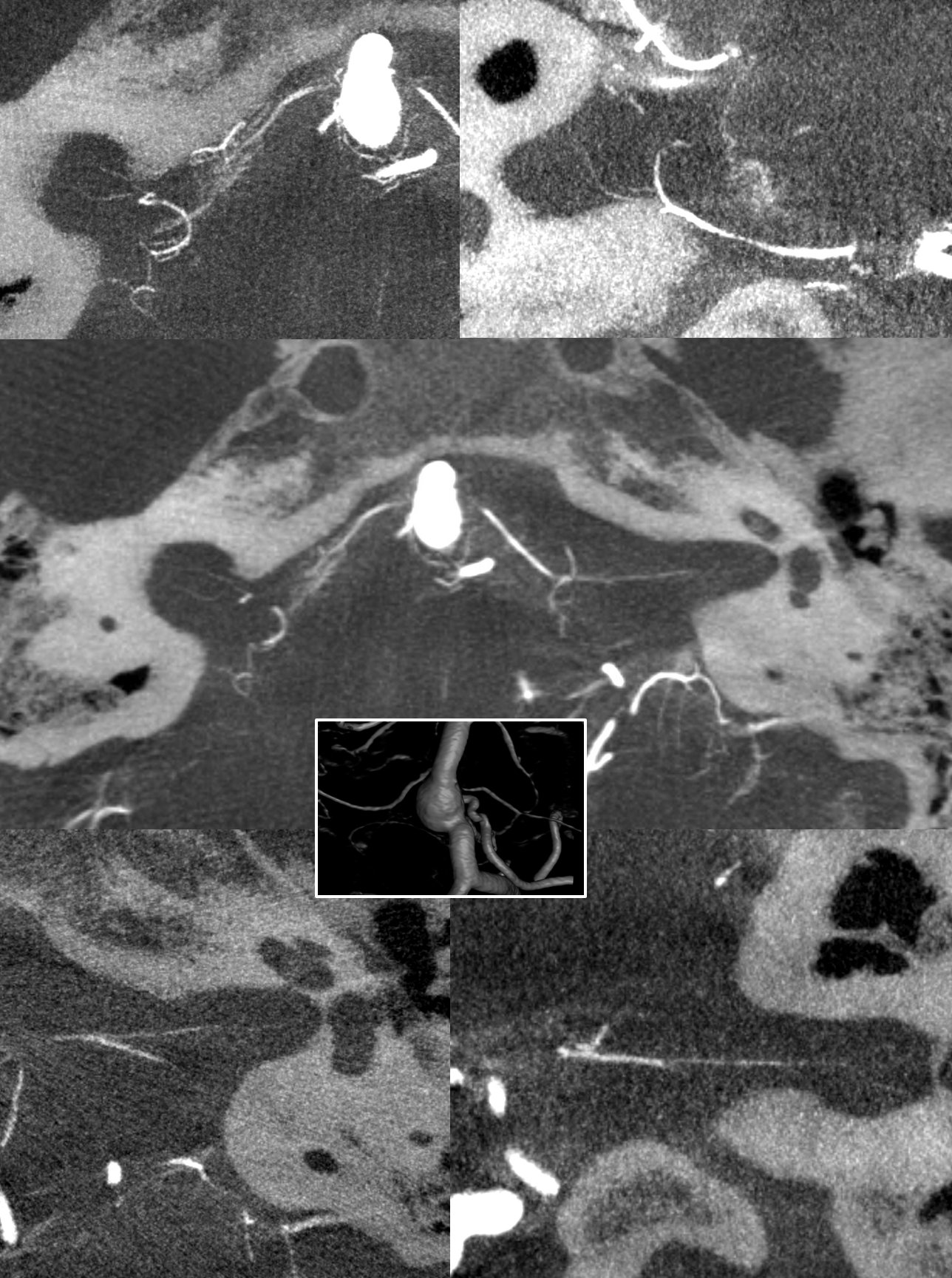
Less constant than the labyrinthin artery. Piodural vessel — like the Davidoff-Schechter or the Wolfschlaegger and Wolfschlaegger. Usually seen in the petromastoid (aka subarcuate) canal. Supplies the adjacent bone and dura. It is this artery, presumably, which participates in the piodural anastomoses of dural fistulas such as petrous apex type. Essentially never seen on DSA in normal states. Can be seen on Cone Beam CT.
