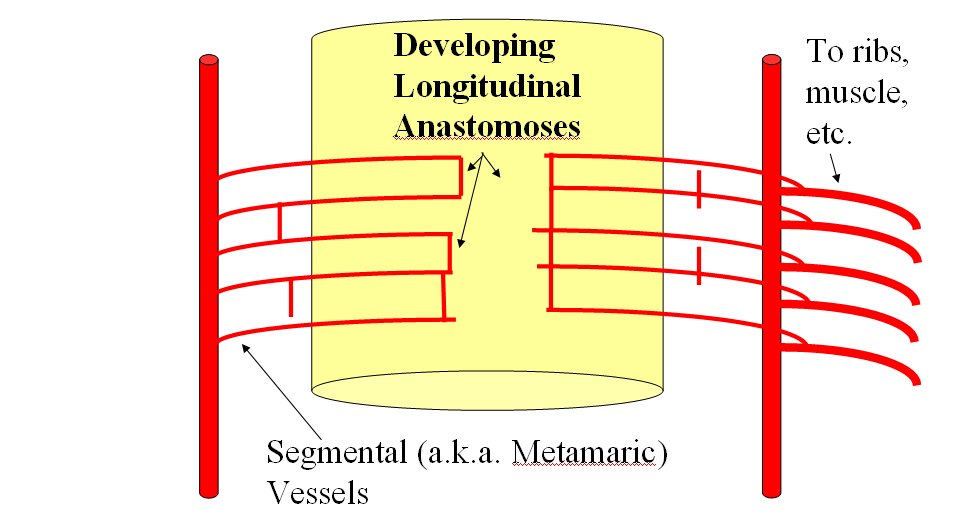
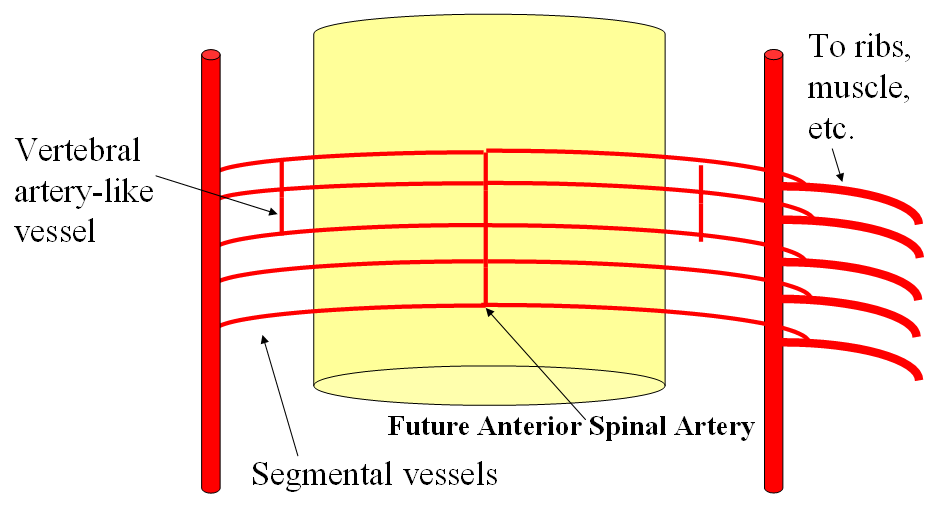
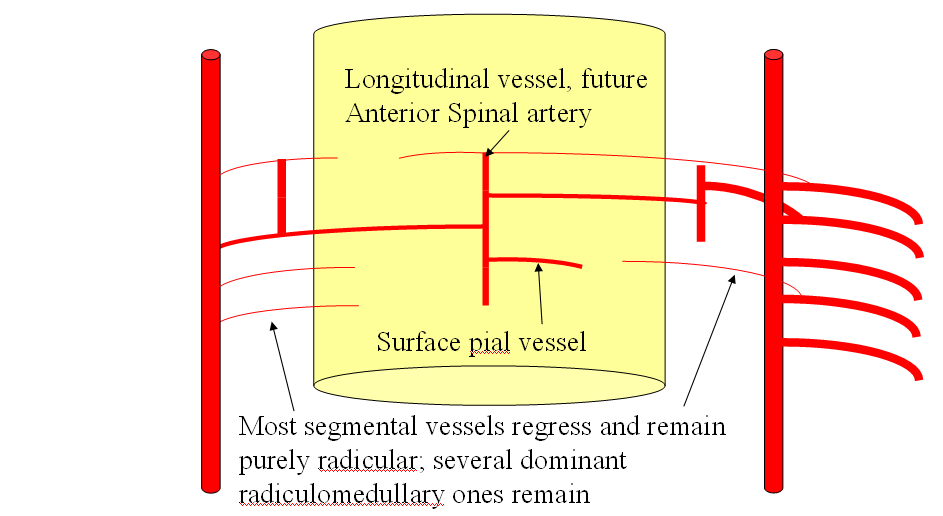
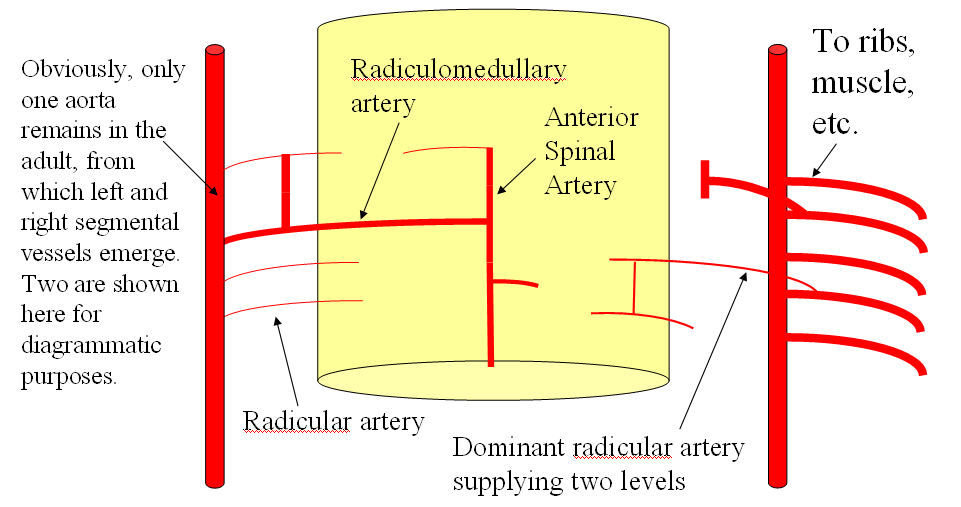
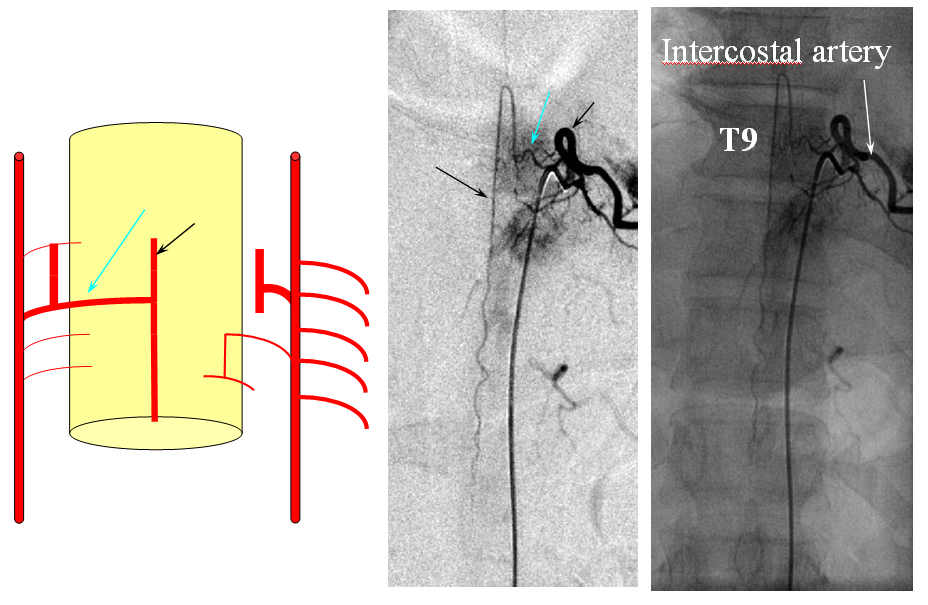
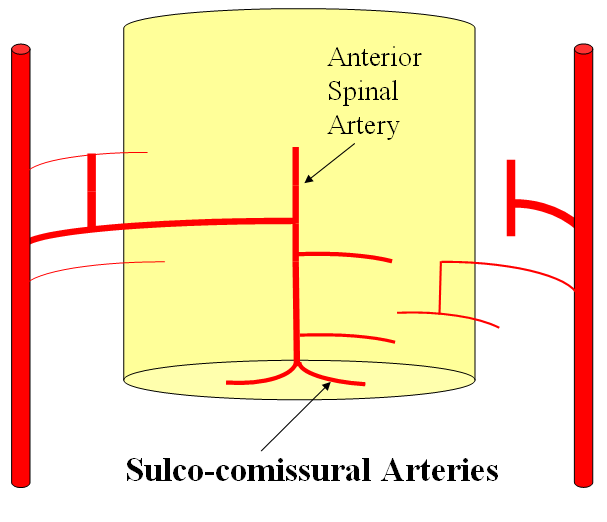
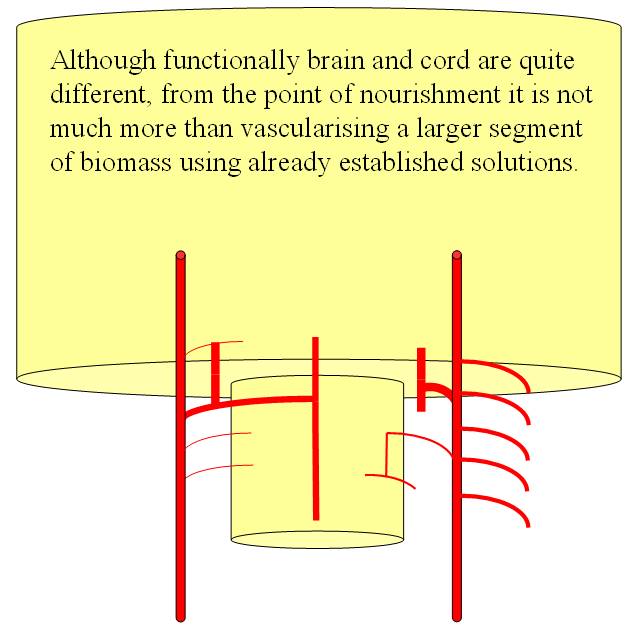
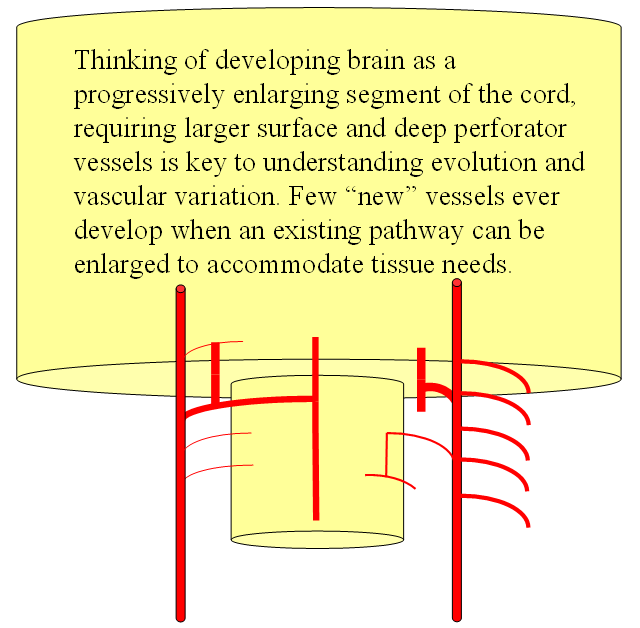
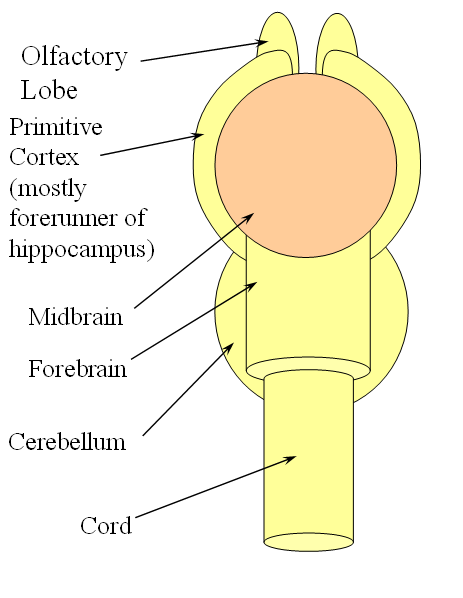
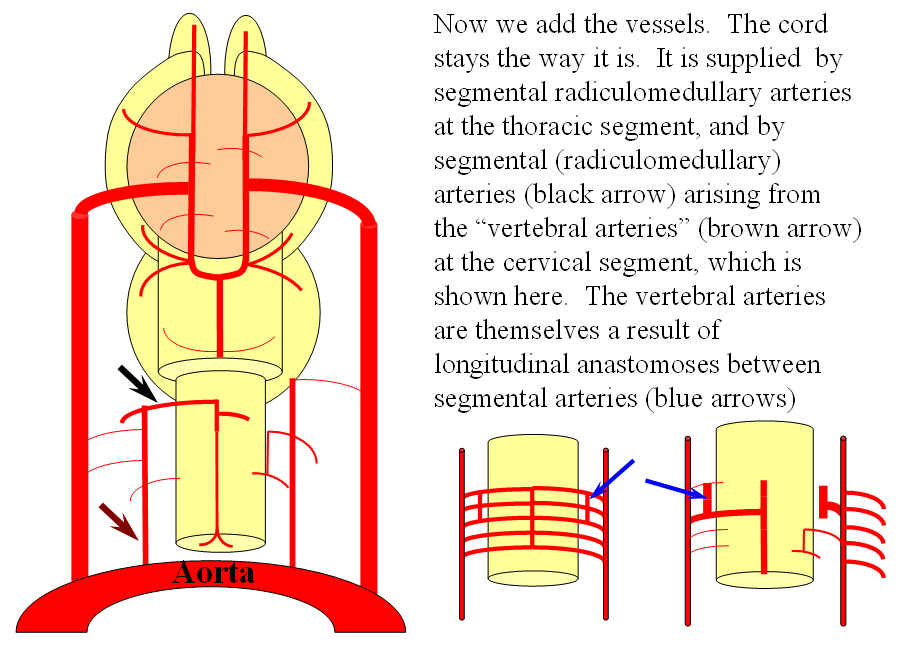
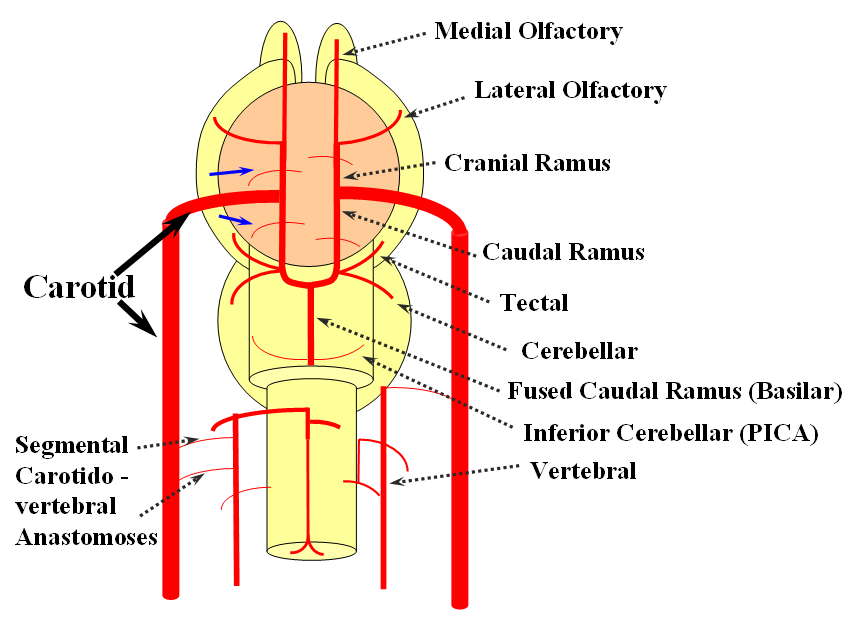
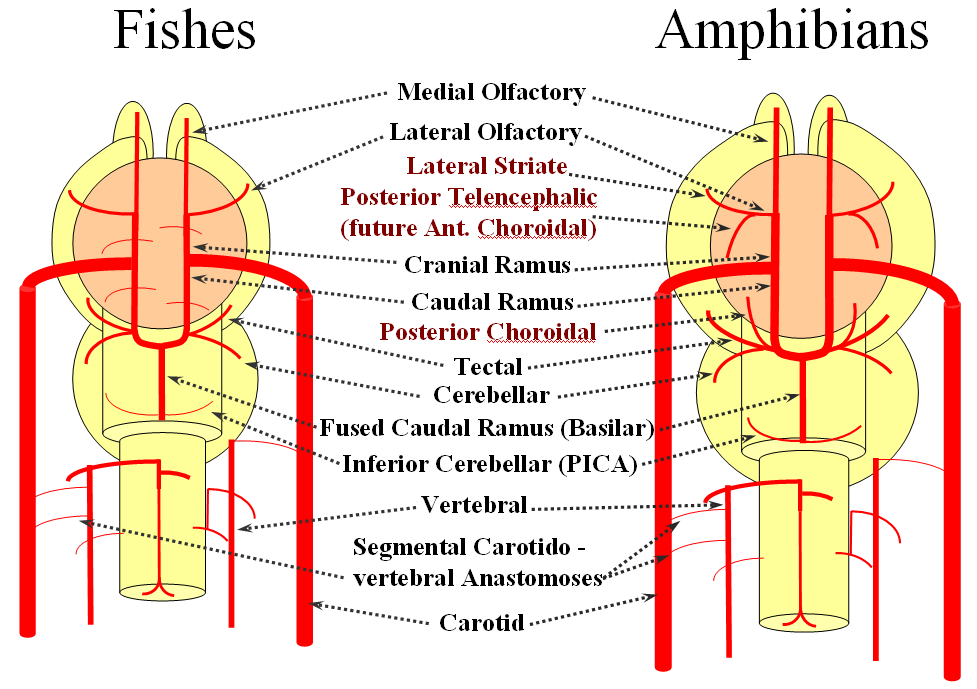
FISHES
The arrangement in the brain is similar (see diagram below). Two large longitudinal vessels – the carotids – ascend the neck and enter the cranial cavity, in a way similar to that of segmental radiculomedullary vessels in the cord (the carotid artery is also made of segments, which will be discussed later). Inside the cranium, the internal carotid artery gives rise to paired longitudinal arteries which run the length of the brain. The one projecting forward is the “cranial ramus” and the one extending caudally is the “caudal ramus”.
The “cranial ramus” is the forerunner of the anterior cerebral and middle cerebral arteries. The caudal ramus – of the PCOM and parts of the basilar, which is already fused at midline No functionally