This page was developed as a follow-up to collaboration on a JNIS publication with Drs. Melanie Walker, Kate T. Carroll, Michael R. Levitt, Eytan Raz, Erez Nossek, Nader Delavari, Osman Mir, and Peter Kim Nelson.
The image below was expertly created by Jonathan Dimes of JDIMES Medivisual Communications
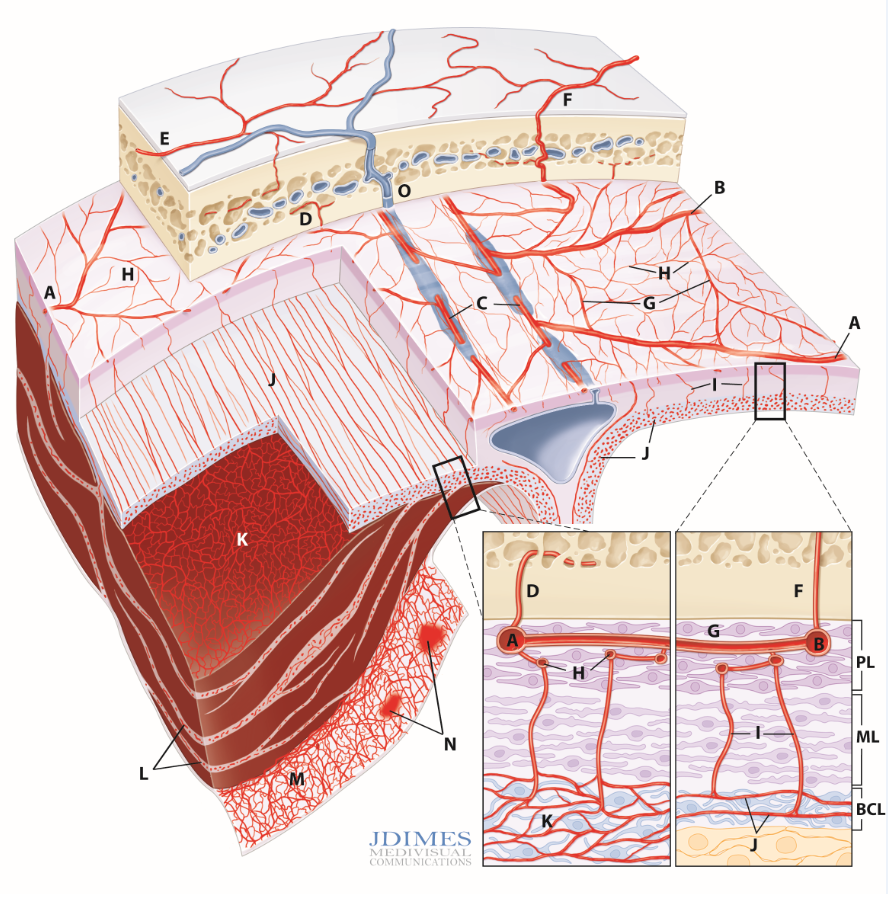
Normal and pathologic dural vasculature. PL – periosteal layer; ML – meningeal layer; BCL – border cell layer; A, B – main MMA branches such as frontal (~400-800 um); C – arterial network in walls of sinuses (variable size); D – meningeal branch supplying skull; E – cutaneous branch (superficial temporal, occipital, etc) supplying skull; F – cutaneous branch supplying skull and meninges (usually occipital over temporal/parietal regions); G – primary anastomotic network of the outer layer, connecting larger dural branches (100-300 um); H – secondary anastsomotic network in the outer dura; I – penetrating vessels in the relatively avascular middle layer; J – normal inner capillary network in the border zone layer; K – proliferating border zone / inner network vessels in a subdural hematoma – the vessels are located within membranes (L) and on the inner surface