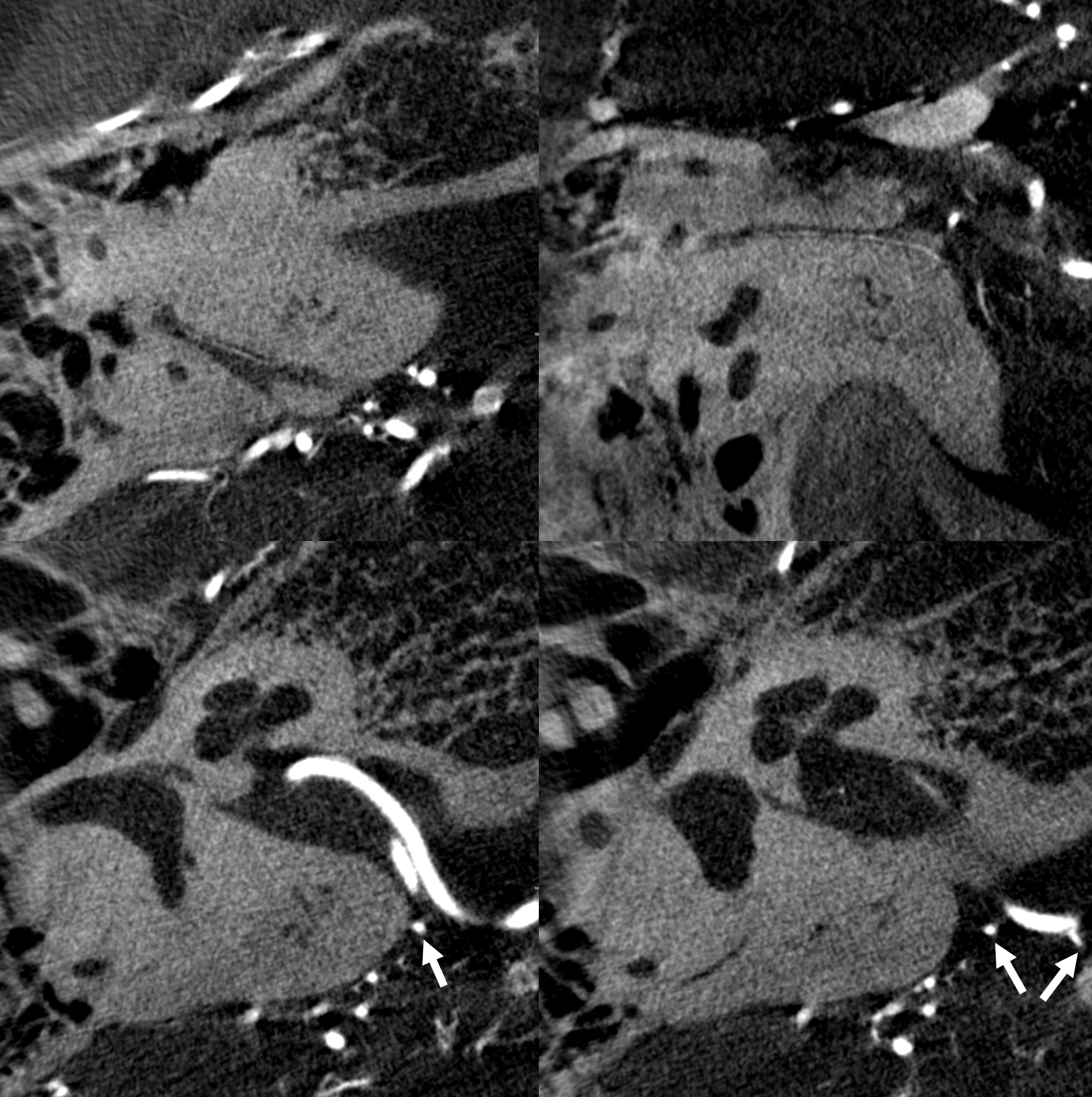
Normal appearance (dashed arrows) in the petromastoid canal. Solid arrows point to an AICA branch from which it arises. Arrowhead points to the labyrinthine artery.