significant anastomosis between the caudal ramus and the spinal cord / vertebral artery system yet exists. The entire brain (including prosencephalon and cerebellum) is supplied by what passes for internal carotid arteries.
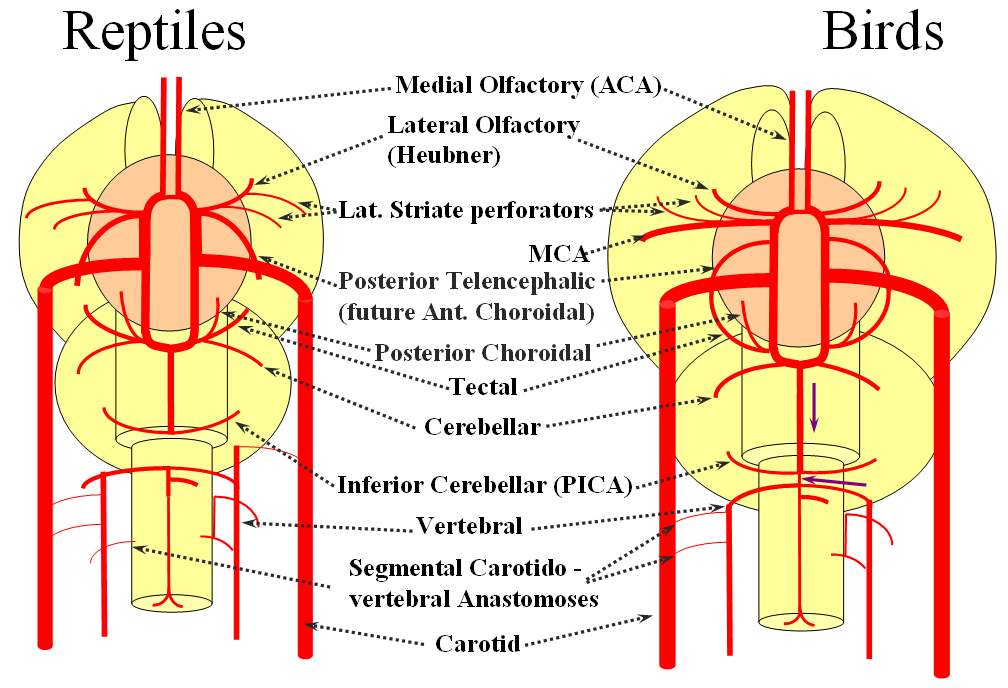
The cranial ramus has two named branches –medial olfactory artery, forerunner of the ACA; and lateral olfactory branch. It is the forerunner of the Heubner and anterior choroidal. The caudal ramus sports a tectal artery which supplies the posterior top of the brainstem; from this vessel comes off a cerebellar artery supplying the primitive cerebellum – forerunner of the superior cerebellar artery. Numerous little perforators (blue arrows) come off all these vessels, so we are talking here about the larger named channels only.
Segmental anastomoses also exist, usually in very small form, between the carotid and vertebral systems. These persist in animals and human in diminutive form. In the human embryo, these pathways are quite functional at early stages. Subsequently as the vertebrobasilar system develops they regress. Occasional persistence of these channels leads to variables such as trigeminal, hypoglossal, and proatlantal arteries, depending on segmental level.