of the hematoma (M) which is also covered by membranes; N– area of leakage / extravasation; O – venous sinuses adjacent to larger and smaller caliber dural arteries; P – dural venous drainage into diploic vein
Intrinsic Dural Vasculature
This section deals specifically with intrinsic arterial vasculature of the dura. For discussion of individual arteries, see Anterior, Middle Meningeal Artery as well as the Umbrella Meningeal Vessels pages. The separate venous page is here.
As of 2020, this topic remains new. Very few studies on this, ex- or in-vivo. However, this vasculature is extremely important in understanding dural-based diseases such as dural fistula and chronic subdural hematoma. Modern angiographic equipment can see a lot. So, here we go.
The basic anatomy is like this (see figure above): convexity dura has three layers — periosteal, meningeal, and border zone. Each layer is associated with its vascular network. Periosteal layer with “outer layer network”, meningeal with “meningeal network” and border zone with “inner layer” network. See picture above
Outer Layer Network
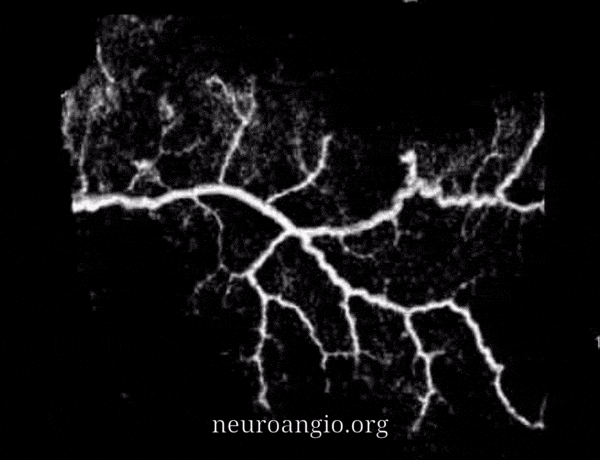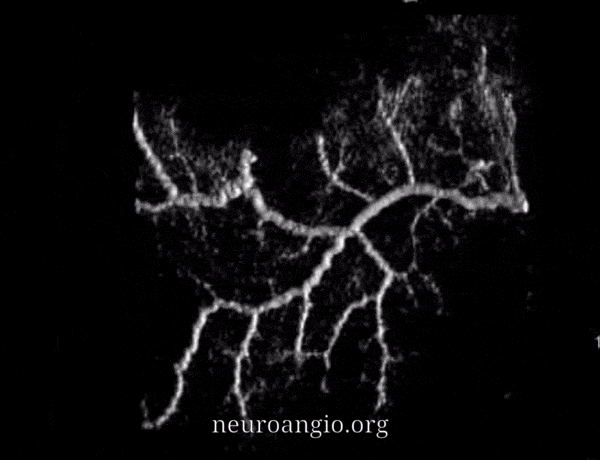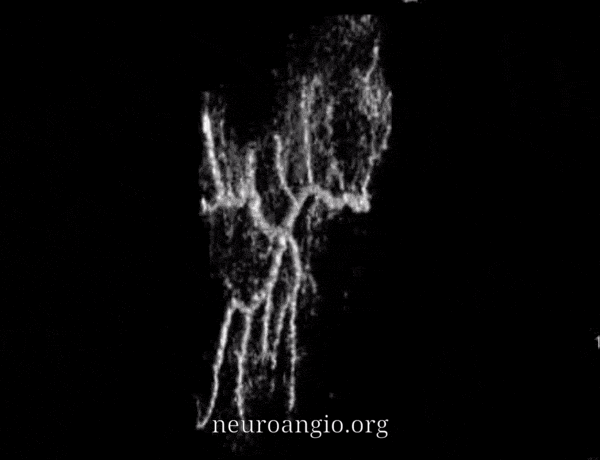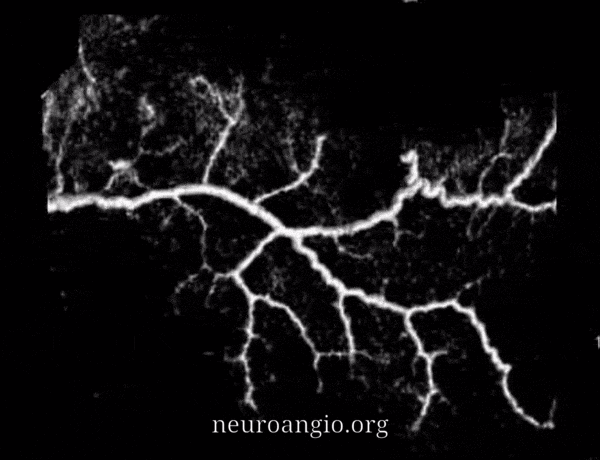