Without labels
Another one
Another one — not in the canal — they are usually very small…
Subarcuate Artery and Dural Fistulas
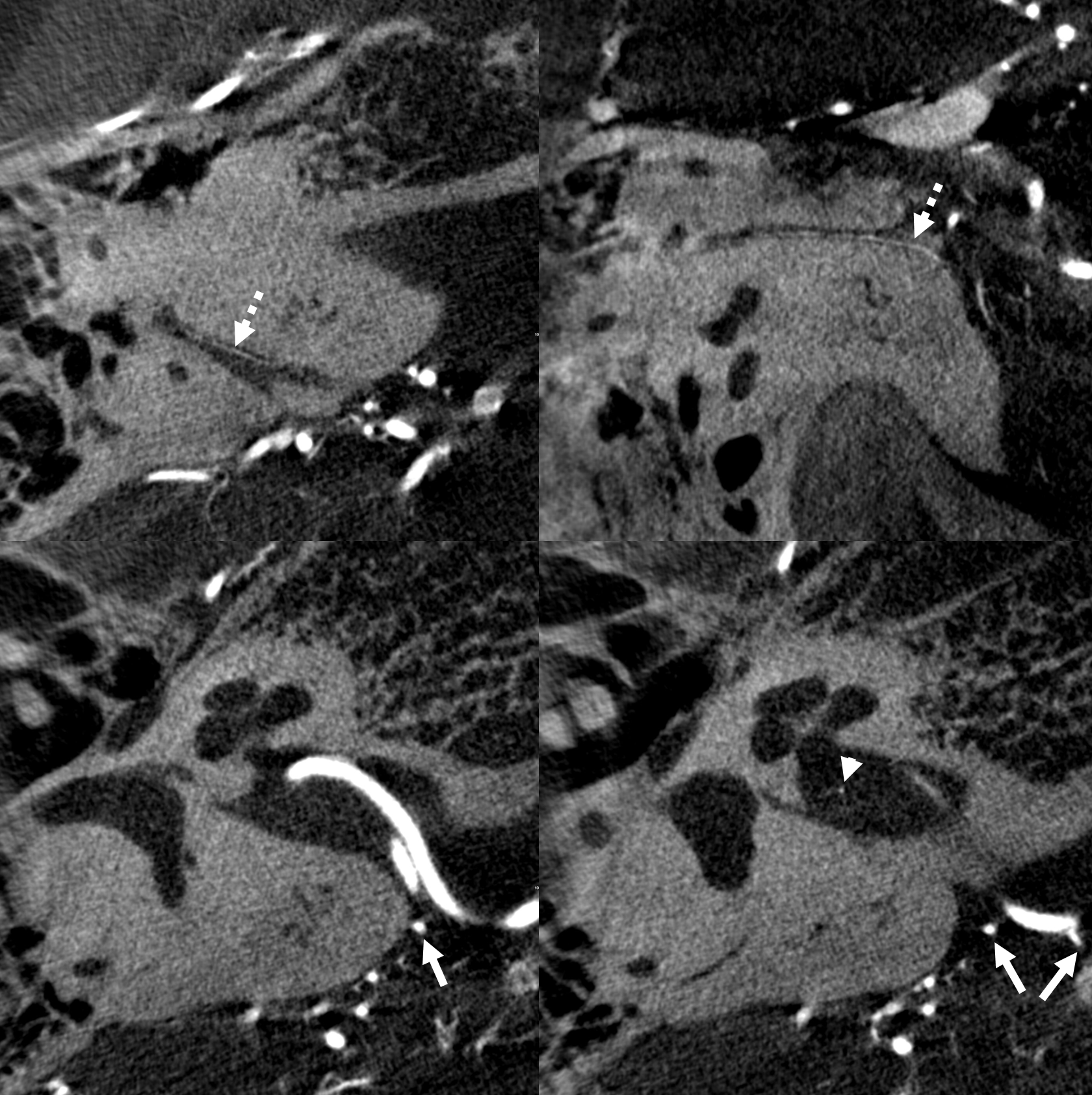
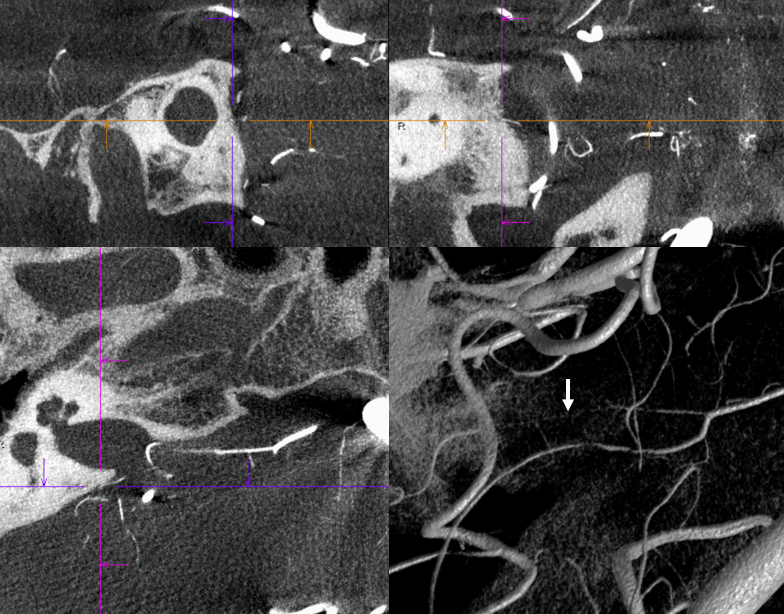
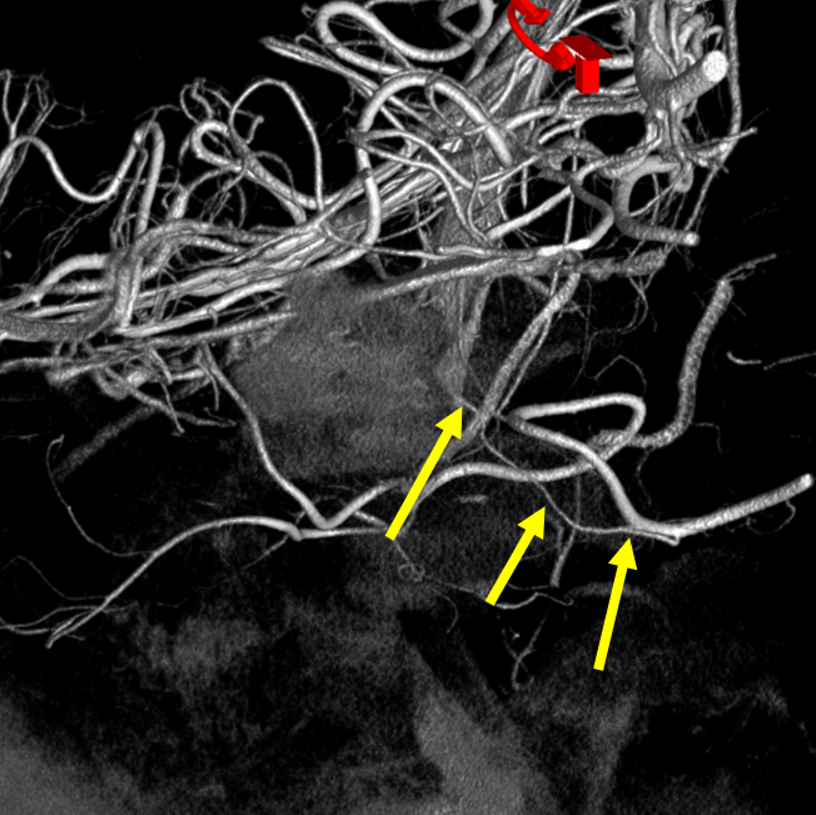
One time we can see this artery a bit more consistently is high flow shunt states — dural