FISHES
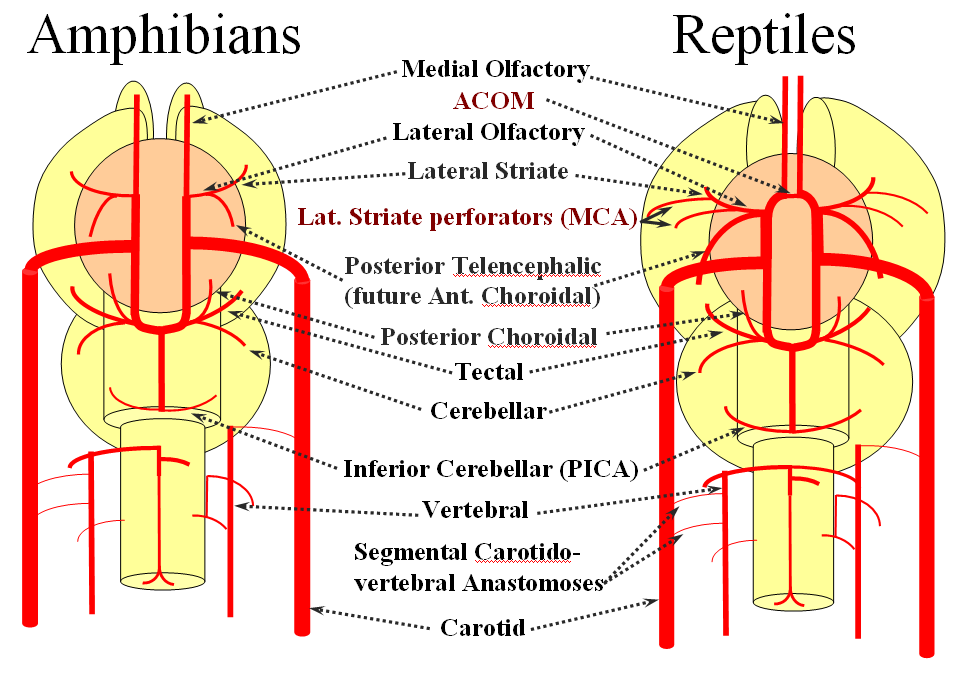
Now the amphibians. The brain continues to grow, with a primitive hippocampus and enlarging “higher” centers which are essentially representatives of the human basal ganglia. For example, visual processing takes place in the homologs of thalamic/tectal region. The cerebellum is also enlarging. The larger size of the brain leads to hypertrophy of some branches arising from existing channels and coalescence of smaller feeders into larger named branches. The lateral olfactory branch has enlarged with additional named vessels and now consists of two main branches – the lateral striate artery (forerunner of the Heubner, MCA, and MCA perforators); and the posterior telencephalic branch, forerunner of the anterior choroidal artery. On the caudal ramus, the tectal/cerebellar complex assumes supply of a developing choroid plexus with a posterior choroidal branch. There is still no effective anasomosis with the vertebral system, which is responsible for supplying the spinal cord.
Moving on to the reptiles. From the lateral striate (future Heubner) branch of the rostral division arise multiple “perforator” vessels supplying the developing hemisphere. These will eventually become the MCA – thus the very large MCA in the human is phylogenetically a relatively late acquisition and is, in fact, a hypertrophied perforator branch of the anterior cerebral artery. A midline olfactory artery fusion is observed in some reptiles, the new ACOM. The Posterior Telencephalic artery, in fact the future anterior choroidal, has also enlarged and supplies the posterior portions of the cerebral hemisphere – a function it will continue to fulfill until very late in mammalian evolution, when its territory will be annexed by a growing posterior cerebral artery. This particular arrangement caused much confusion in the literature, with anterior choroidal erroneously assumed to be the PCA by early investigators. The PCA does not yet exist as such, but its forerunner, the tectal artery, is alive and well, content with a much smaller territory. An enlarging cerebellum, particularly the more caudal vermian portion, is nourished from distal “basilar” system by the homolog of the PICA.
The birds, collectively, represent a developmental stage where a discrete MCA emerges from as a dominant perforator from among a collection of lateral striate vessels, driven by rapidly enlarging cortical volume. The perforator vessels arise from all sections of the circle of Willis, and notably from the MCA itself. Anterior cerebral artery segment dominant perforator analogous to the Heubner is present. The bird “PCA” – really the anterior choroidal, continues as the dominant parieto-occipital supply source. The tectal artery (the real PCA) extends its coverage and forms a functional anastomosis with the anterior choroidal system, setting stage for future acquisition of its territory. A growing “PICA” continues to be supplied by anterior circulation via craniopetal flow into the basilar artery. The vertebral system remains confined to the cord or in some cases lower medulla/vermian region.
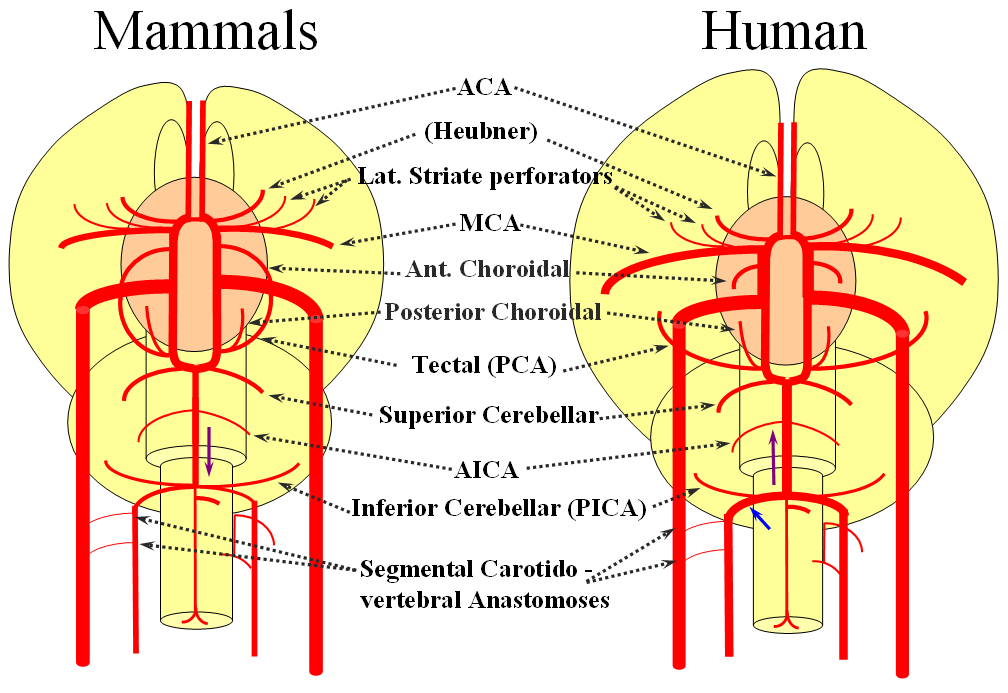
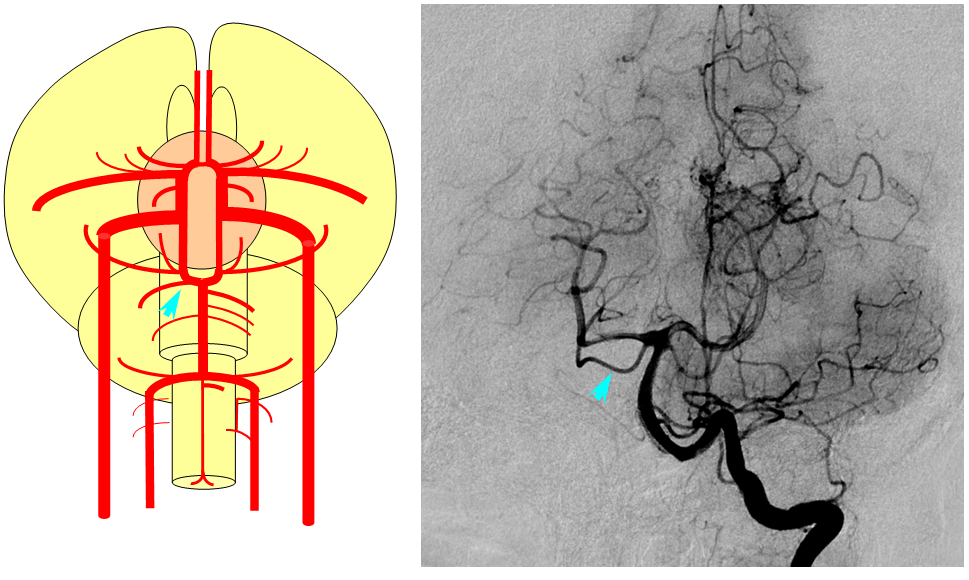
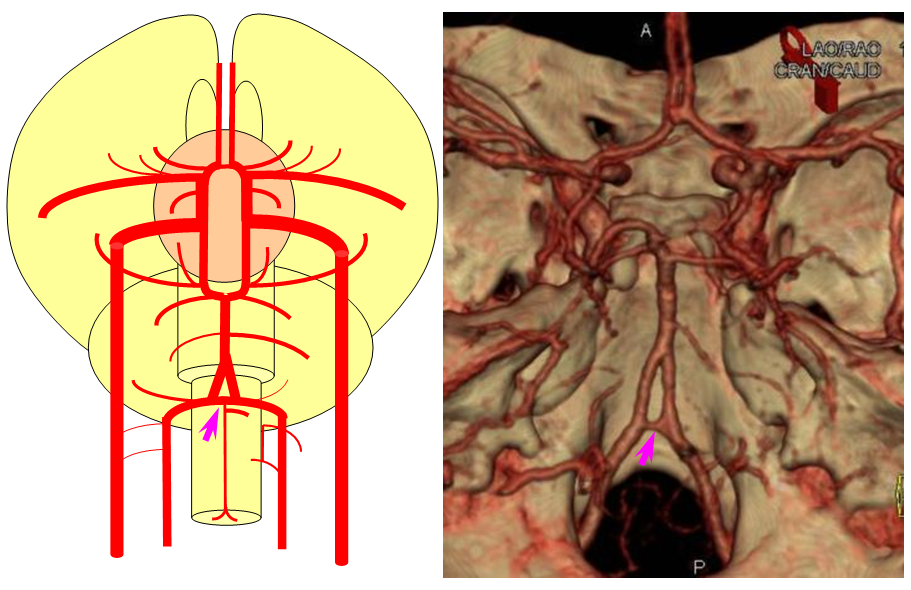
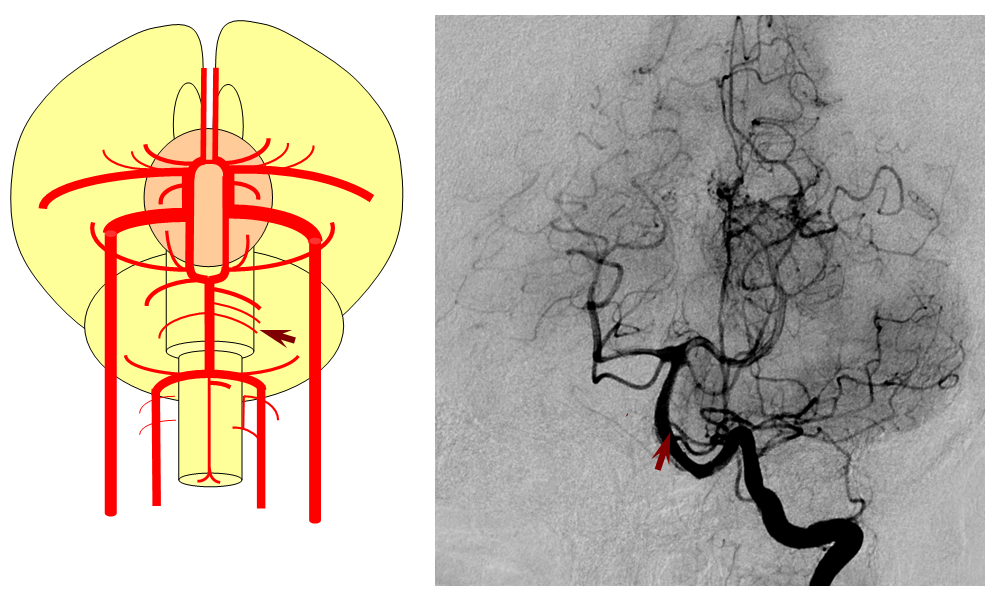
Birds to Mammals: Important changes now concern development of the caudal ramus, where enlarging cerebellum and posterior telencephalon place increasing demands on the carotid system. Nevertheless, even in mammals as “advanced” as the sheep, the vertebral system does not contribute to brain supply, with the basilar artery continuing to demonstrate craniopetal flow from the internal cerebral system (purple arrow). However, in the yet higher mammals the carotid system appears to reach its functional limit, leading to progressive annexation of brainstem and cerebellar territory by the vertebrobasilar system. At first, this is confined to the PICA region. Progressively, however, the vertebral artery annexes portions of basilar territory, resulting in reversal of heretofore craniopetal basilar flow to the craniofugal form observed in monkeys, apes, and humans.