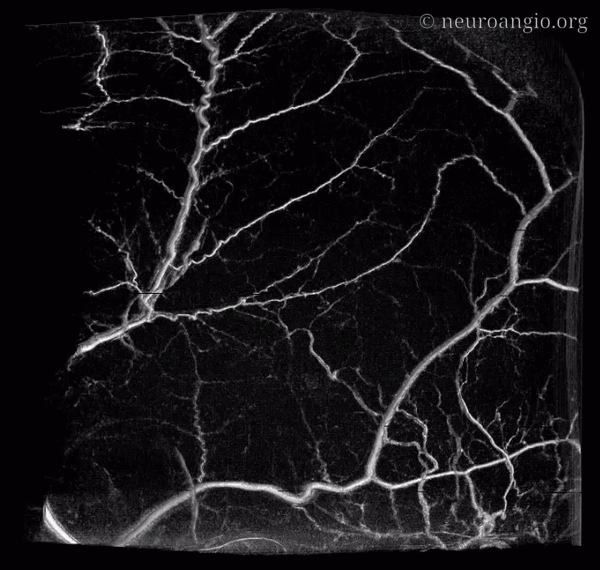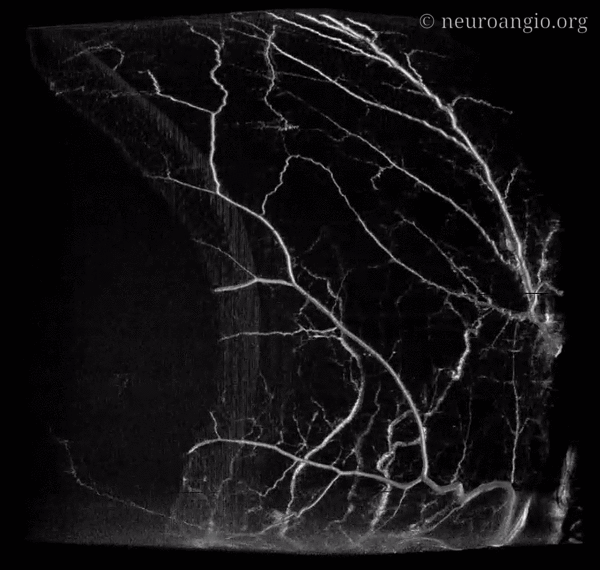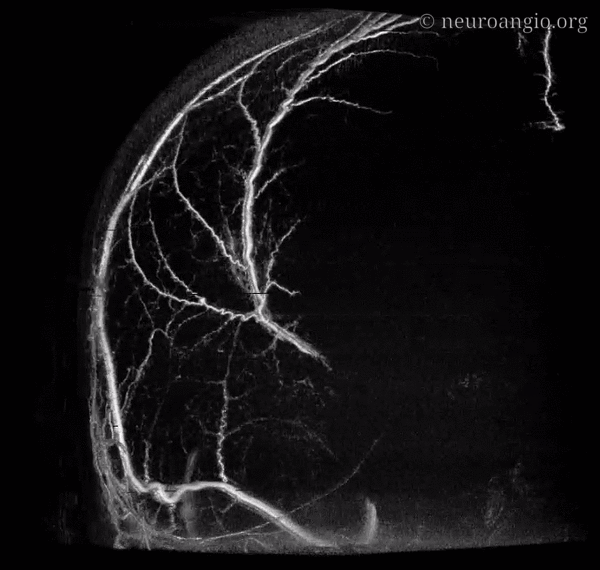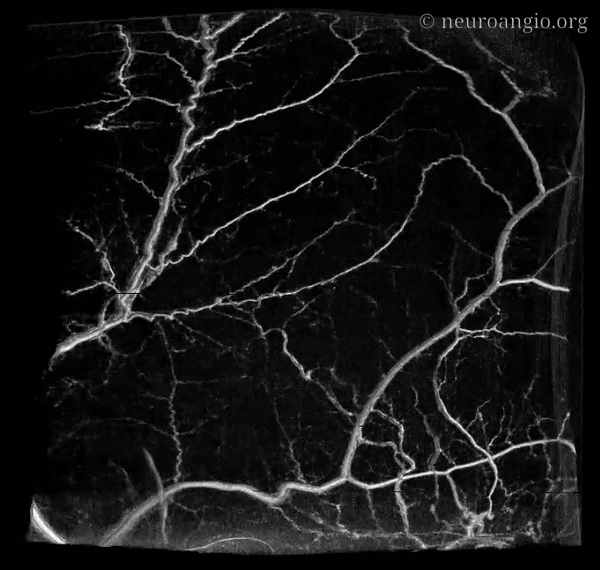
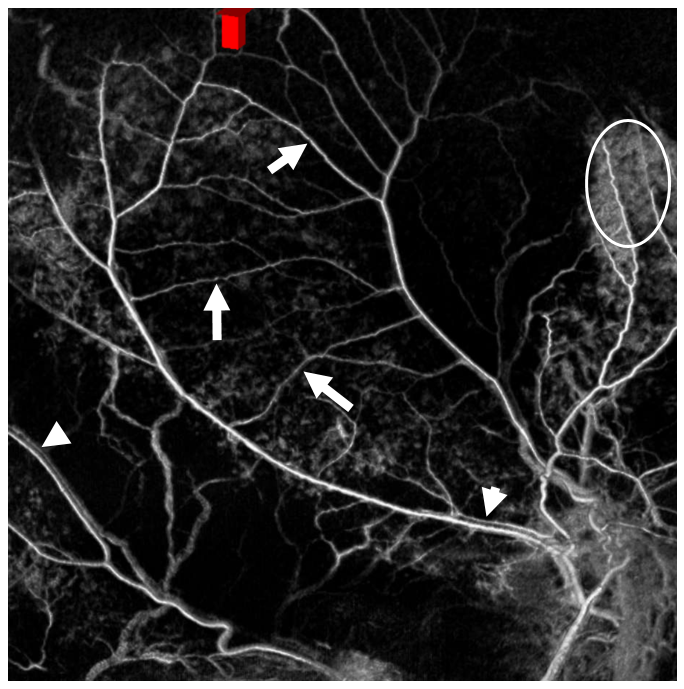
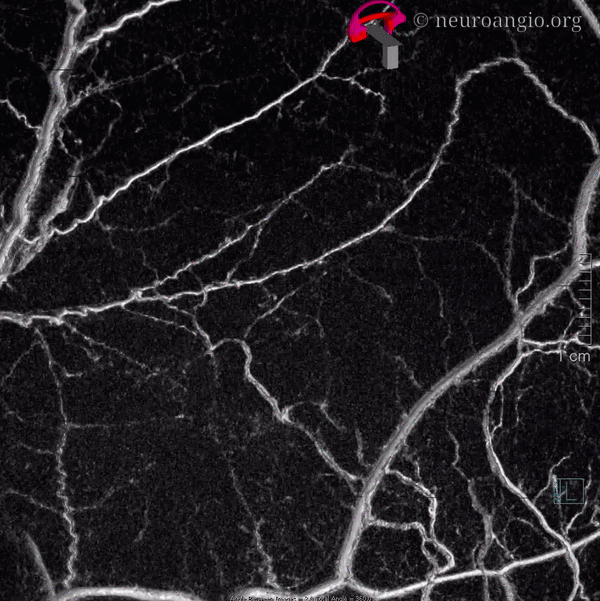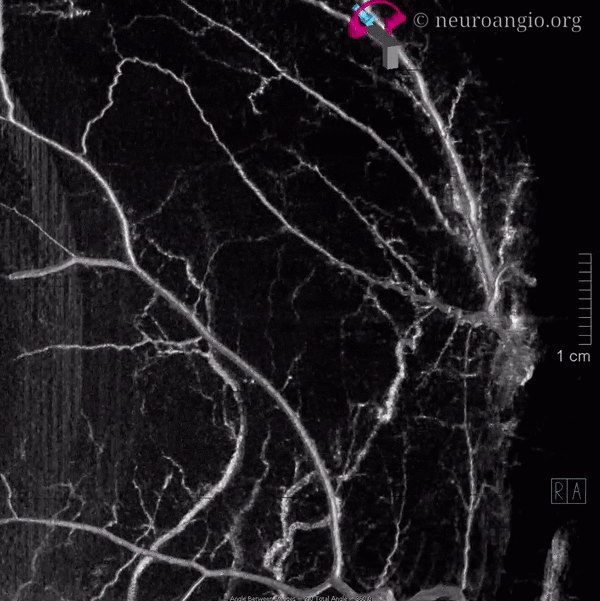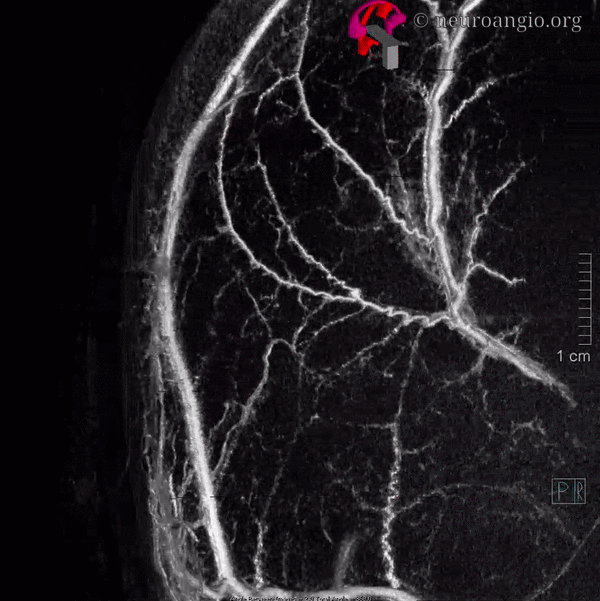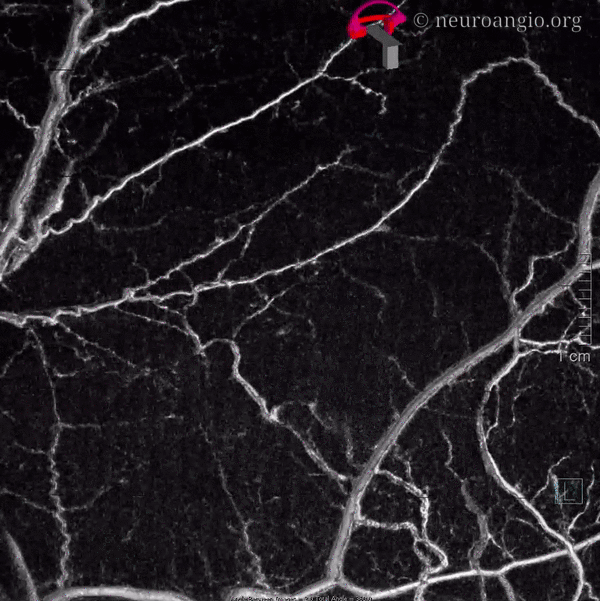
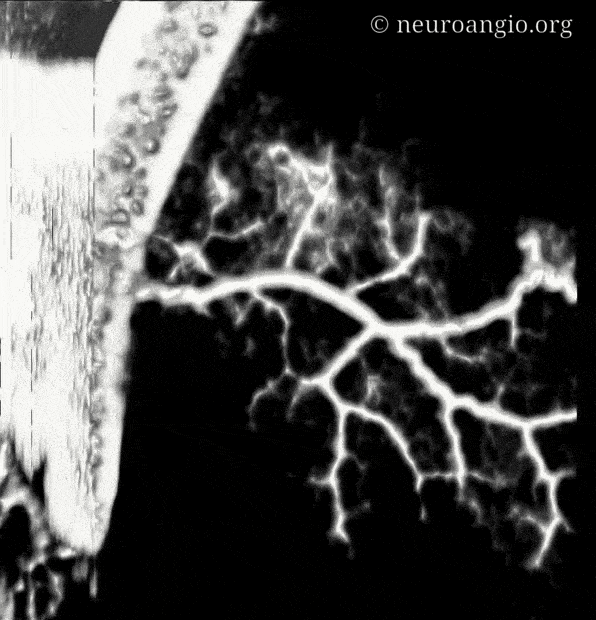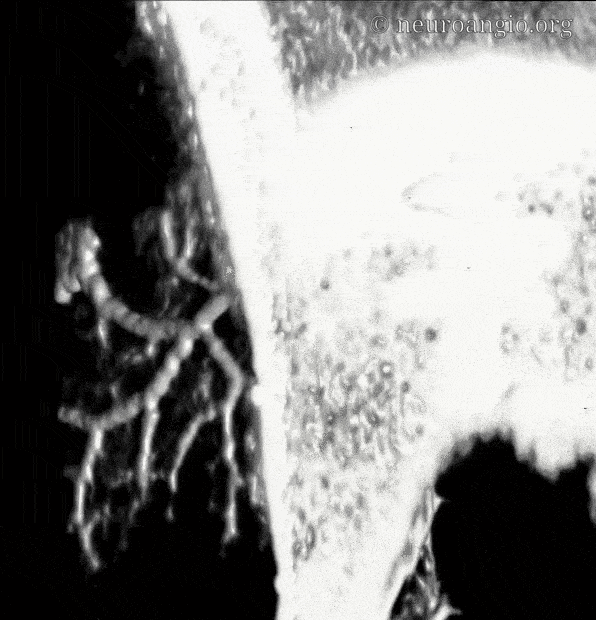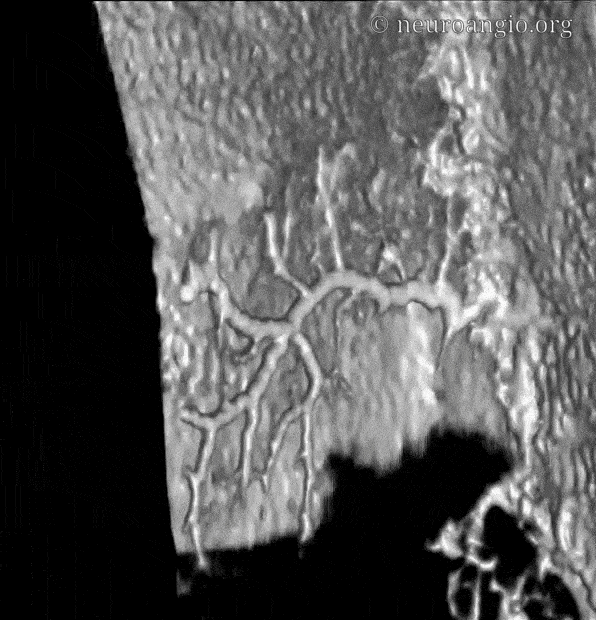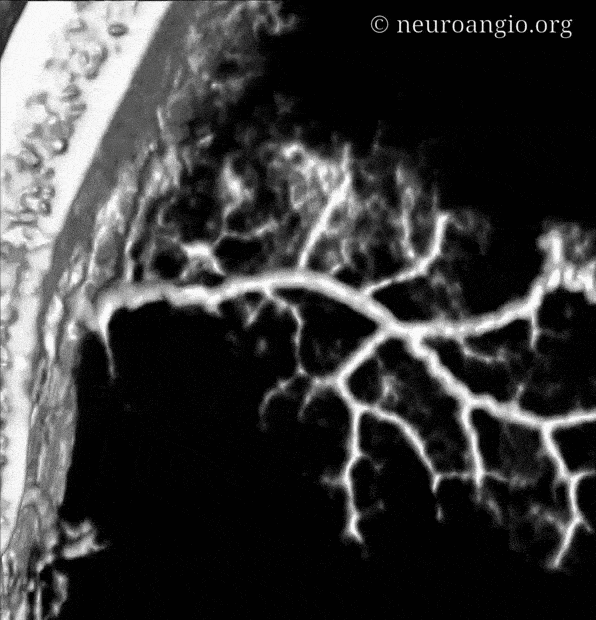
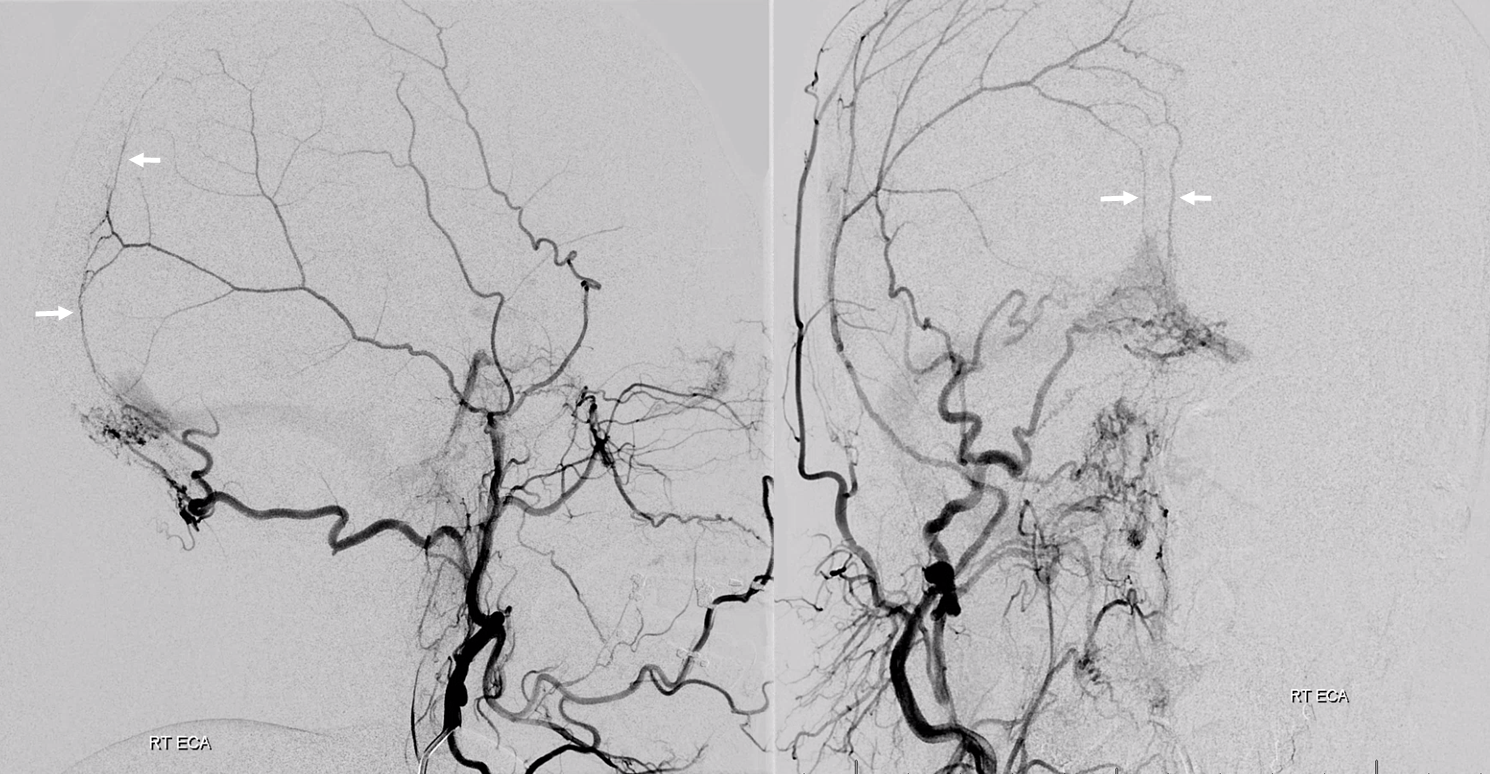
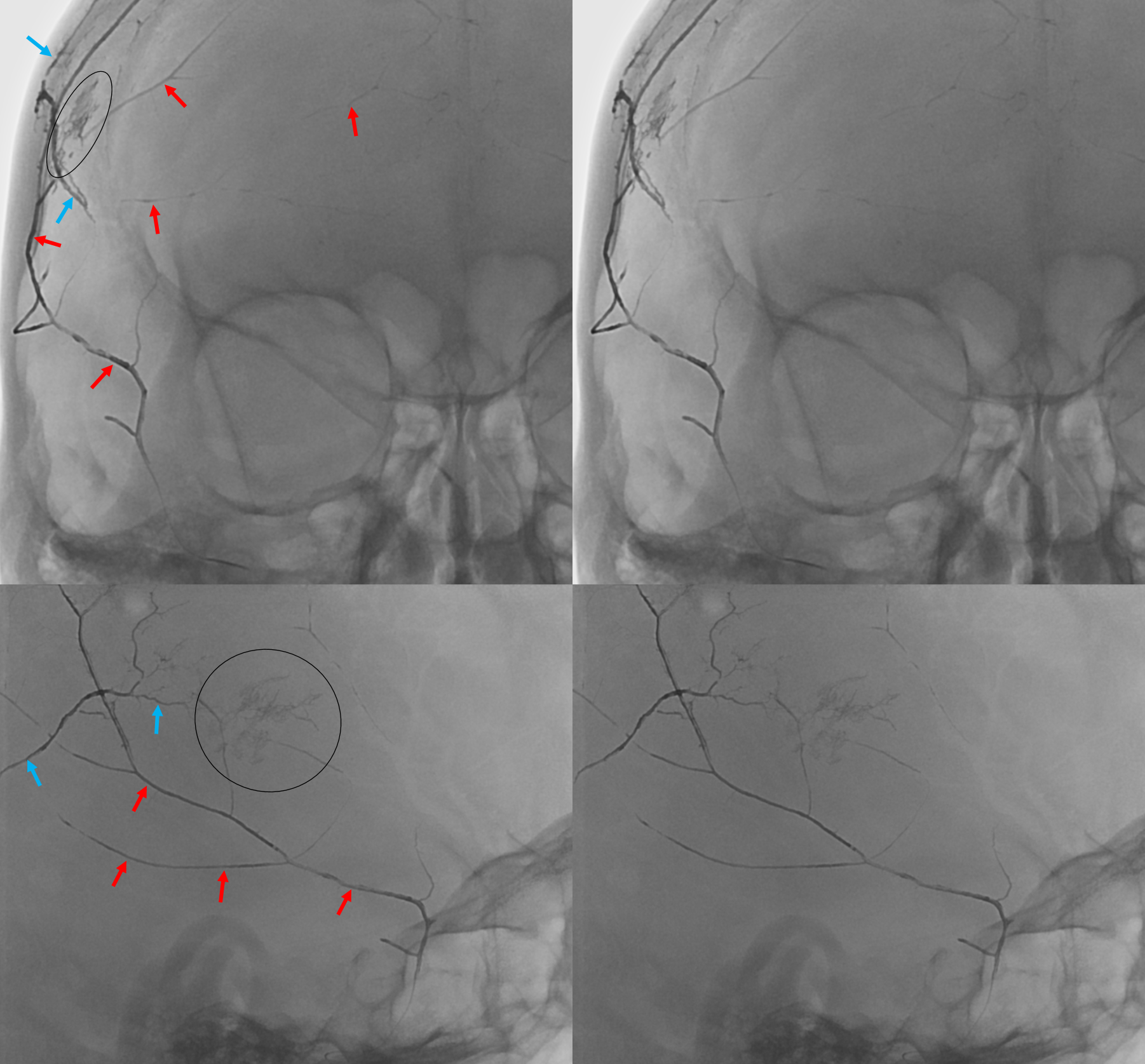
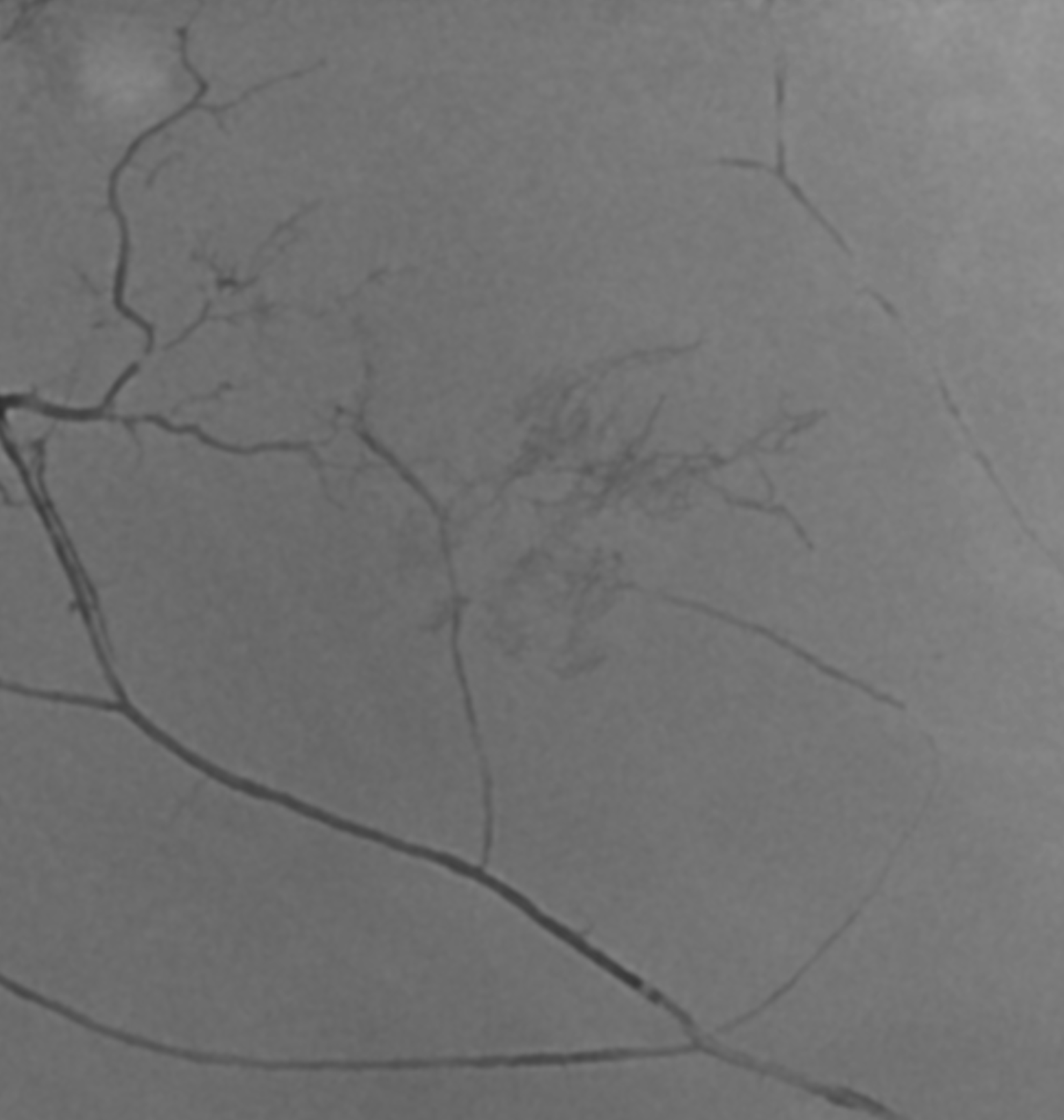
The outer layer network contains all the vessels you know — MMA, its major branches, and connections between them. Its a fractal structure — there are “primary” and “secondary” outer layer networks. The “primary outer layer” connections between major MMA branches are something like 50-90 um in diameter. You can see them on DSA — below catheter is in the perosquamosal branch. Injection opacifies multiple primary outer layer anastomoses (white arrows) between petrosquamosal and parietal MMA branches, and between posterior meningeal territory (black arrows). Also notice supply to the skull (arrowhead)