fistulas specifically. Here is a sigmoid sinus one, courtesy Dr. Eytan Raz, with pial contribution mainly from the SCA. A very small amount of AICA supply is present also, but impossible to say more without DYNA
DYNA CT MIP images show the arcuate artery supplying fistula. The cochlear branch is too small to see (might be the very small lower one)
VR image
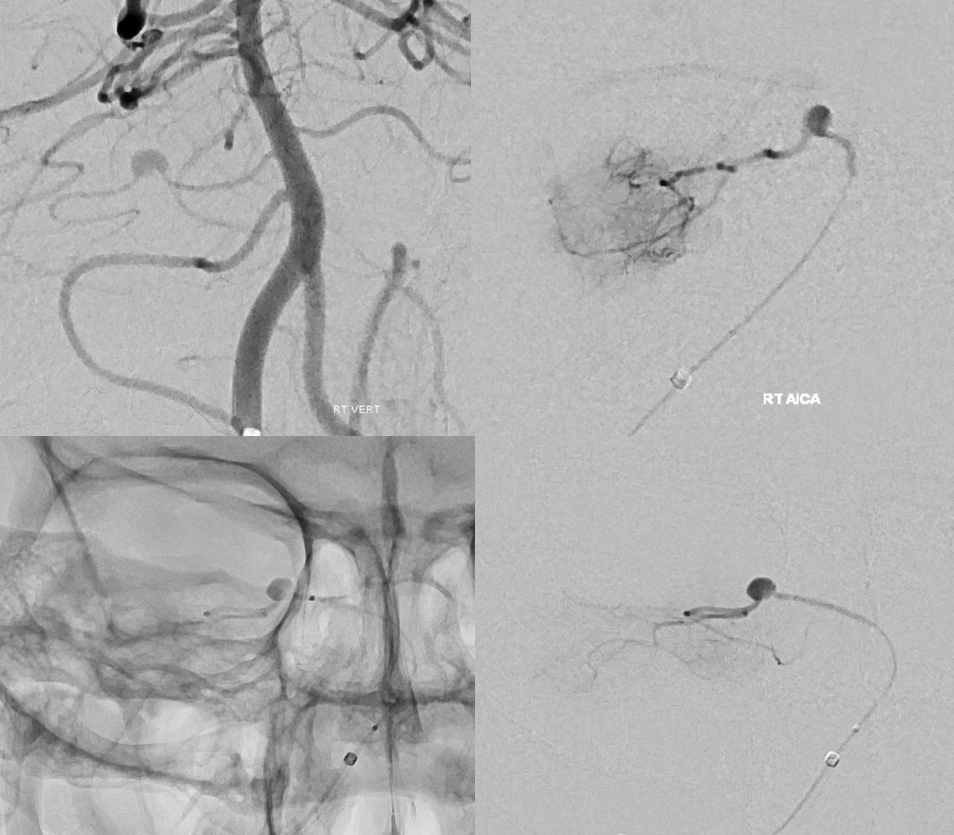
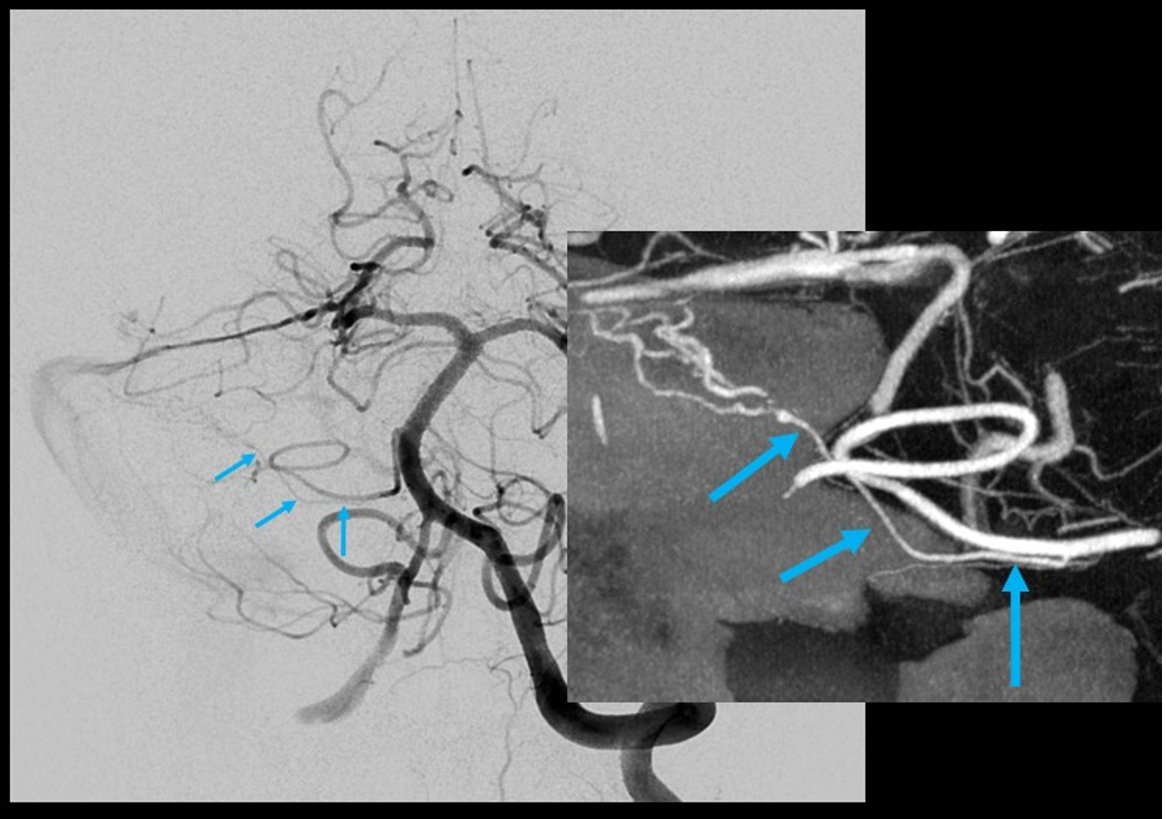
It is not a good idea to use AICA as embolization route — too small and too eloquent. Below different case example shows why not.
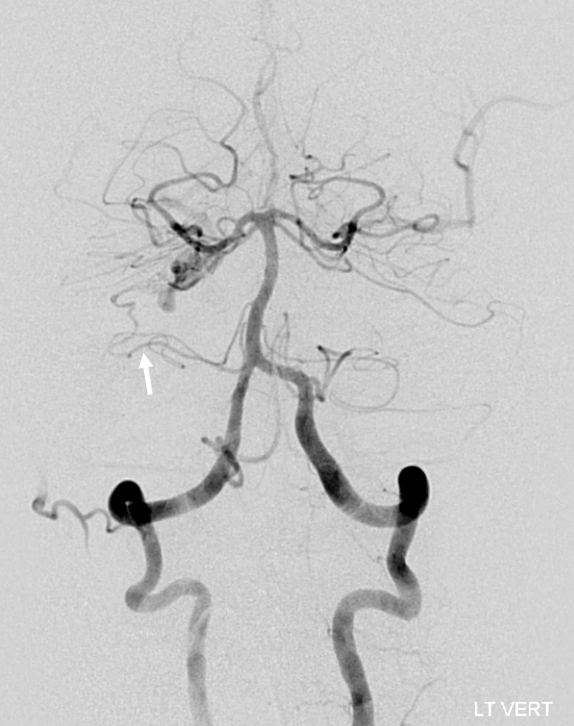
Here, a petrous apex fistula is supplied by SCA, AICA (arrow), and MMA (not shown)