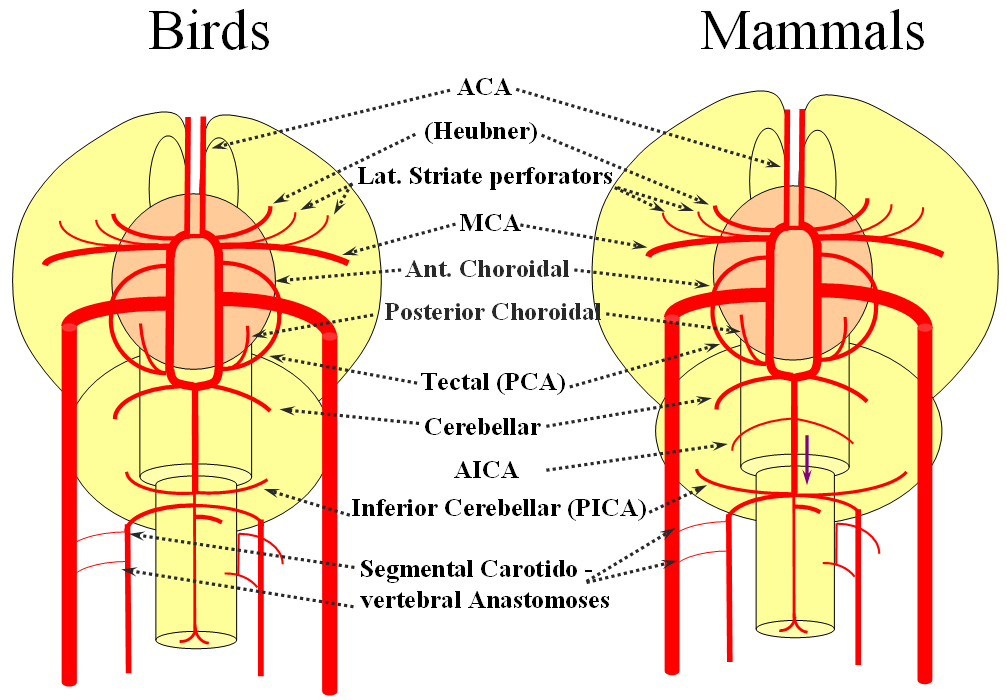
The phylogenetically novel participation of vertebral system in brain supply is maximized in the human, where the posterior cerebral artery, previously known as the tectal artery in the “lower” species, is now functionally acquired by the posterior circulation, even though developmentally it belongs squarely with the carotids. Even as such, frequent occurrence of “fetal” PCOM disposition attests to as yet tenuous acquisition of the PCA by the vertebrobasilar system. The PICA is now squarely in the vertebral territory region. Recent territory shifts in this region are borne out by marked variability in PICA-AICA dominance, whereas the phylogenetically older superior cerebellar artery shows little variability. Superior cerebellar duplications are relatively common, but transfer of overall territory to another cerebellar branch is quite unusual.
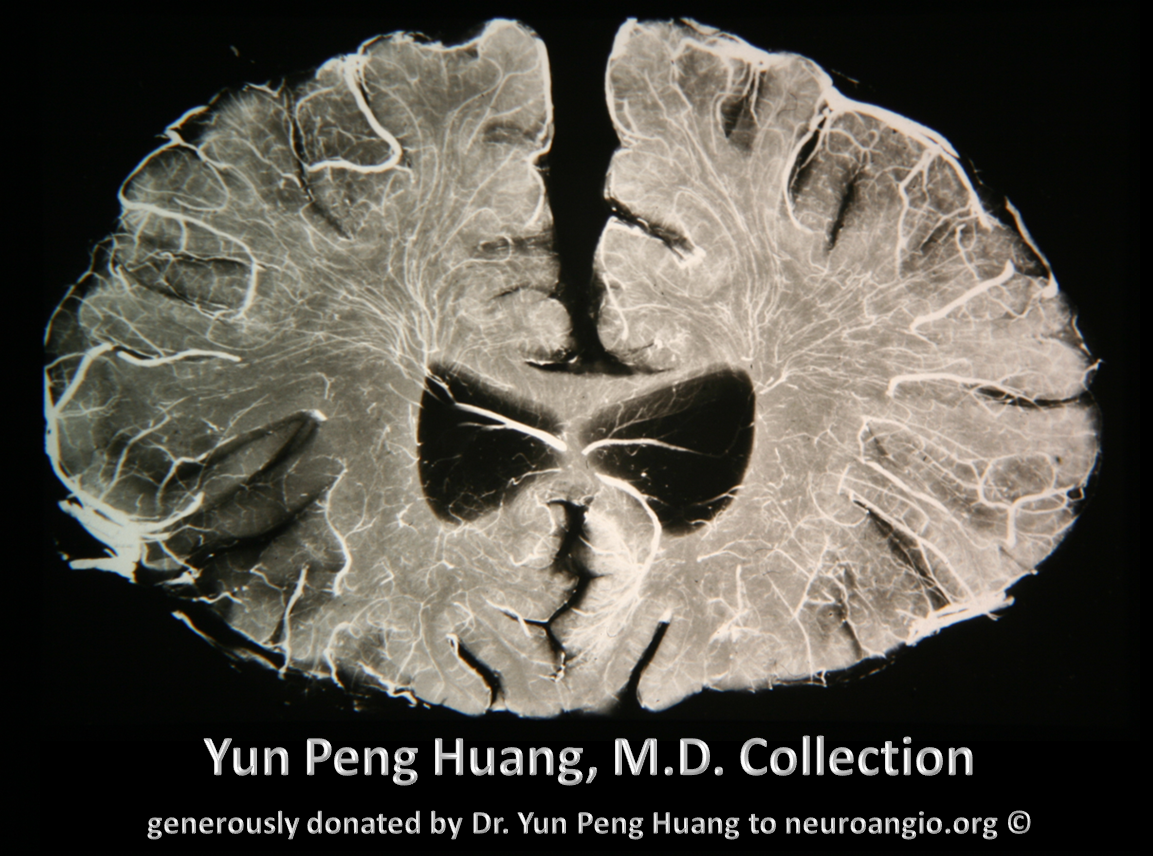
The homology between vascular blueprints of spinal cord and brain can be strikingly obvious even in the adult state. Here is a bifrontal specimen from the collection of the great Yun Peng Huang, M.D., the first angiographer to extensively study cerebral veins, establishing the importance of medullary veins and their relationship to what is now known as the Developmental Venous Anomaly (see Deep Venous System). Here is a cut specimen of the frontal lobe, looking more than a little bit like the spinal cord:
Practical Aspects of Neurophylogeny and Neuroembryology
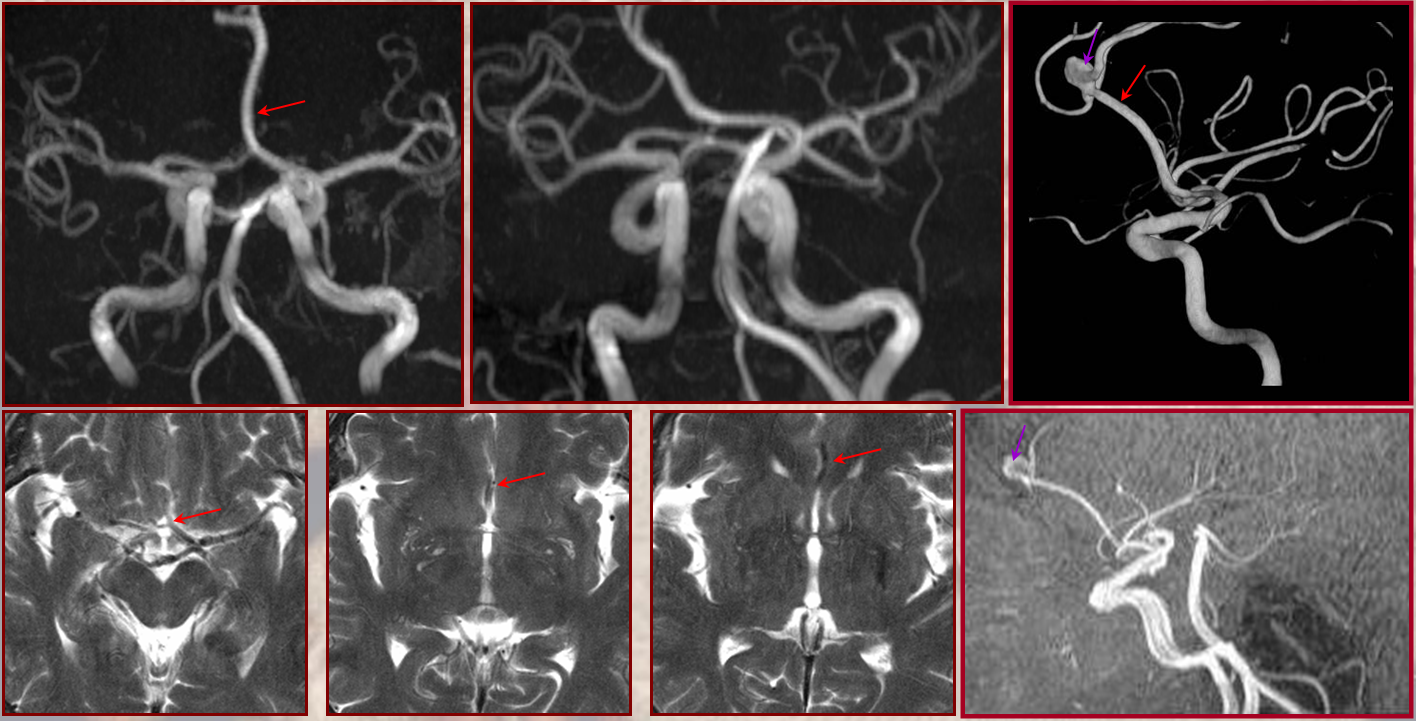
Fetal posterior communicating artery
This dime-a-dozen configuration is the most common “variant” of the cerebral circulation. As you can see, fetal PCOM (blue) reflects effective persistence in carotid supply of the PCA. The P1 segment of the PCA (brown) is hypoplastic. A unilateral fetal PCOM is seen in 25% of cases, bilateral in 0.25×0.25=0.05 cases. The more important implication of PCOM origin as an ICA branch is that existence of a “duplicated PCOM” is not possible — as may be confused on occasion with a larger anterior choroidal artery.