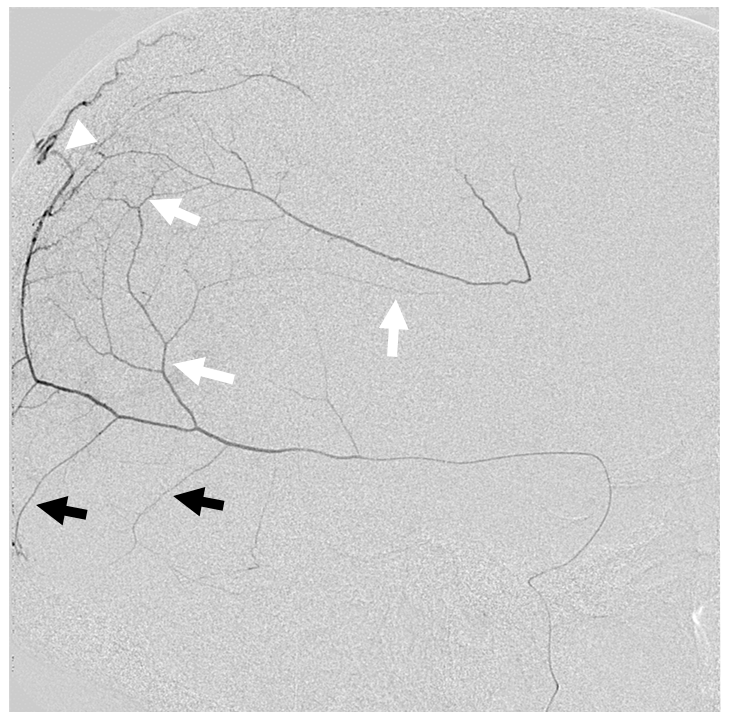
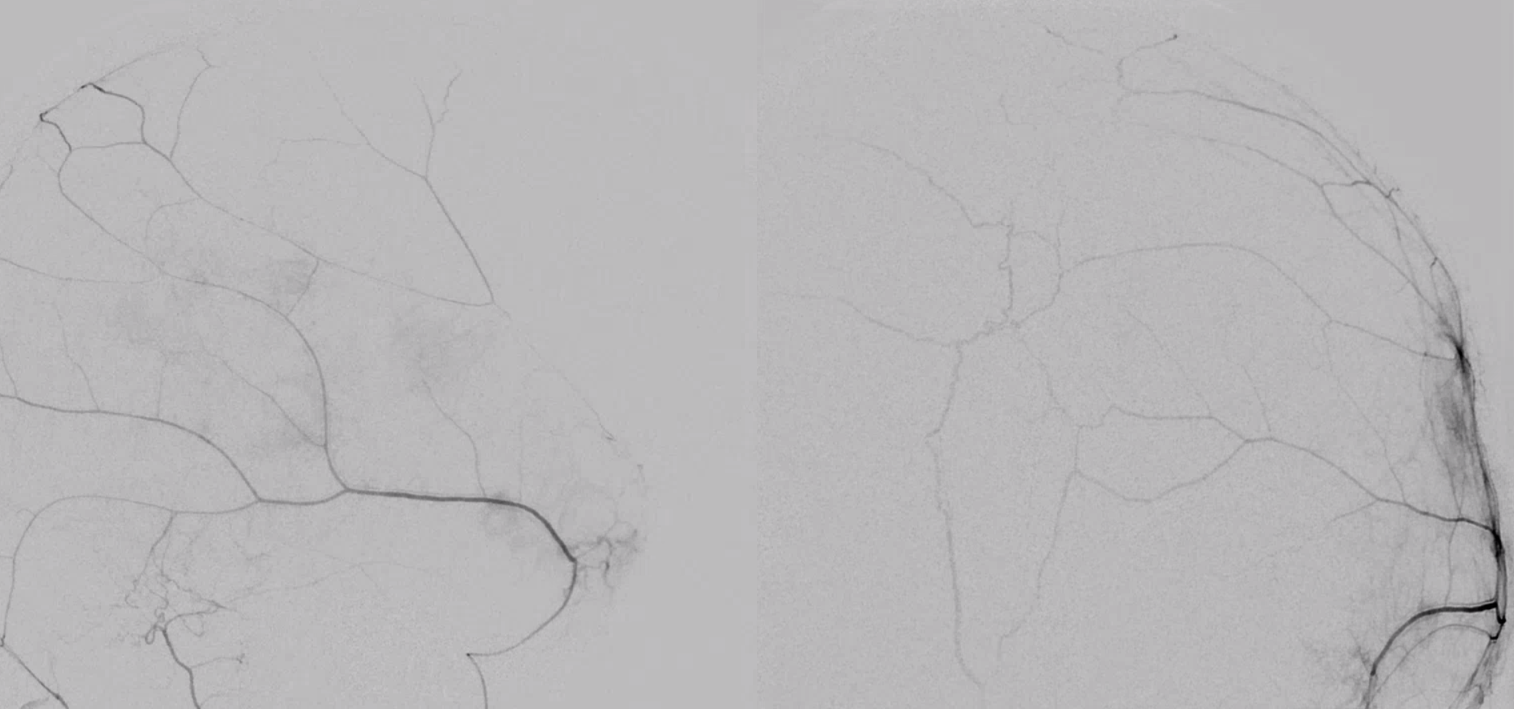
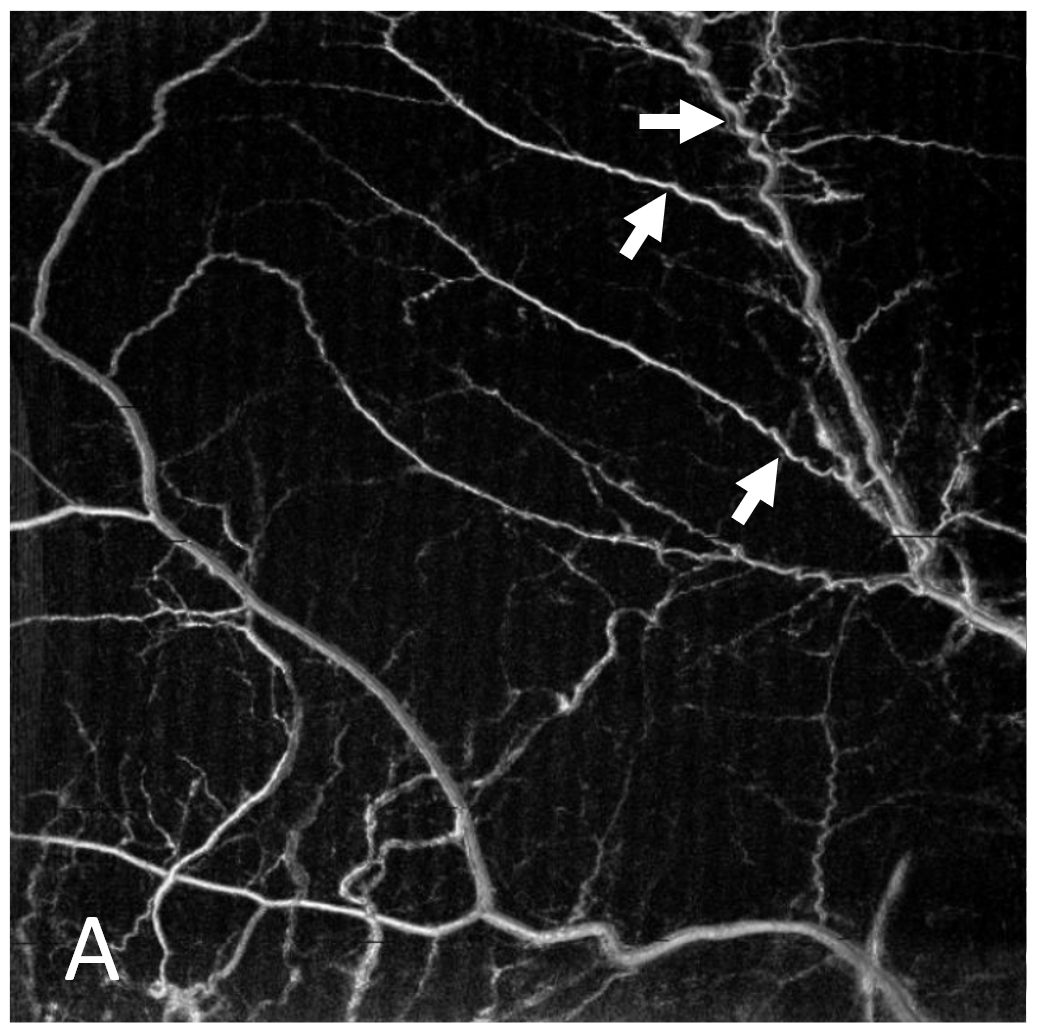
Another example of excellent outer layer anastomoses. The primary anastomotic network extends across the entire dura — there is no reason why it should respect midline, as shown below. Notice also anastomoses below with the posterior meningeal territory
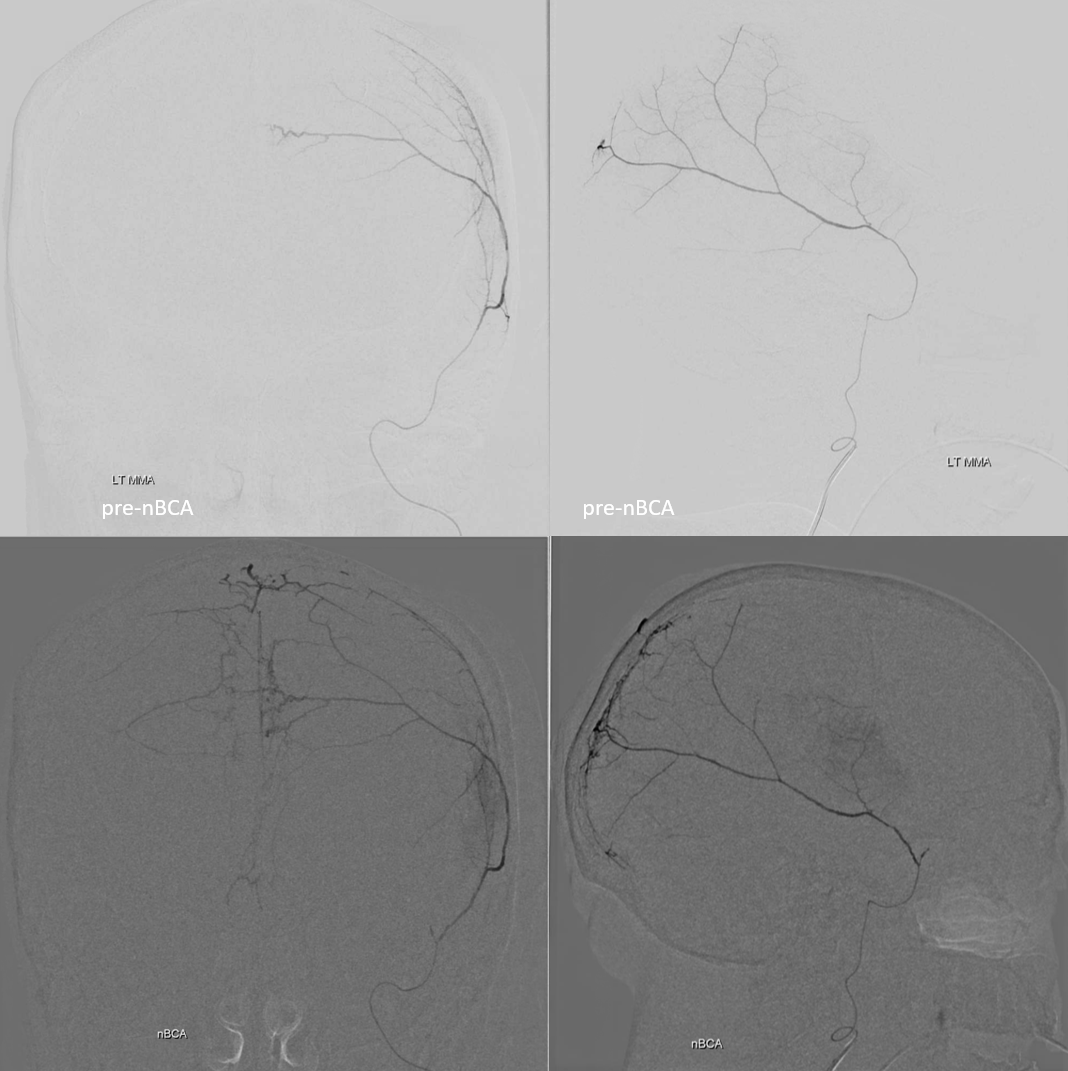
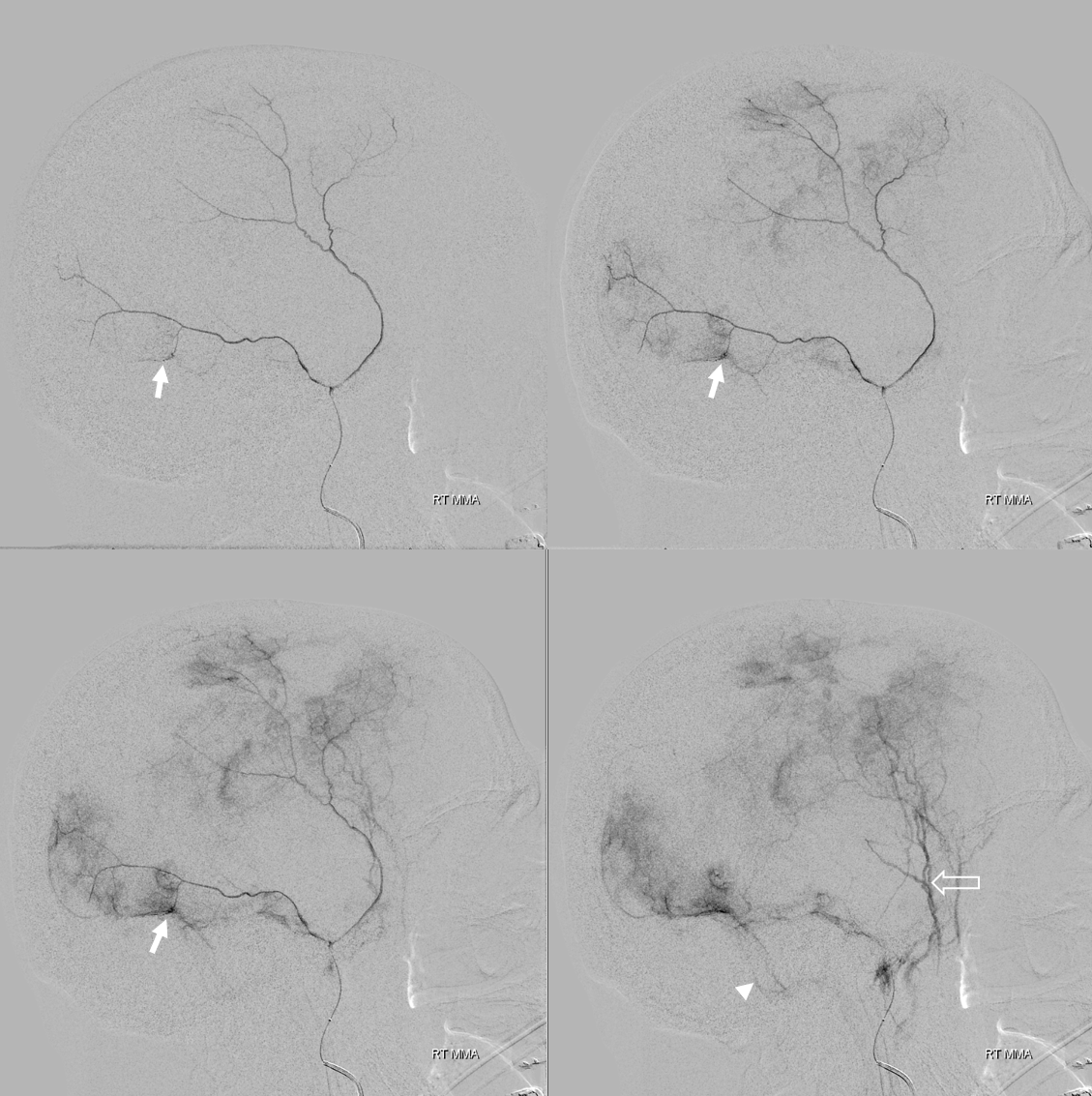
To really evaluate the “goodness” primary outer network anastomoses, your microcatheter should be in a “wedge position” — to hydraulically push contrast into the network. Just because you don’t see the whole thing on an injection does not mean its not there. Even when there is “wedge” or “flow control” position, still… Look below — a wedge position of the microcatheter produces the top set of images. However, the subsequent nBCA injection — bottom images — is an altogether different matter — look at how far the glue goes! And be careful while watching…. 🙂
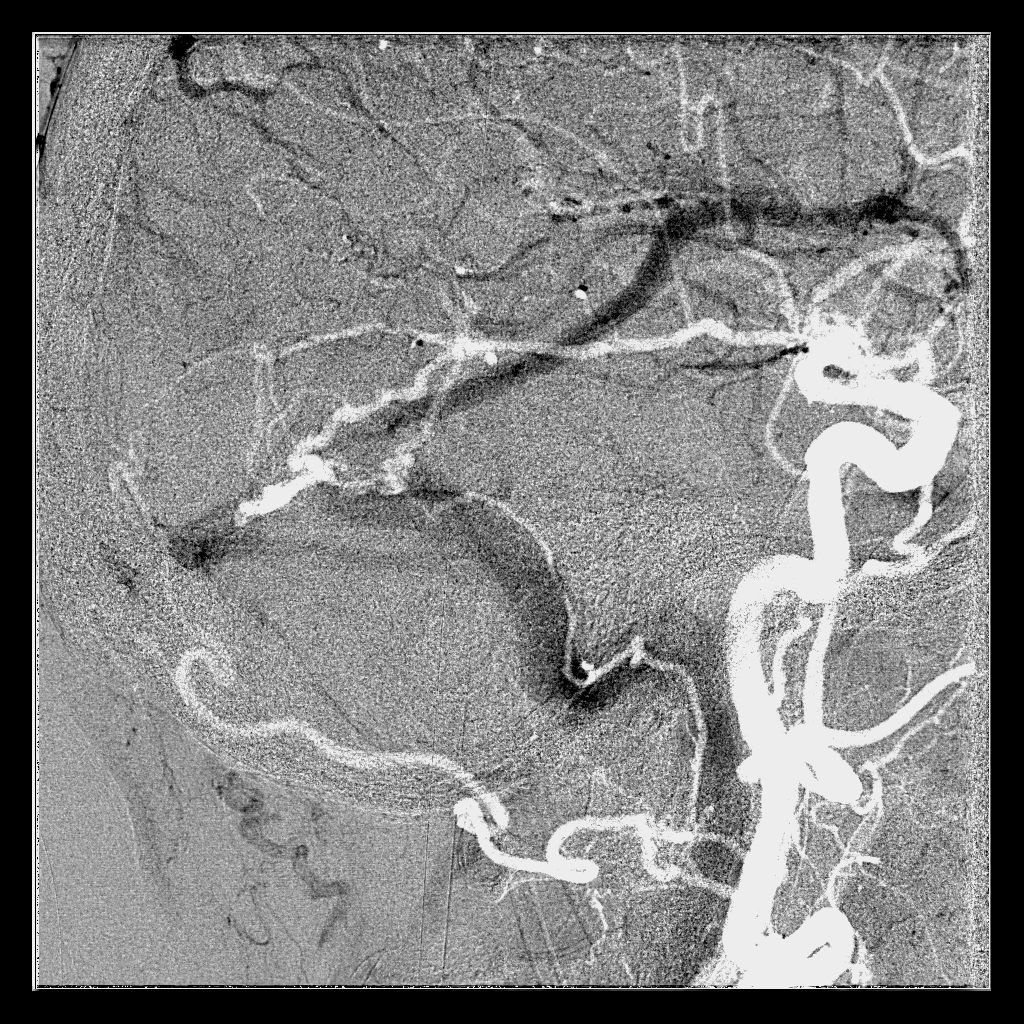