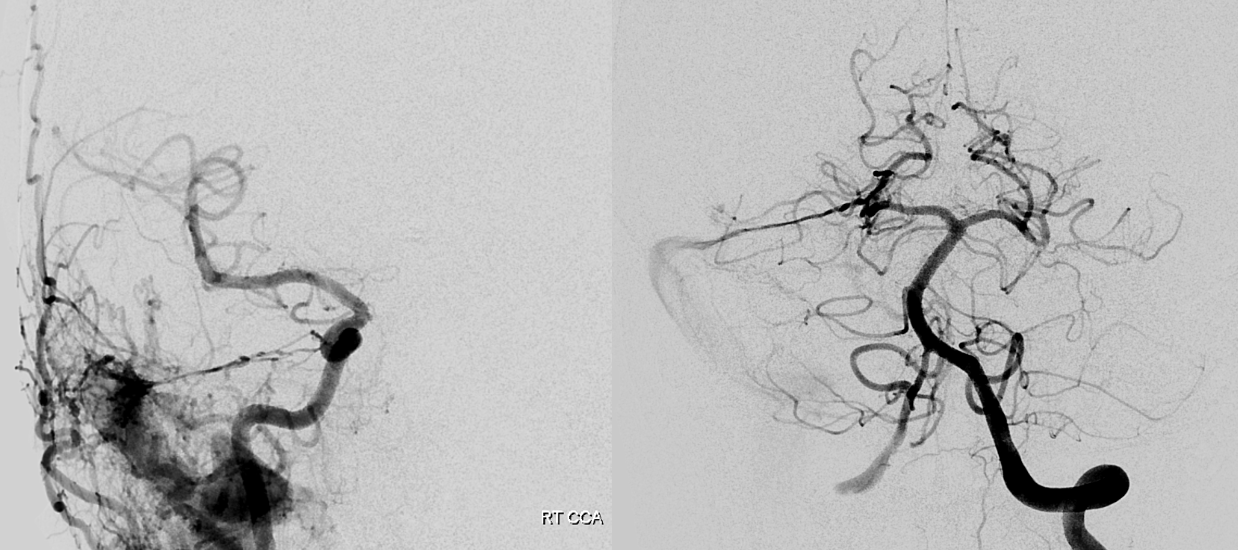
An ill-advised catheterization of the AICA (left image) results in a perforation sustained during attempted navigation of the AICA loop (right image arrow), which spontaneously seals after minimal bleeding. The catheter was wedged in the AICA, and perforation was not the problem…