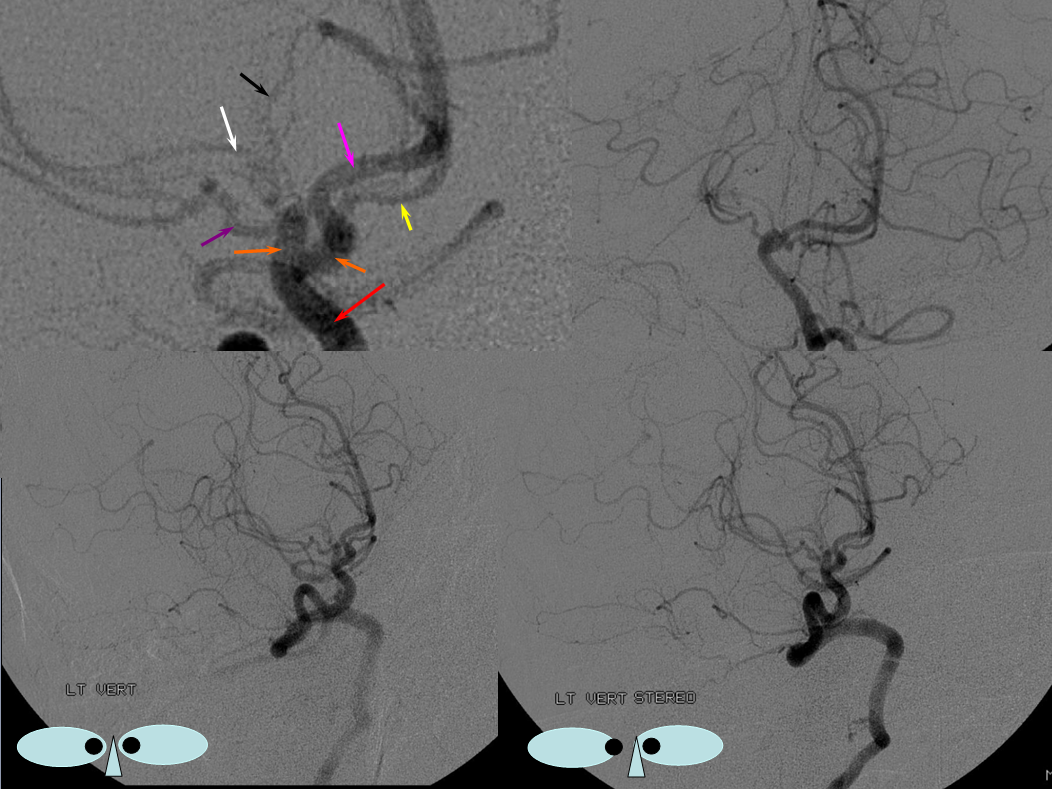
Fetal PCOM on angio marked in red on left ICA lateral injection. AP vert injection shows a diminuitive left P1 segment (orange), as compared with normal course of the right PCA (green). Notice other unusual arrangements in this case, including a somewhta low origin of the left superior cerebellar artery (yellow) and a basilar fenestration (brown)
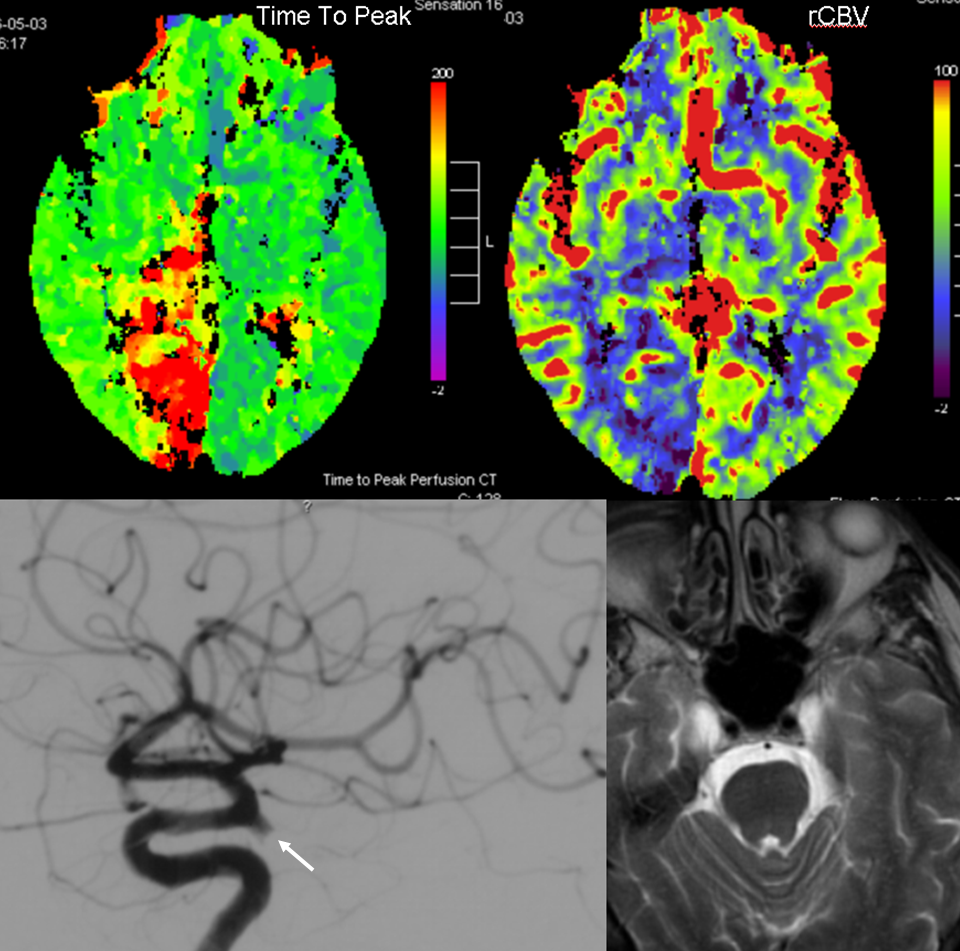
PCA territory infarction in a case of “fetal PCOM”. Time to Peak and rCBV maps (top) demonstrate a completed infarction in right PCA territory. The embolus (right lateral ICA injection on bottom left) originated from the right ICA. Typical diminuitive apperarance of the basilar atery in a patient with bilateral fetal PCOMs is seen on bottom right T2 image.
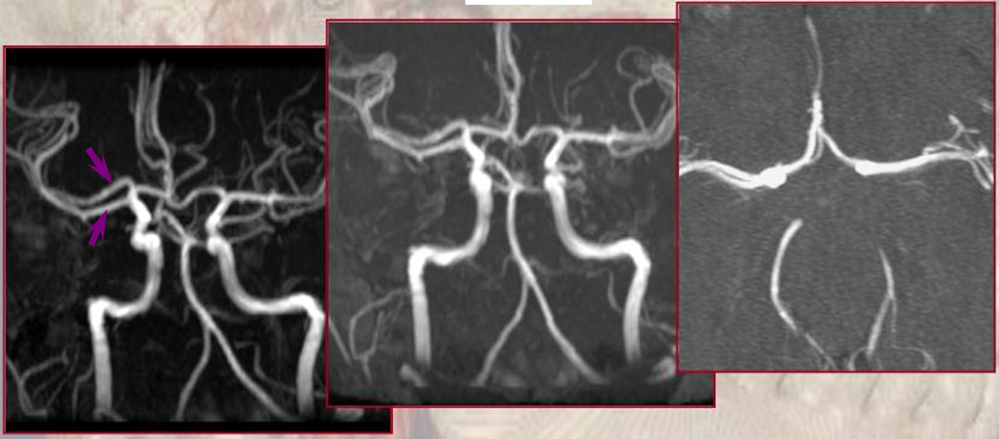
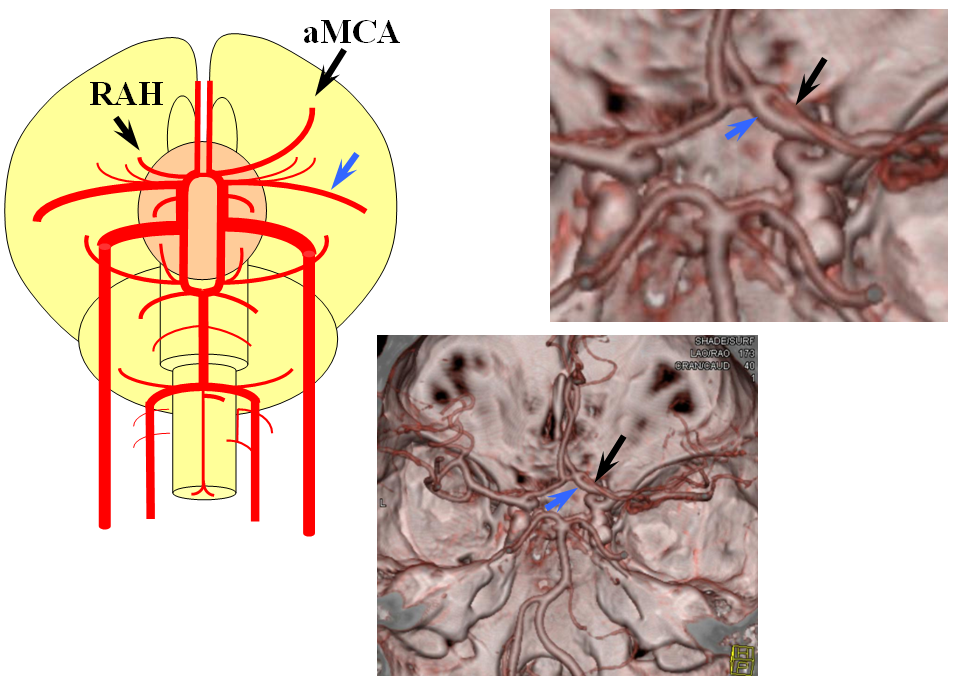
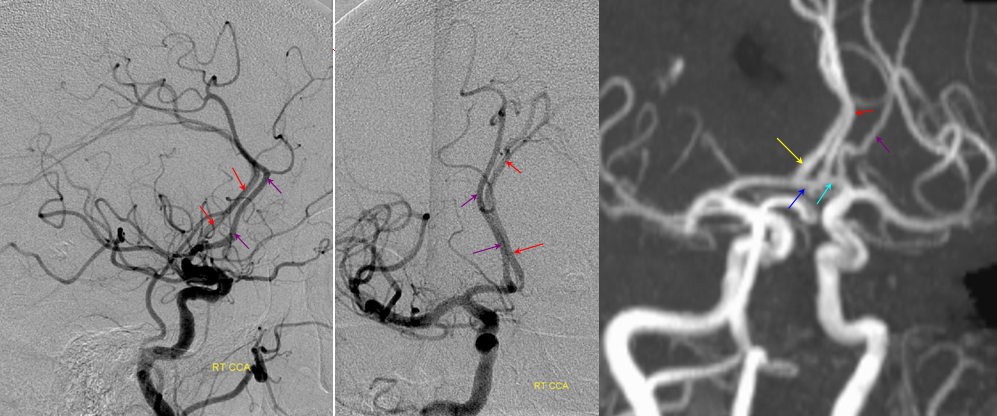
Accessory MCA
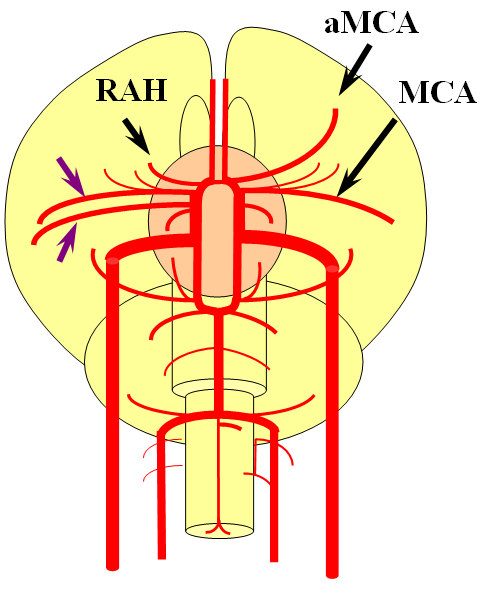
Recall that MCA (purple) arises as a dominant vessel from a number lateral striate perforators of the anterior cerebral artery in the reptile. As such, a disposition may arise where two perforators remain dominant, thus resulting in the “accessory MCA” The accessory vessel is, in fact, either a hypertrophied Recurrent Artery of Heubner (RAH) – a medial ACA perforator, or another perforator-like vessel. To qualify as an MCA, the vessel must have cortical territory. In this cartoon, the schematic on the LEFT shows aMCA configuration where both branches (purple) appear to originate proximal to the A1 complex (which is here defined as segment past the more “distal” MCA branch. These are known as Manelfe type 1 or 2 – depending on which branch is larger. The important feature however is to note from which vessel the perforators originate, and whether they are medial or lateral. The schematic on the RIGHT shows the Heubner-type aMCA, known as “Manelfe Type 3.