There is a lot of between-subject and within-subject variation — some people have lots of good primary connections, others don’t. In the example below, the connections between major MMA branches are bad. However, we see very well contrast enhancement of the skull (arrows). More on this below.
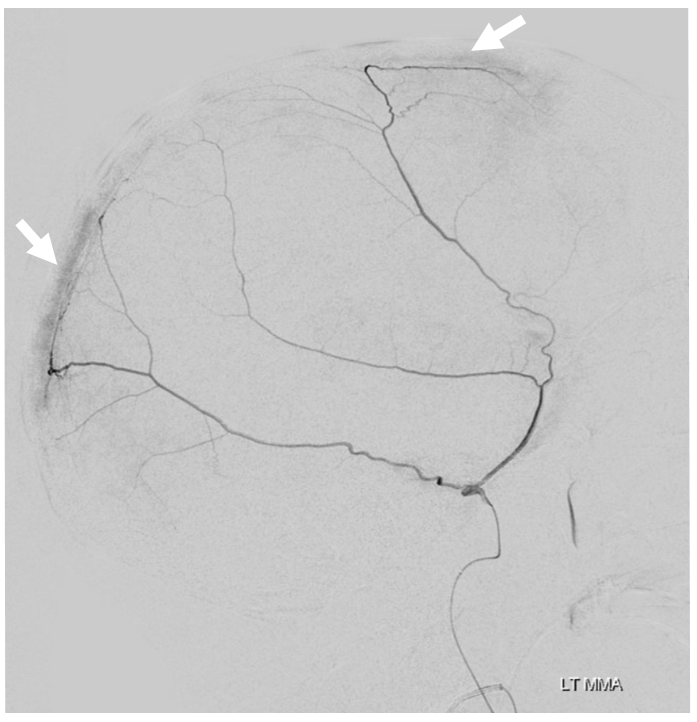
Here is another example of relatively poor outer layer anastomoses — with the caveat that you really need to have a “wedge position” to visualize them well, as above. However, look at that venous pouch in wall of the sigmoid sinus (arrows). Late phase images show that pouch draining into the sigmoid sinus (arrowhead) as well as the temporalis venous plexus (open arrow). See dural venous page for more info.
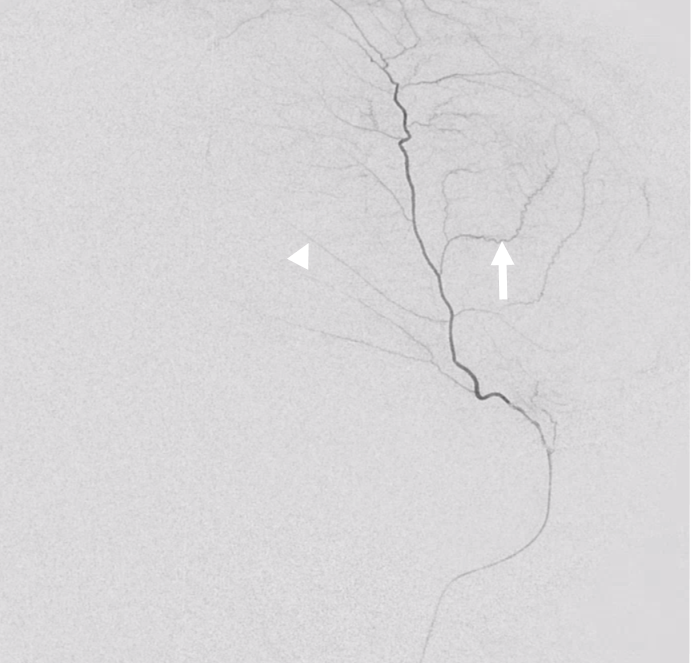
Some authors, particularly Roland et al et el described variation of primary anastomotic network vessels, some being straight (arrowhead), others corkscrew like (arrow). No one knows why.
Yet others are ark-like, as below. No one knows why either. Also see a few contrast accumulation spots in this person with a cSDH.