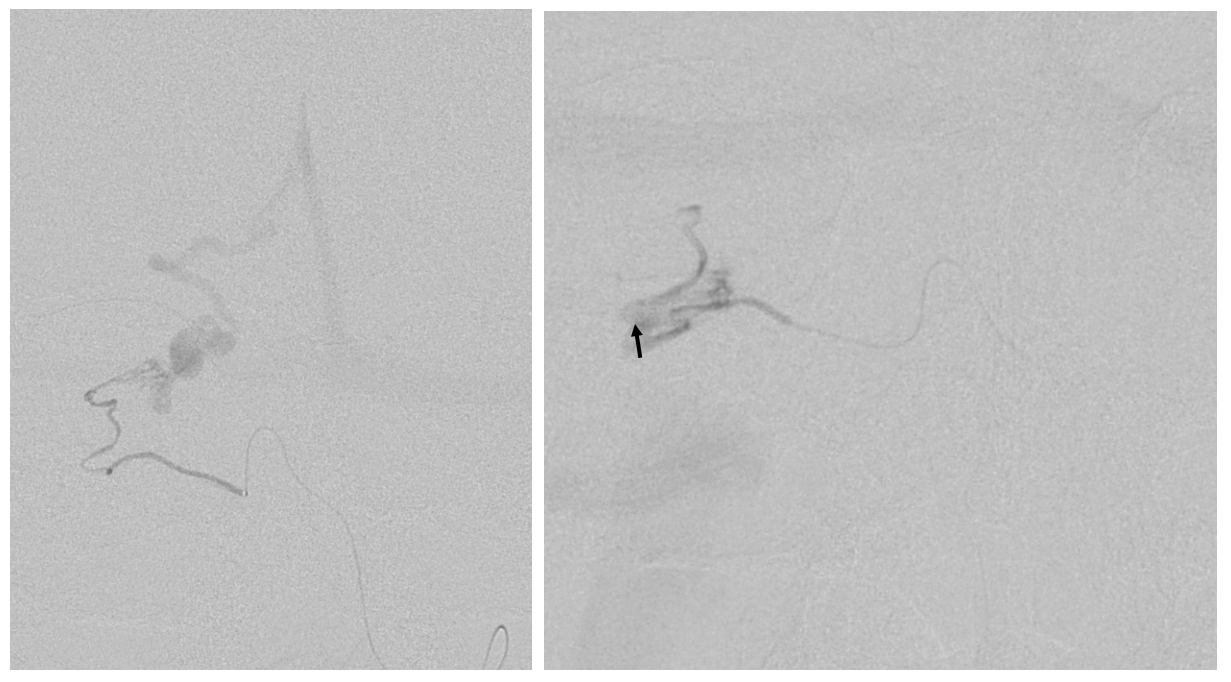
The fistula is cured via MMA onyx injection. However, at end the distal AICA has gone down by thrombosis after perforation (compare pre-embo left and post-embo right images). The patient lost hearing about 12 hours after procedure despite permissive hypertension — probably because collaterals to inner ear went down.