Type I/II accessory MCA on MRA
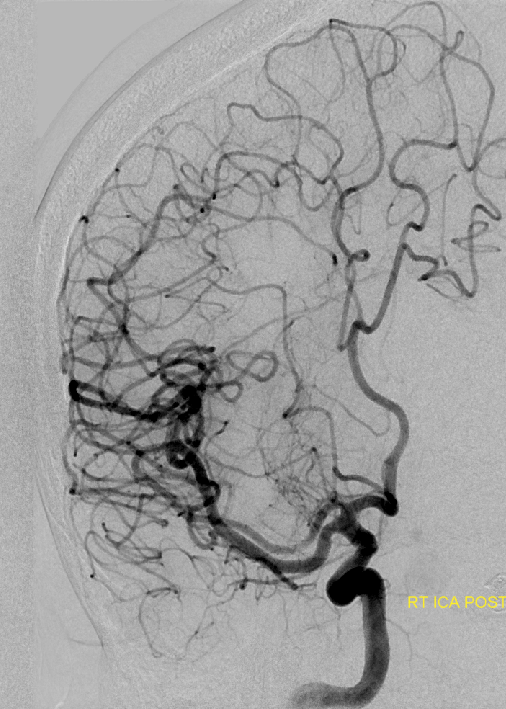
Catheter angiogram of the same patient. Notice how well MCA perforators can be seen, originating from the smaller and more distal of the two MCA vessels.
Type III (Heubner-like) aMCA originating from the ACOM complex
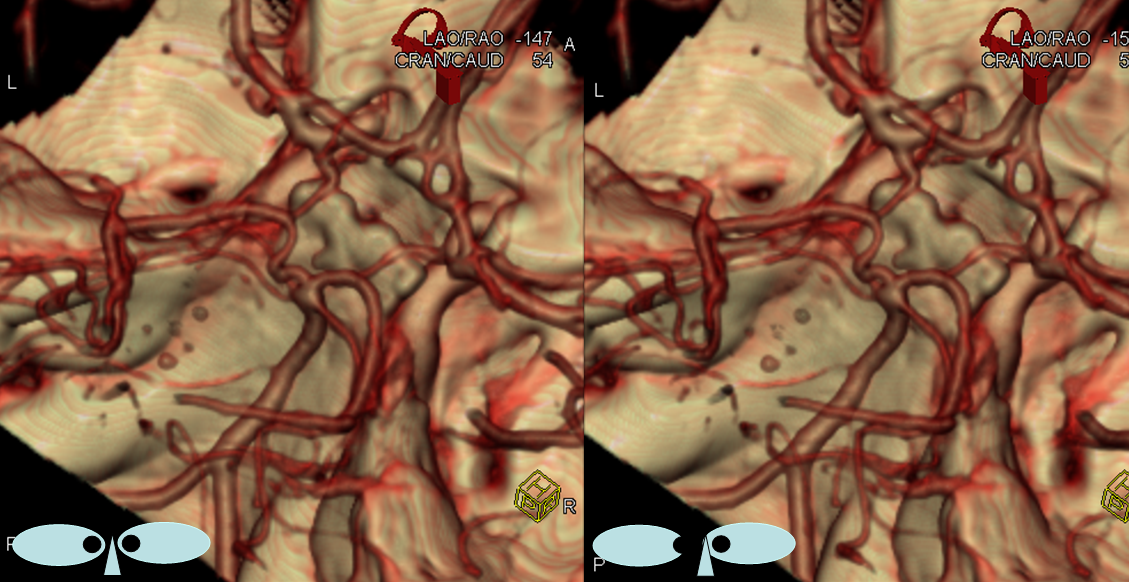
This series of images shows a prominent RAH, as a correlation to the aMCA case above. The bottom set of images (with the blue eyes) is a stereo pair.
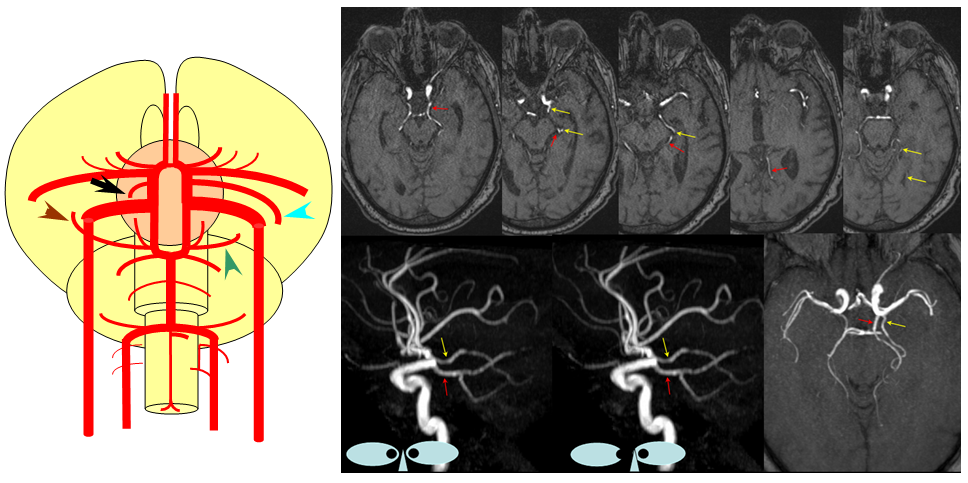
Dominant anterior choroidal artery
As you remember, the anterior choroidal artery (black), until late in evolution, supplies a substantial portion of cortical territory, which in the human is annexed by the PCA (brown). On occasion, the degree of annexation is incomplete, with large portions of the cortex remaining under anterior choroidal control (blue) while PCA contribution is correspondingly reduced (green). This is sometimes erroneously considered a “duplicated PCA”. Mistaking the choroidal artery for the PCOM can have disastrous surgical consequences. On MRA images, the PCOM is labelred in red, and choroidal in yellow.
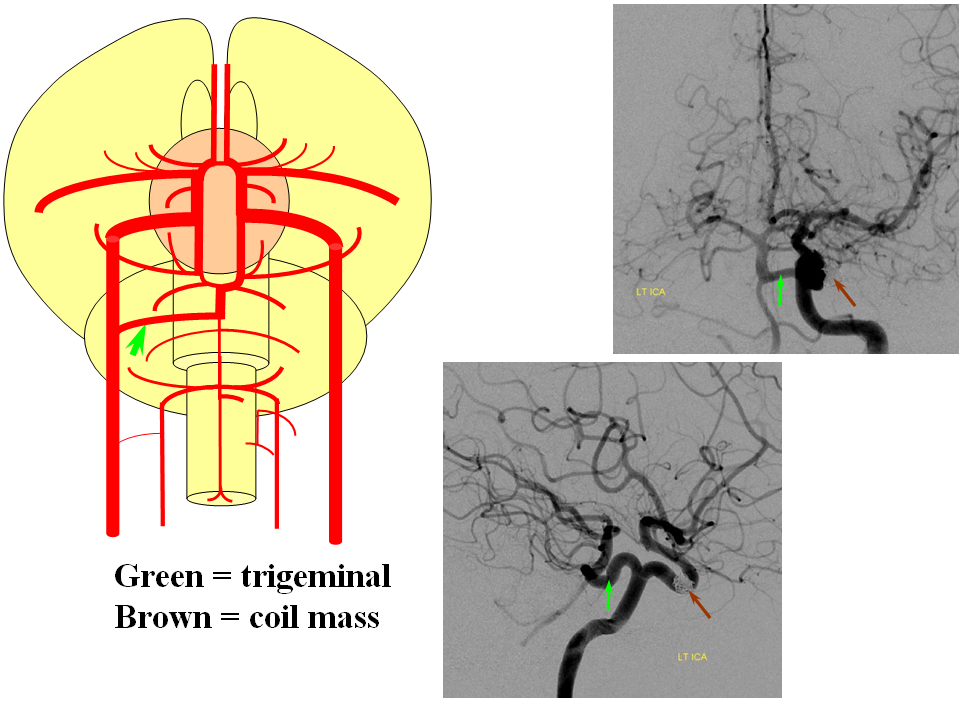
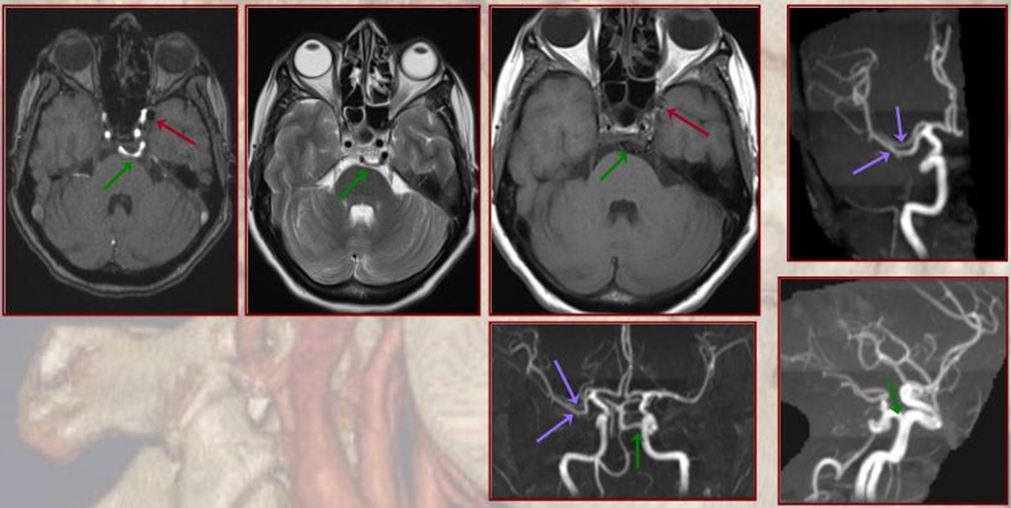
Persistent Trigeminal Artery
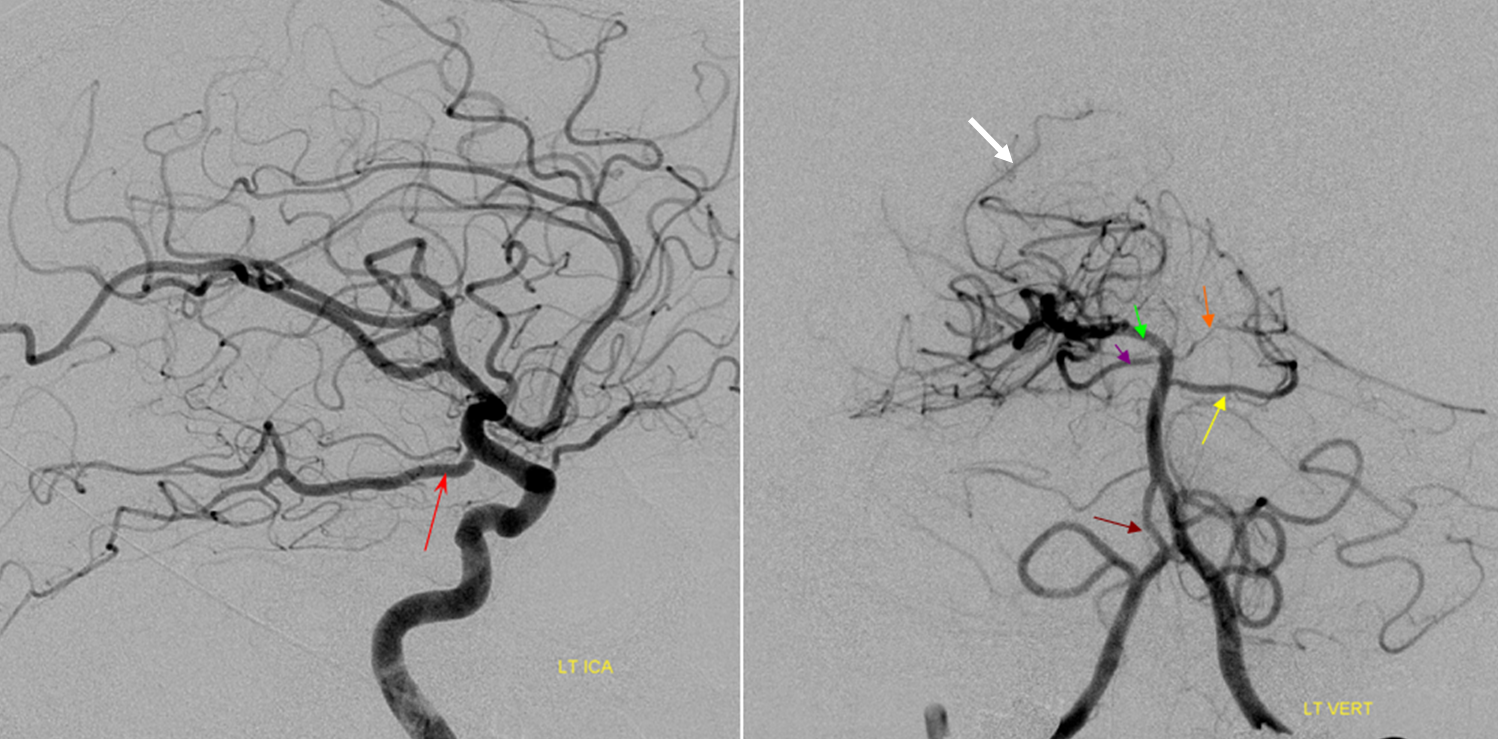
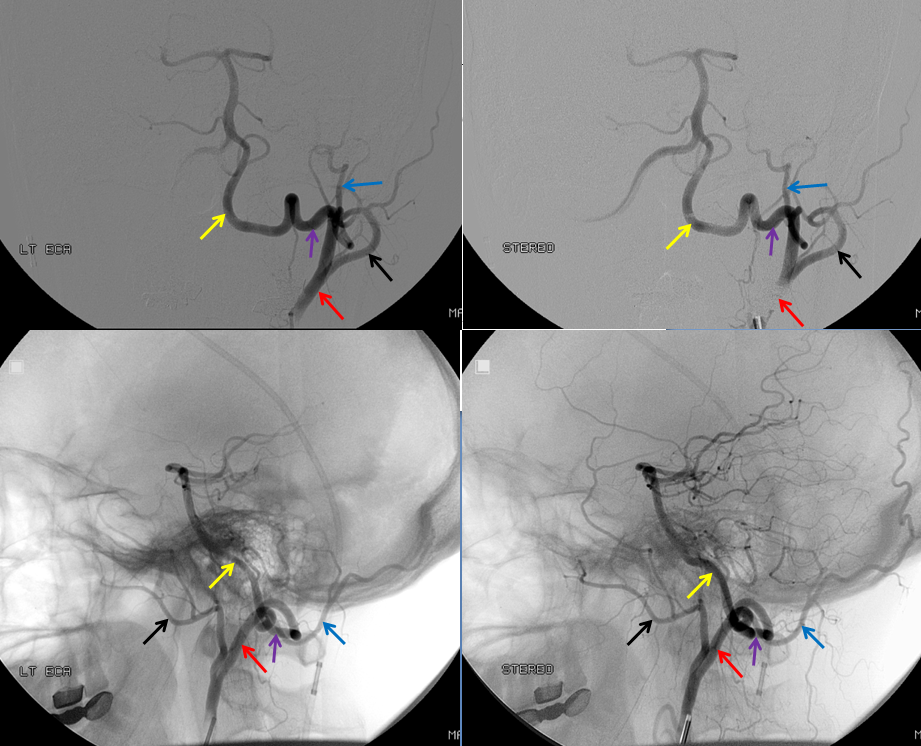