The “dual volume” DYNAs can be amazing here — a pre-contrast DYNA is subtracted from a postcontrast one — with really awesome results — unfortunately they are still inconsistent. Look at the network here. Tram-tracking veins are also present (arrowheads) — more on that in venous section. The oval outlines skull enhancement — not dura. How do we know? — see below.
Here is another example — with that corkscrew morphology again
GIF
Movie — pause and scroll thru individual frames
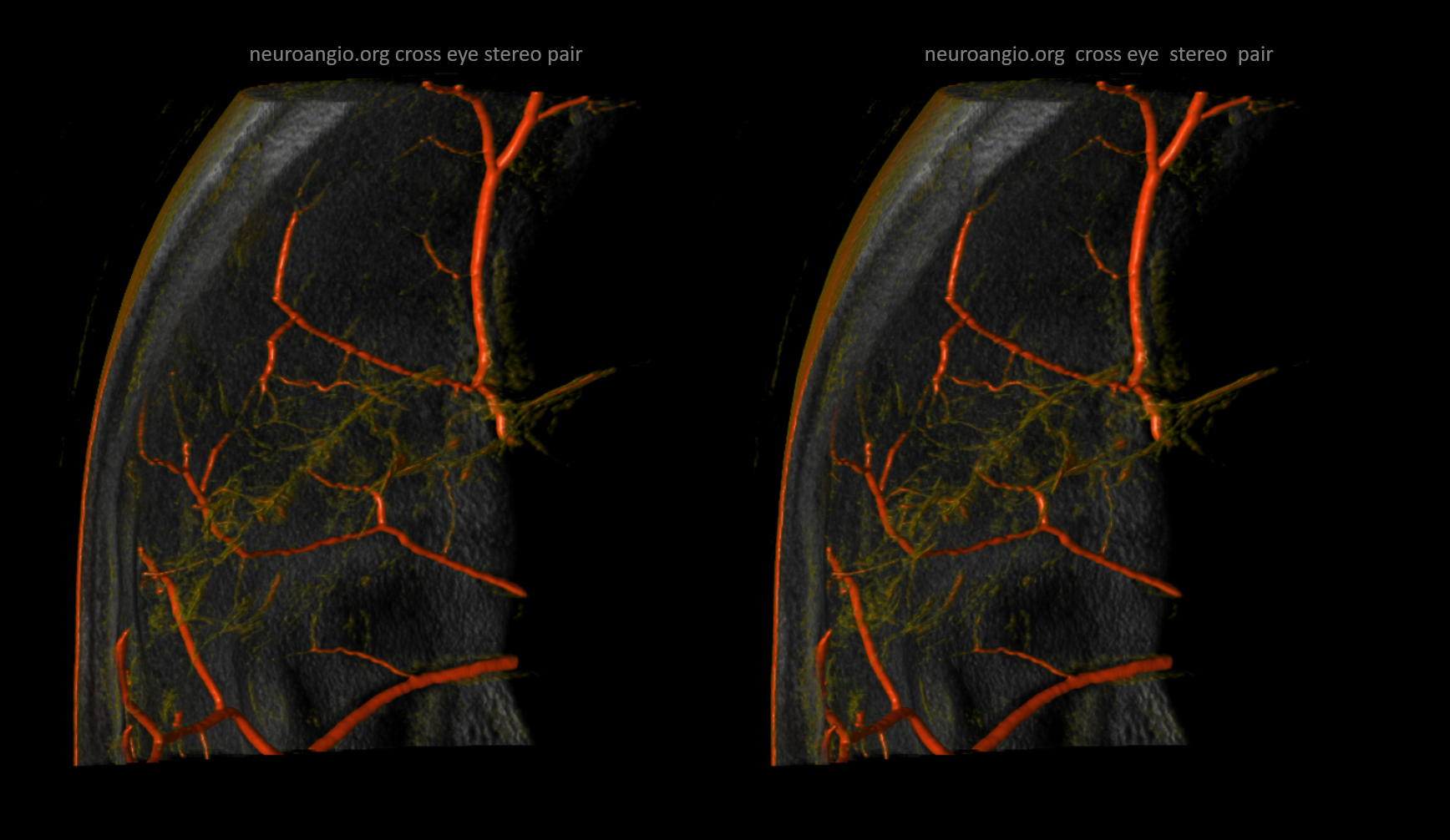
Some really nice stereo cross-eye and anaglyph images
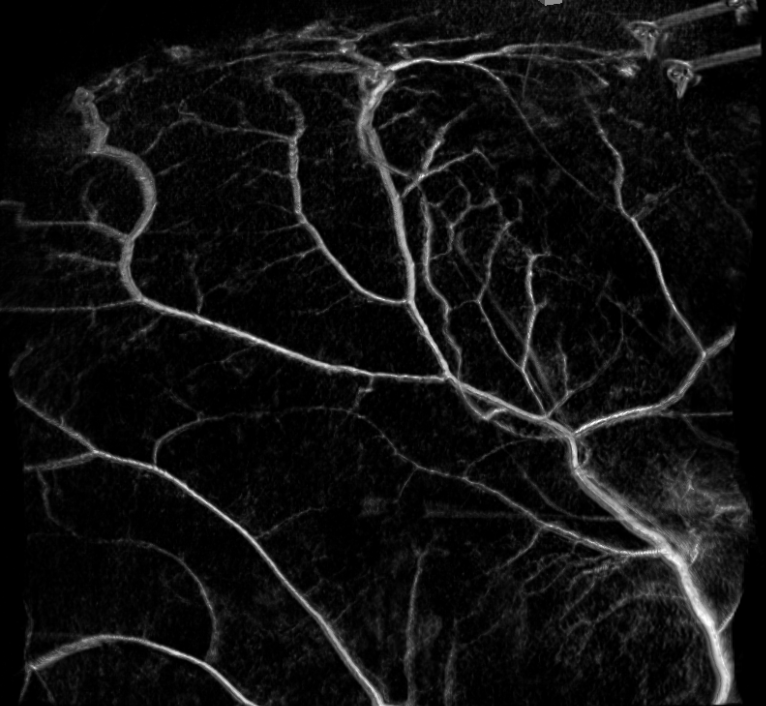
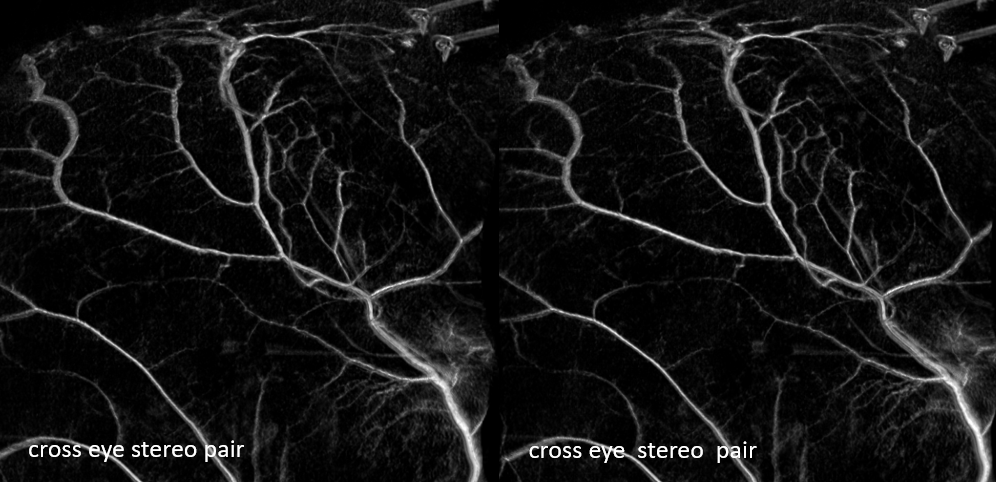
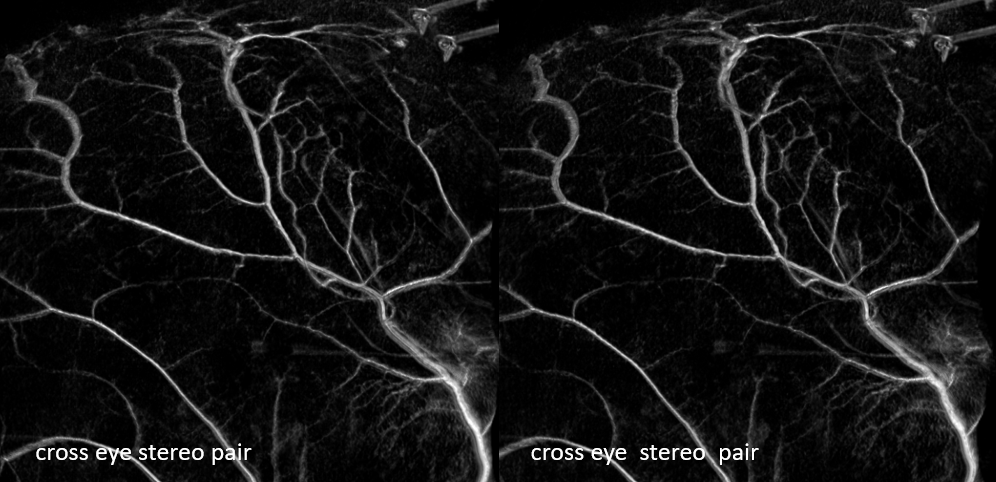
Movie — you can pause and scroll thru individual frames
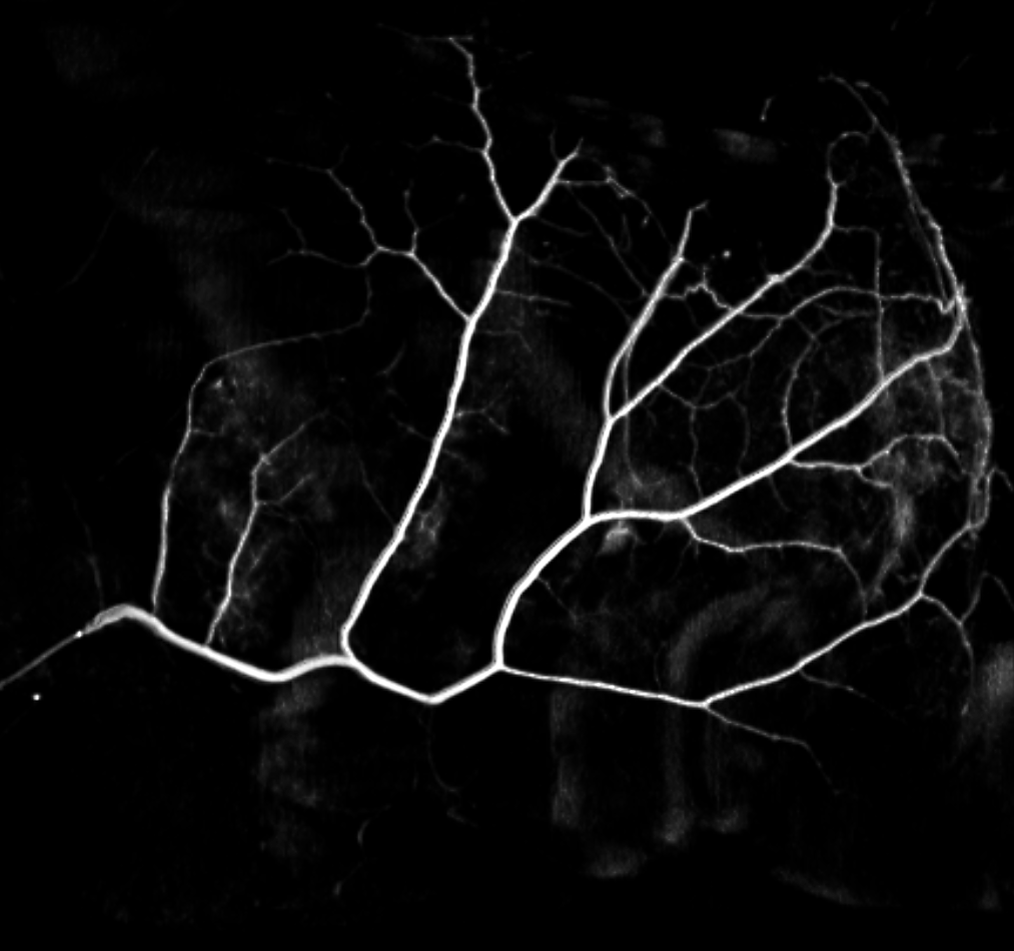
Another few nice examples of intrinsic arterial vasculature — these are obtained with a 6 second injection duration, which minimizes the presence of veins. This vessel is in the falx — again subtraction is harder near bone, so we are working on that.
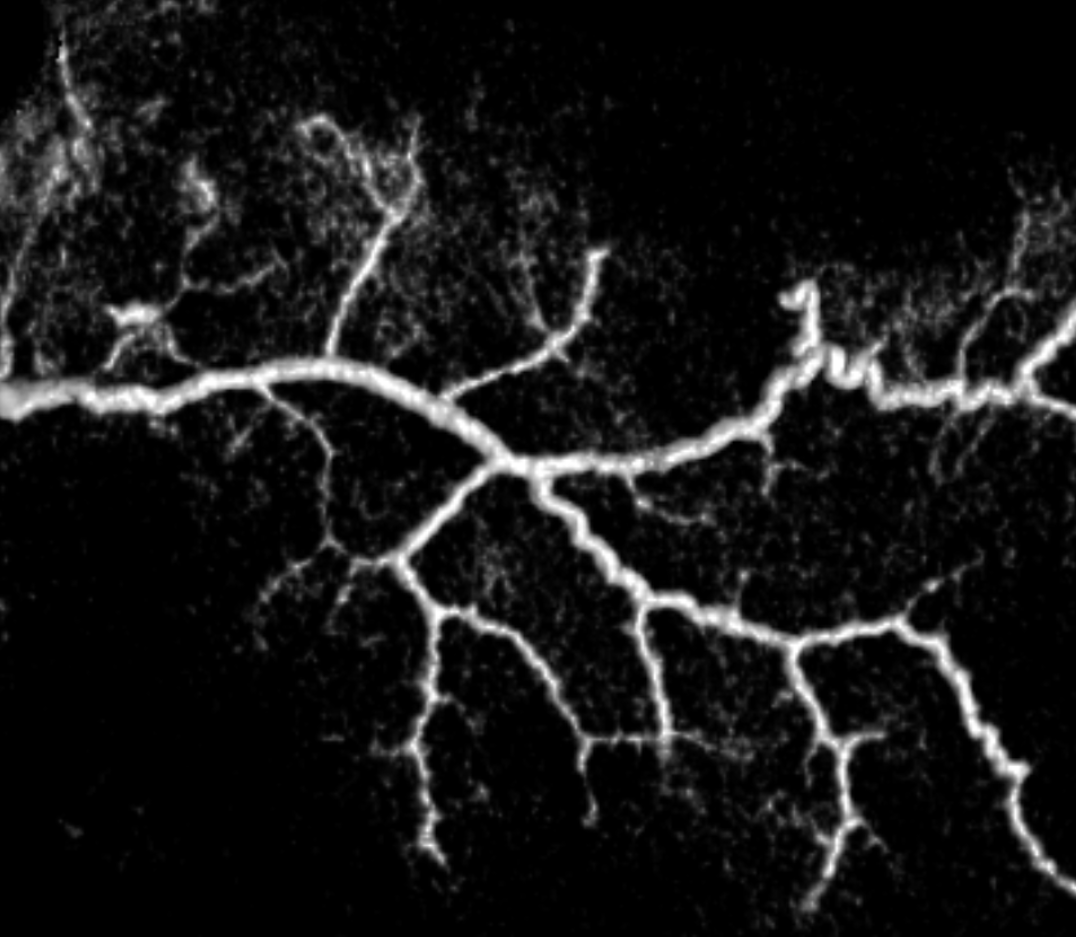
GIF for u
Movie of the same — u can pause it to scroll
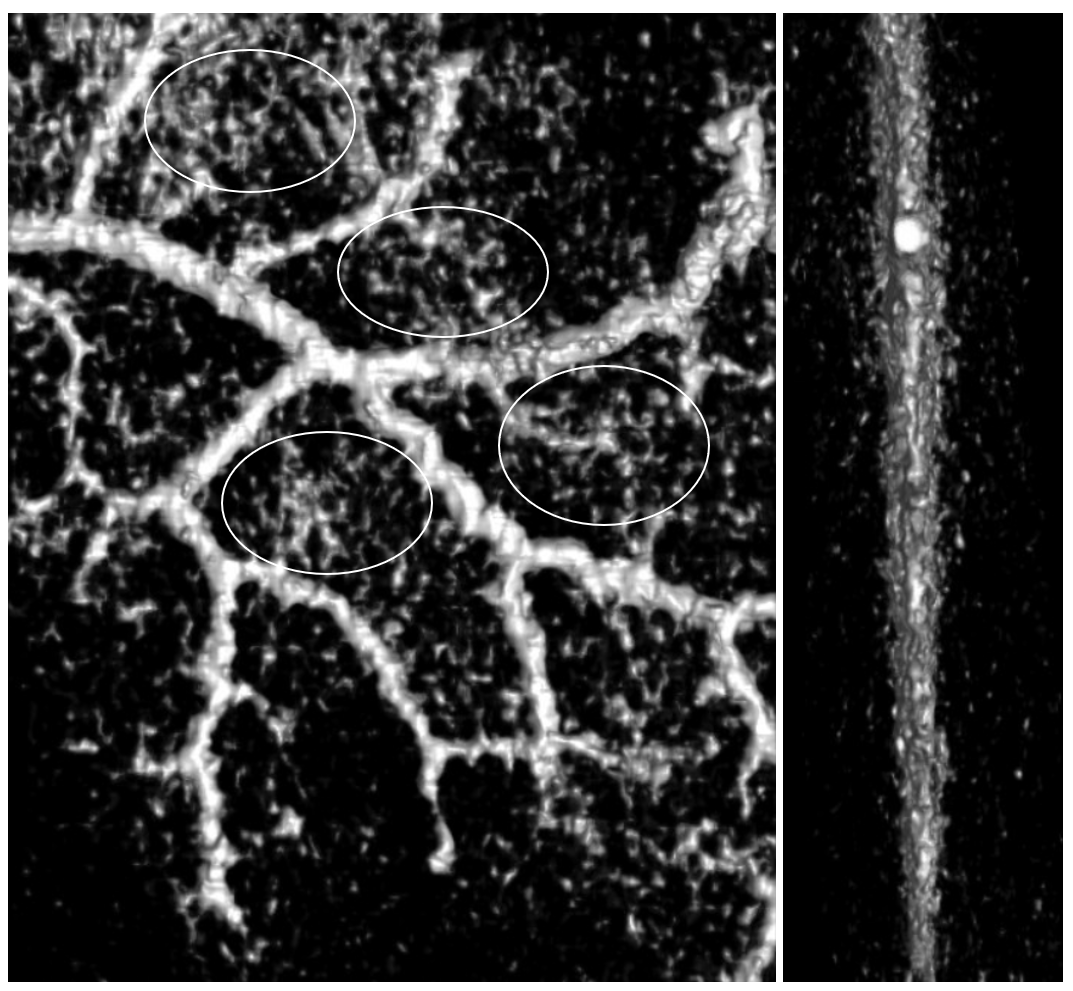
In the above image we can see a hint of secondary anastomotic network in the outer dura — those honeycombing dots and lines are probably this network. we are at the edge of current in vivo resolution.