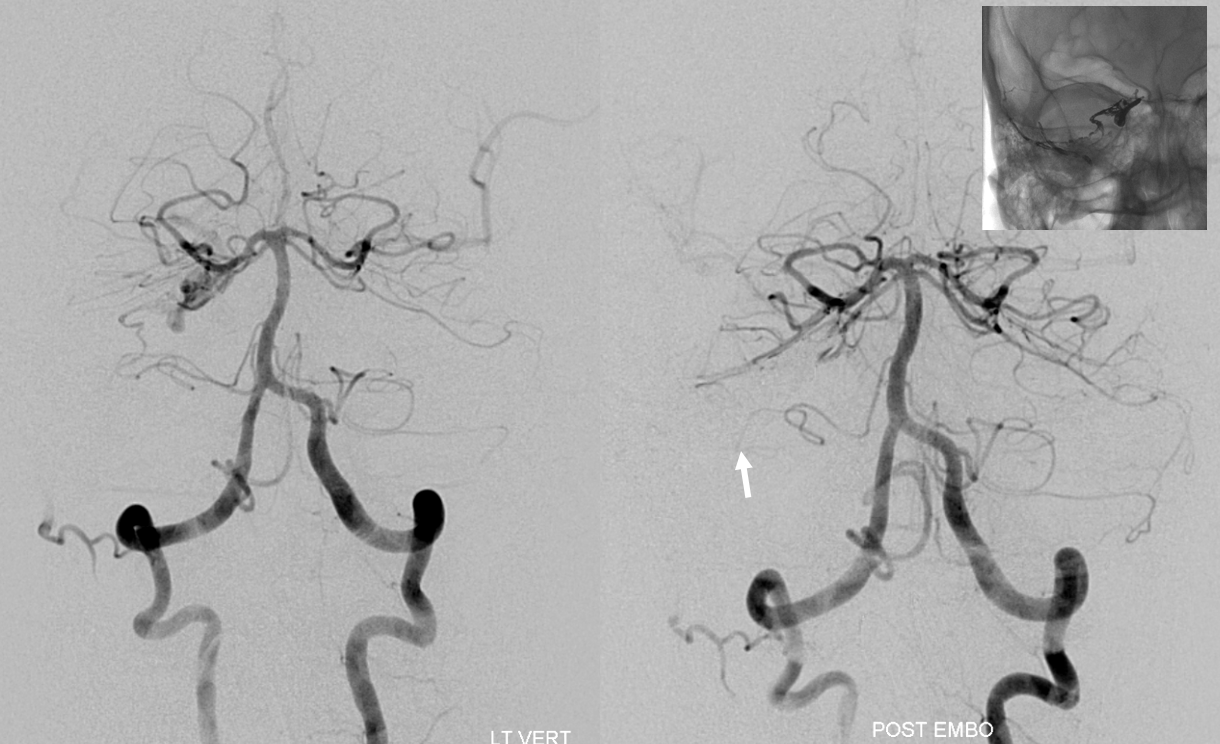
Let this page end with a warning — getting beyond the AICA loop is technically challenging, and deafness is bad enough — but the often-associated tinnitus can be even worse. Be careful