This most common embryonic carotid-vertebrobasilar anastomosis at the mid-basilar level represents persistence of segmental ICA-basilar connection along the trigeminal nerve. The posterior communicating artery is usually small in these cases. The case below illustrates a persistent trigeminal, an accessory MCA, and an associated aneurysm treated with coils.
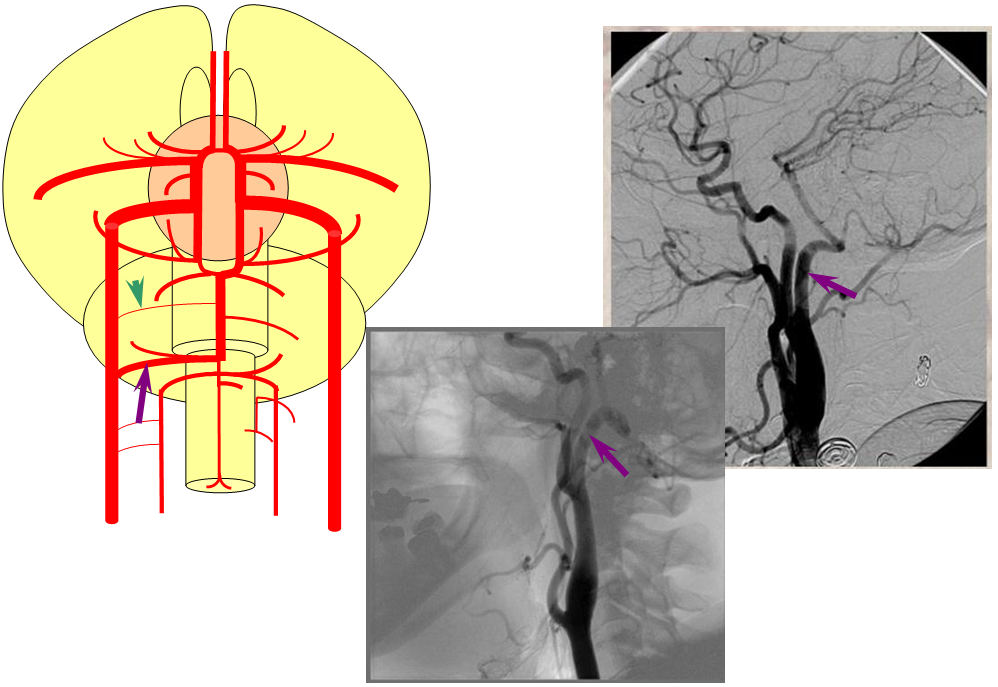
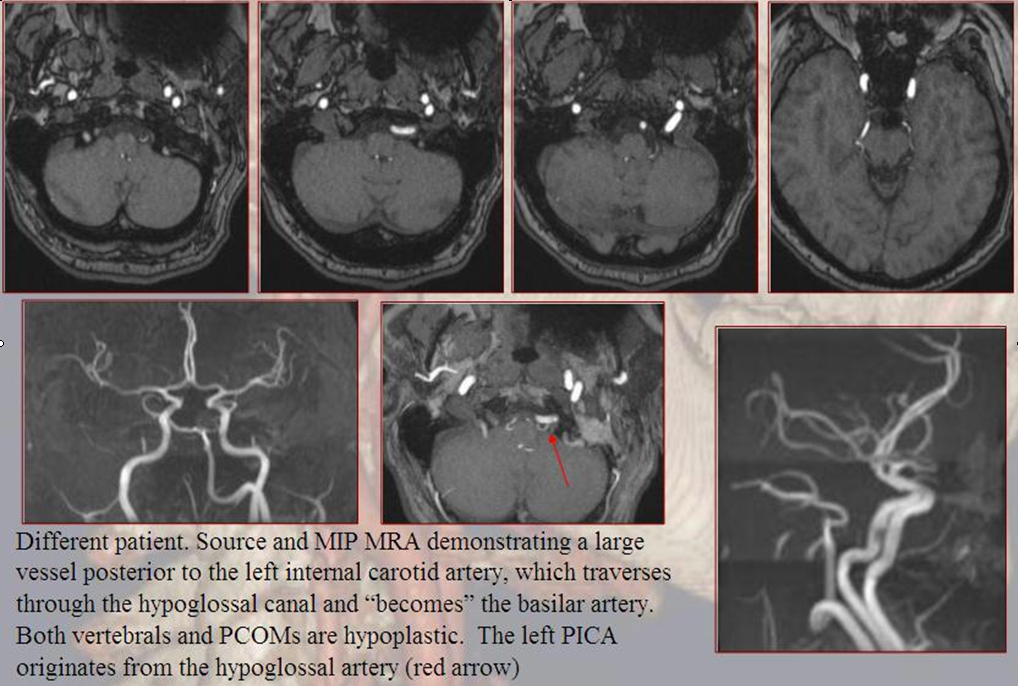
Persistent Hypoglossal Artery
More caudal than the persistent trigeminal artery (green), persistent hypoglossal artery (purple) enters the cranial vault through the hypoglossal canal, connecting the distal vertebral and the proximal internal carotid arteries. It is, like trigeminal and proatlantal vessels, a segmental artery.
Proatlantal Artery
An embryonic carotido-vertebral anastomosis, the proatlantal artery is, in fact, the occipital artery. A C1 segmental connection between the occipital and vert is the Proatlantal type I; C2 level connection is Proatlantal type II (a different classification based on whether the proatlantal comes off the ECA or ICA exists also, to generate confusion, — embryologically, the classification according to cervical level of occipital-vertebral anastomosis makes more sense.) Usually, the vertebral artery proximal to the proatlantal segment is hypoplastic.
Stereo AP and Lateral views of left ECA injection, opacifying the vertebrobasilar system(yellow) via the C1 segmental artery (purple) connection to the occipital artery (red). This is the Proatlantal I type; the proximal occipital artery (red) is the proatlantal. Occipital artery distal to the vertebral anastomosis is blue, and IMAX is black.