In the image below, the network is shown somewhat better — look at he vessel-like structures within the ovals. They are not simple noise — a tangential view of the falx (right slim image) shows that whatever we are looking at is in the falx itself. We need better resolution, but so far its promising
Below is a subtracted 6 second image — the falx vessel is visible also, better in stereos below
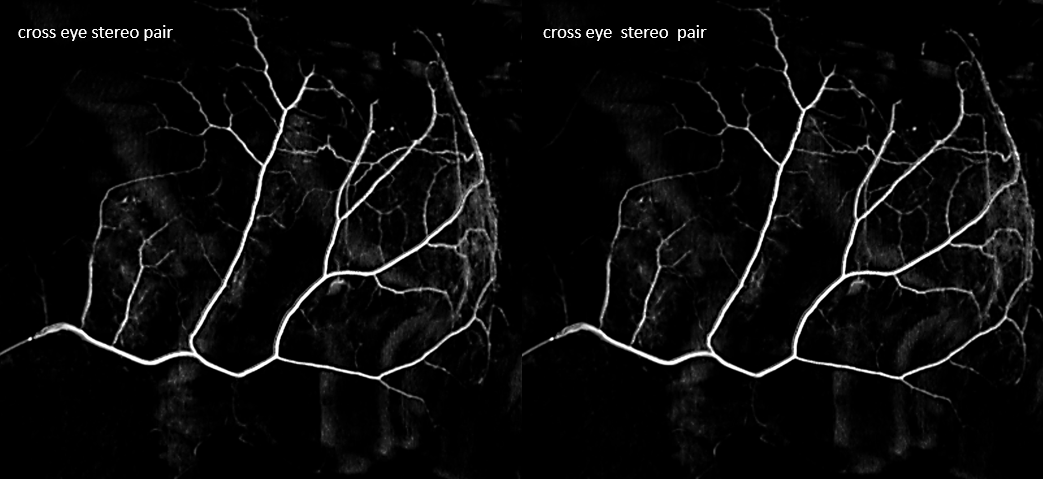
Anaglyph
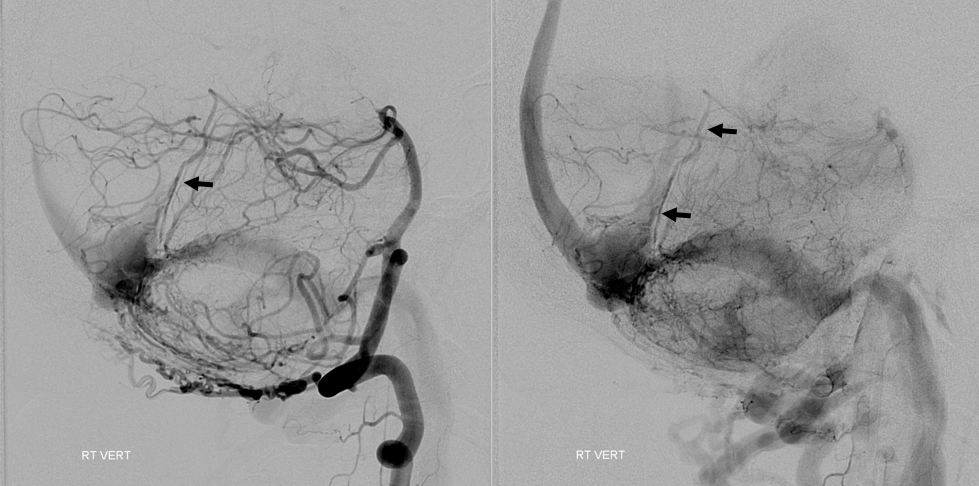
One of the MMAs is usually dominant in supply of the falx (arrowhead). Dashed arrows point to arterial arcade in the wall of SSS. Arrow shows the same corkscrew-vessel as above. Ball arrow points to a supraorbital branch that should not be mistaken for a lacrimal one.
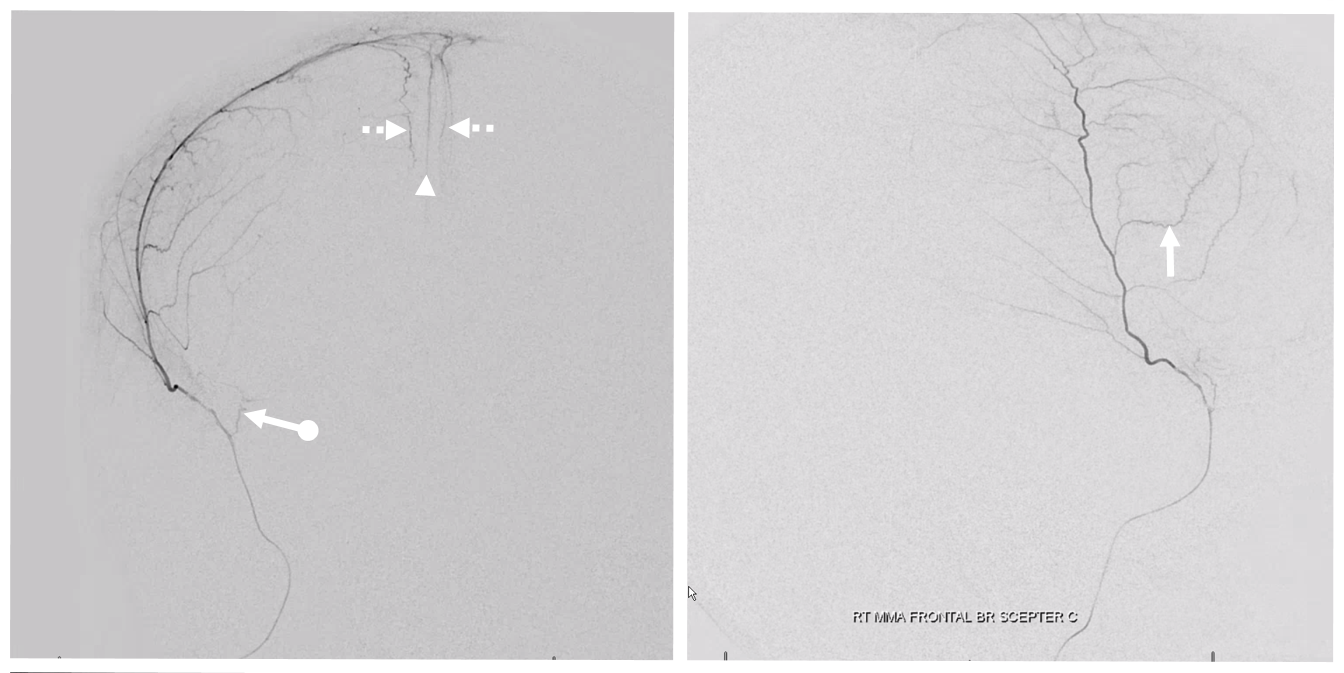
Another example of falx supply, in this person with a subdural extending to falx also
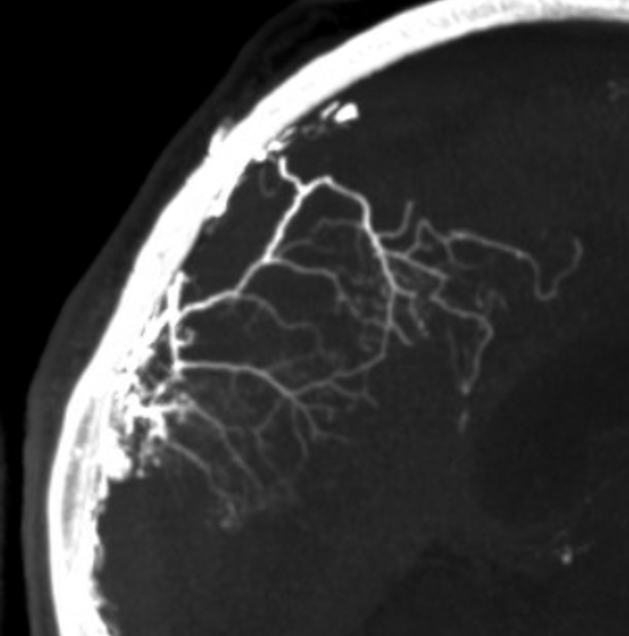
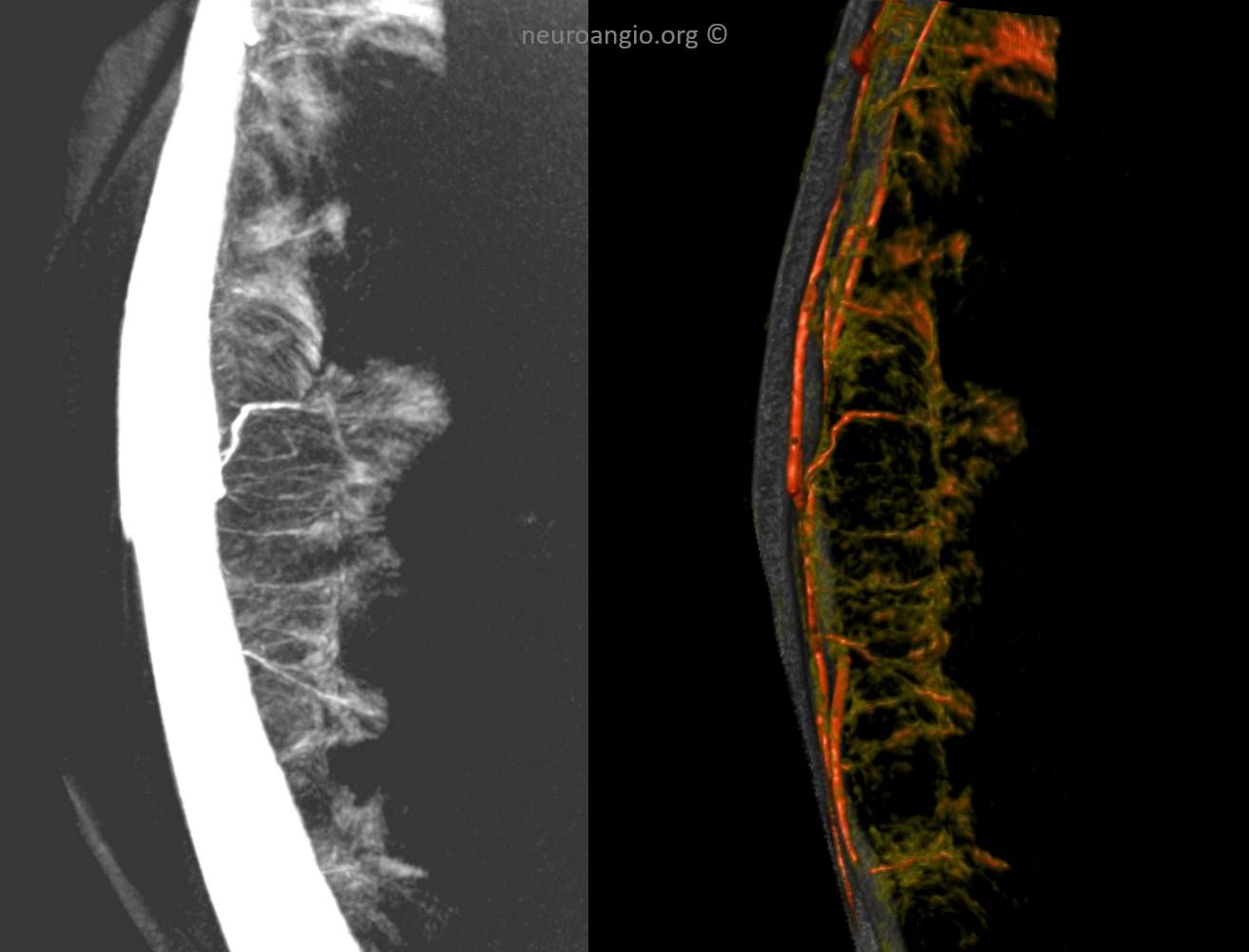
The secondary outer layer anastomoses are 20-40 microns in diameter and not seen on DSA. They can be relatively consistently seen on DYNA CT (2020 advanced imaging modality) in the falx cerebri and tentorium cerebelli. Its harder to see them over the convexity because of adjacent skull. Subtracting a precontrast DYNA from contrast one (registration) can help, but its still not great. Here is an example of secondary network in the falx — the smaller vessels are below DSA resolution. This, in fact, is a picture obtained after the bilateral nBCA injection shown above — probably a mix of glue and stagnant contrast in the falx vasculature — be careful!