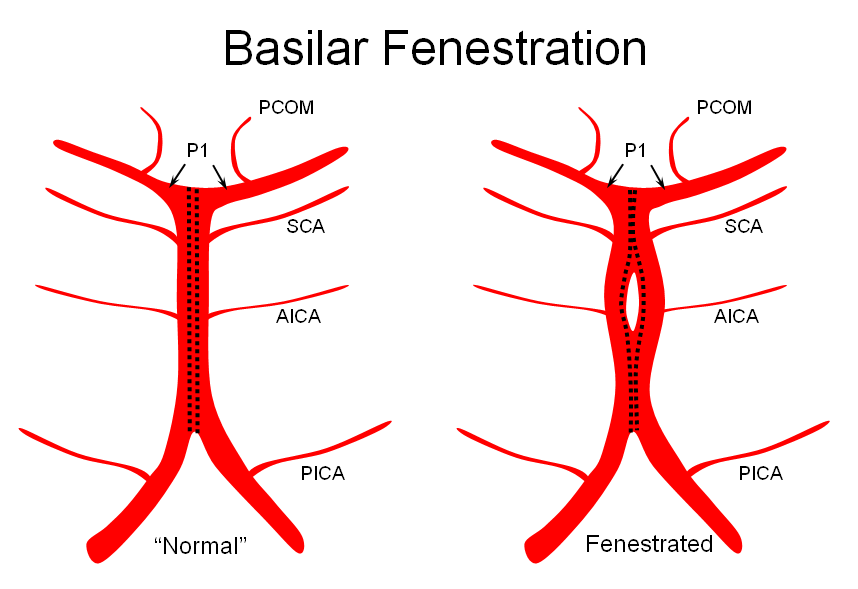
Variations in fusion patterns of the basilar artery
The basilar artery is formed via coalescence of multiple channels belonging to the longitudinal neural system, following development of the anterior circulation. This process sets the stage for multiple basilar artery variations, which can be considered from the standpoint of 1) extent of fusion and 2) completeness of fusion (i.e. fenestrations).
It is conceptually helpful to think of the basilar artery as being “zipped” in the middle, with vertebral and PCA segments being “unzipped”. Thus, both length and the integrity of the zipper determine the final configuration of the artery. As such, the artery may be:
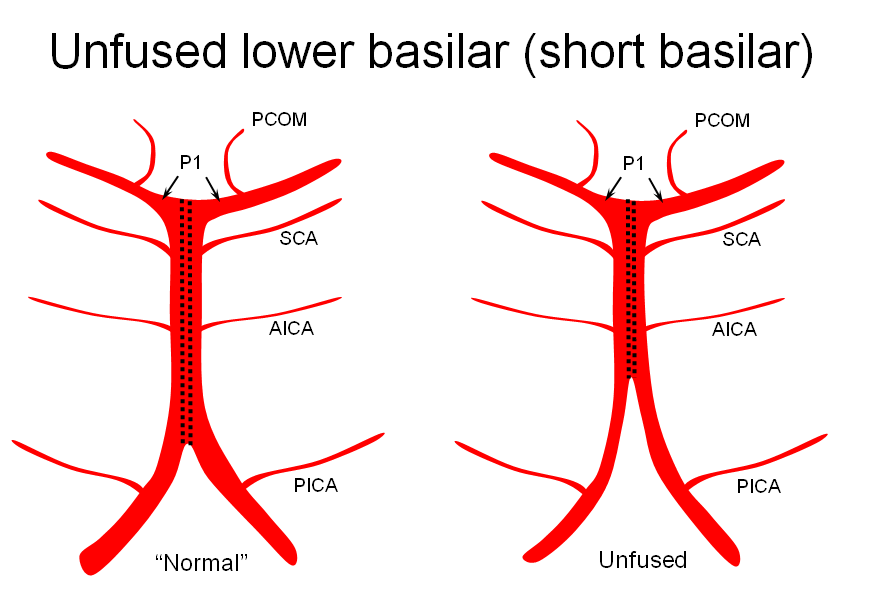
1) “Short” — corresponding to relative lack of fusion of the caudal segment
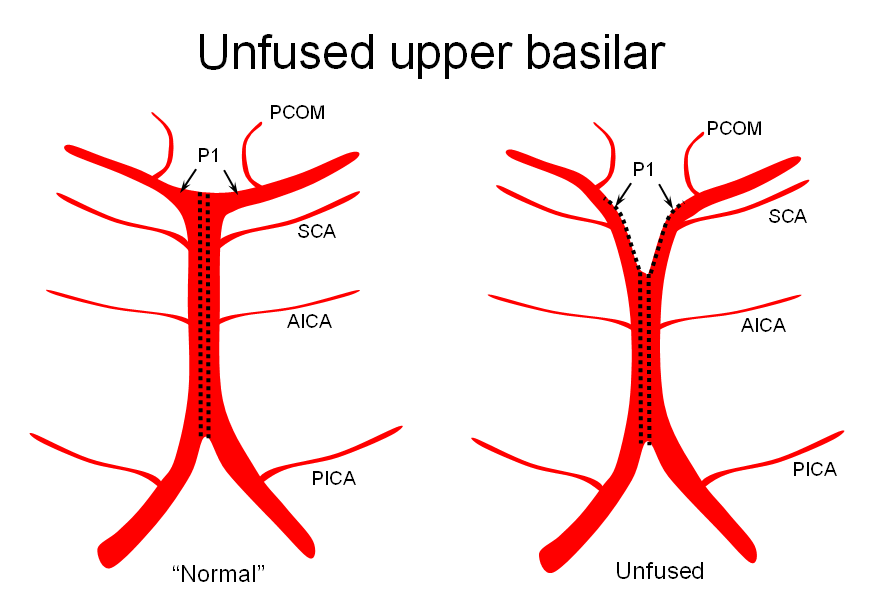
2) Unfused at the top — corresponding to “unzipping” at the basilar tip — less common but much more confusing
3) Fenestrated — broken zipper in the middle
The first one, short basilar, is in fact a continuum in terms of fusion extent. There is no “number” to guide what short or long is, but some basilars are clearly quite foreshortened. (The tortuous basilar of the aged hypertensive is a different matter)
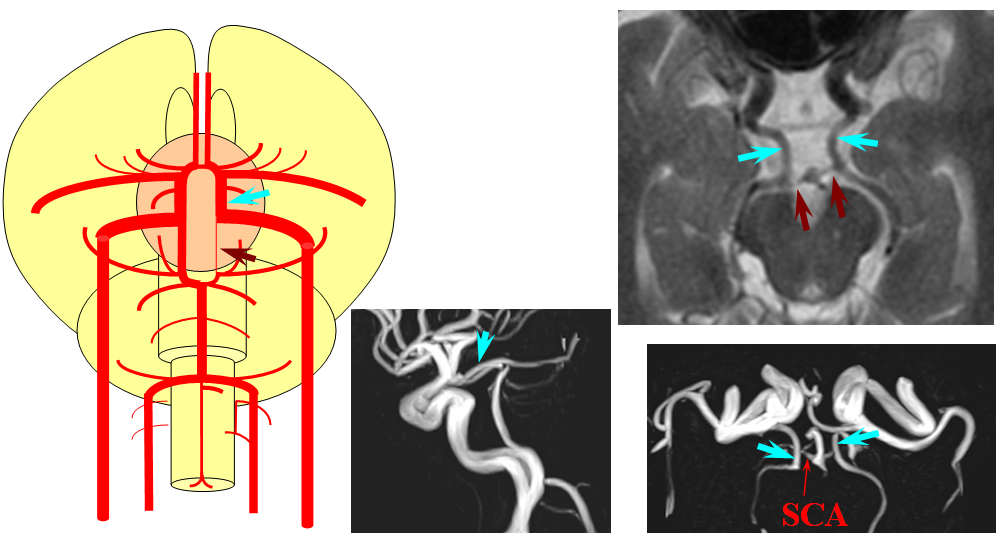
The second variation, involving lack of basilar tip fusion, can generate a lot of confusion. Effectively, the top of the basilar is split in two, so that one or both superior cerebellar arteries originate from the P1 segment. This variant is not, therefore, a primary superior cerebellar artery aberration, but instead a “deficiency” in basilar fusion.
P1 origin of the superior cerebellar artery. The SCA (blue) on the right arises from the P1 segment. Further “Zipping” up would elongate the basilar trunk, foreshorten both P1 segments, and shift the SCA onto the basilar.
When especially prominent, basilar tip “unzipping” can lead to some confusion, as in this patient reported to have a basilar tip aneurysm on CTA. Catheter angio shows an unfused basilar tip with tortuous P1 vessels simulating aneurysm, further complicated by presence of a fetal PCOM on the right.
The third, basilar fenestration, is quite common, and usually of little clinical signficance, except when it is so short as to minic a dissection.
Occasional species, including some monkeys, do not have a basilar artery at all. The caudal rami are unfused throughout, mimiking the anterior cerebral disposition.
Duplications
As in case of accessory MCA, essentially any vessel on occasion is gifted with a twin – not an identical one, since these branches actually supply separate territories. Understanding that arteries in essence are dominant collectors arising from a number of potential homologous candidates clarifies this occurrence. The most commonly dupliated vessel is the superior cerebellar artery. Duplicted AICA (brown) is quite rare.
Fenestrated A1 segment, in stereo
Anterior Cerebral Artery variations
The number of potential ACA variations may seem mind-boggling. Phylogeny and embryology, however, help make sense of frustration and confusion. The appearance of the ACA system can be conceptualized in terms of two factors — extent of fusion and pattern of branch takeoff.
On the fusion side, you can expect anything from no fusion — that is no ACOM, various short segment patterns of fusion (duplicated ACOMS) or elongated segments of fusion (unpaired or azygous arrangements) The unpaired type is one where a long segment of fusion finally breaks up into two branches, each of which gives rise to individual cortical branches to its respective hemisphere. The azygous type is where the ACAs are fused for the ENTIRE length, with individual branches to both hemispheres arising from a common trunk. Thinking of unpaired/azygous arrangements as a kind of “basilar” is helpful, and may not be conceptually incorrect. Many species, such as dogs, have a “normal” azygous arrangement with “variants” of unpaired or “human-type” disposition. In the human, unpaired cases have a much higher incidence of aneurysm formation at the eventual branch point, — not a favorable evolutionary development.
On the takeoff pattern side, you can have any takeoff pattern imaginable, but of particular importance is the arrangement at the ACOM region. The frontopolar branch may arise from the ACOM complex. More importantly, the calossomarginal-pericalossal split may occur anywhere along the length of ACA from ACOM to the calossum genu. Particularly when this split occurs at the ACOM region, a triplicated appearance may result with pericalossal and calossomarginal trunks arising from one side, and regular A2 from the other, for a total of 3 arteries (see below).
Ultimately, ACA analysis in terms of some combination of fusion extent and branch takeoff can clarify any ACA arrangement.
Unpaired ACA. This is a case of an unpaired ACA which eventually trifurcates into a calossomarginal and two pericalossal arteries, with an associated aneurysm.
Images courtecy of Dr. J. Bello and Dr. A. Brook, Montefiore Medical Center, Bronx, NY
Early calossomarginal takeoff at ACOM level (i.e.”triplicated aca”)
Lateral projection angiogram with early calossomarginal takeoff (purple) and pericalossal in red. Corresponding MRA image with left A2 shown in yellow and ACOM in dark blue. This is the appearance of a “triplicated ACA” which is discussed above; it is a misleading term since not 3, but 2 ACA arteries are present. It is the A2 segment on the right that’s absent because of early bifurcation into calossomarginal and pericalossal.
Anterior Cerebral – Posterior Cerebral anastomoses
ACA territory collateral circulation
PCA (particularly posterior pericalossal / splenial branches) can effectively reconstitute distal pericalossal ACA territory. Embryologically this corresponds to a pericallossal/posterior pericalossal anastomosis which exists in some mammals and possibly in the human embryo.
Carotid Occlusion — PCA reconstitution of the ACA territory.
The right ICA is occluded at the origin. Notice how effectively posterior pericalossal branches of the PCA (red) can reconstitute the pericalossal territory of the ACA (yellow), with runoff into the anterior frontal branch (purple)