Another example — arrowheads point to artery in wall of SSS — just as anterior meningeal artery does. This image was obtained after particle embolization of cSDH — stagnation of contrast in dural vasculature allows for this kind of image
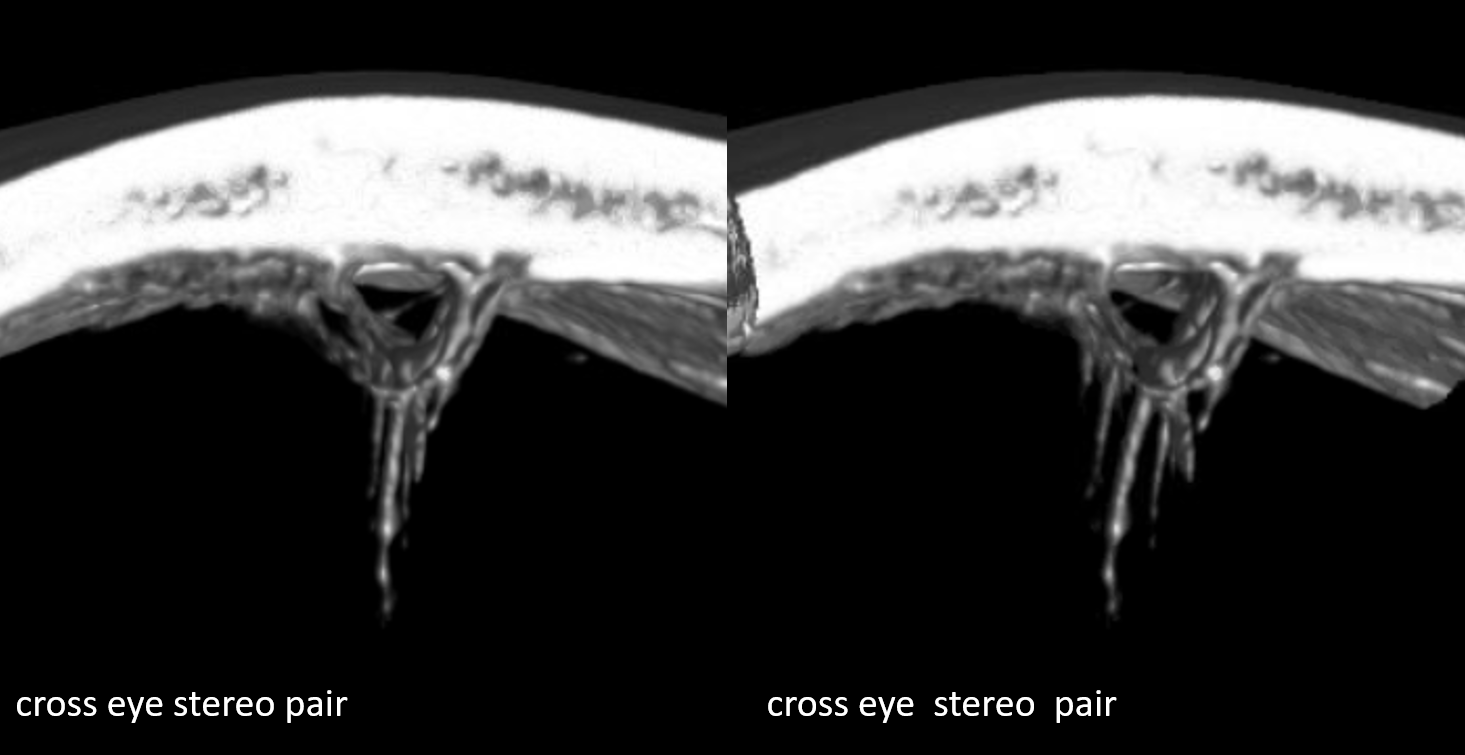
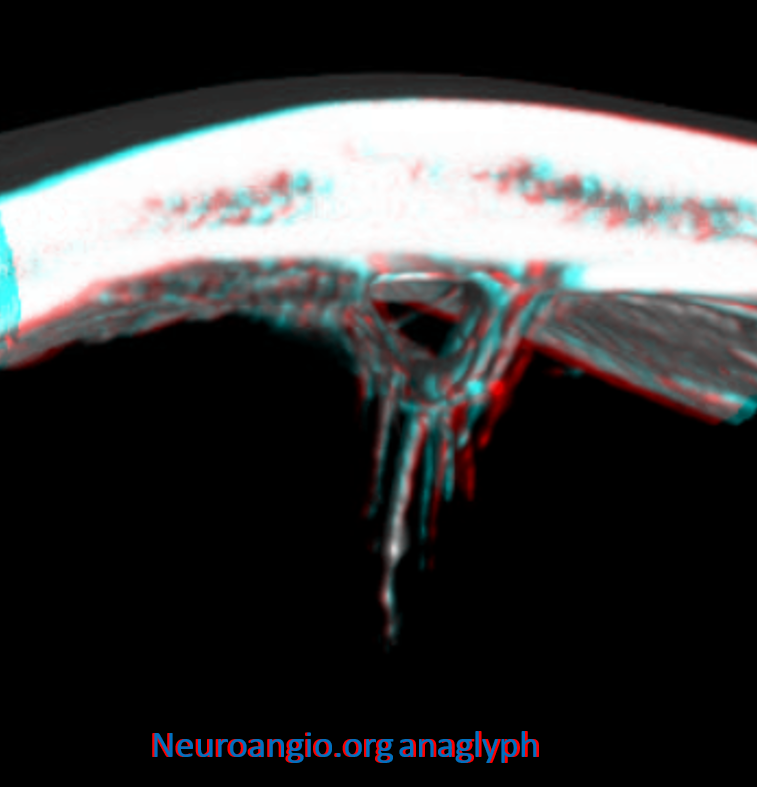
Animated GIF, stereo (cross-eye and anaglyph) of the network in wall of the SSS — how cool is that!

Movie of same — pause it and scroll
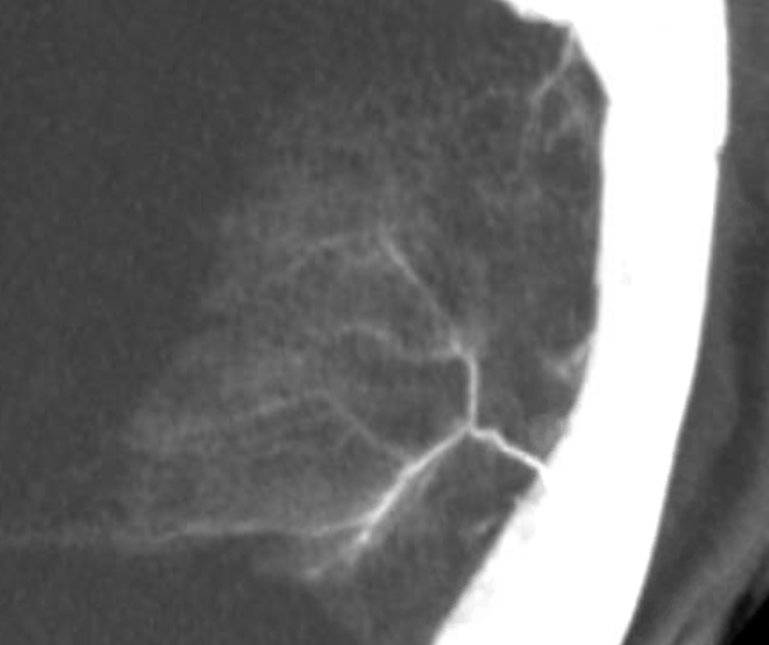
Here is an example of secondary network in the tentorium
Arteries Adjacent to Venous Sinuses — these we have showcased a lot, especially on the MMA page — they are very important in the angioarchitecture of dural fistulas. They can be excellent routes for embolization — see here for example. This network (in the outer layer also) is well seen on all kinds of DSA and DYNA images, and is also discussed below.
Skull blood supply
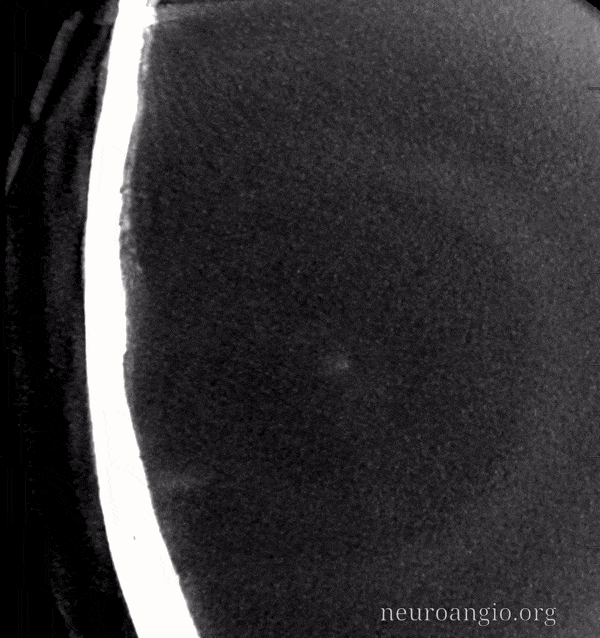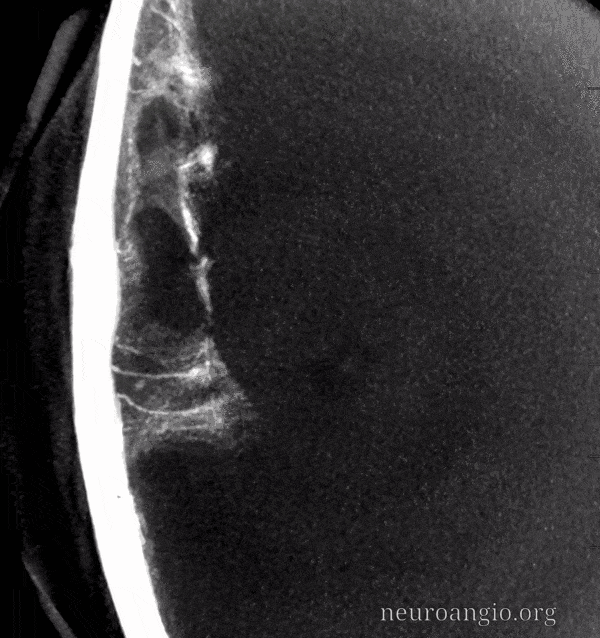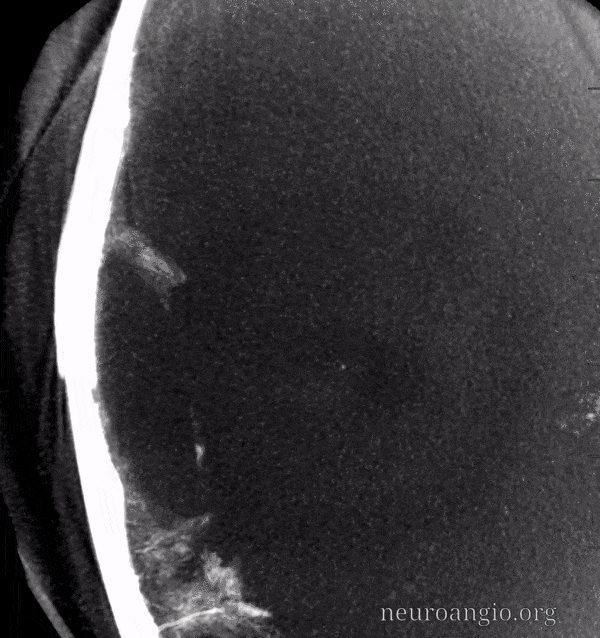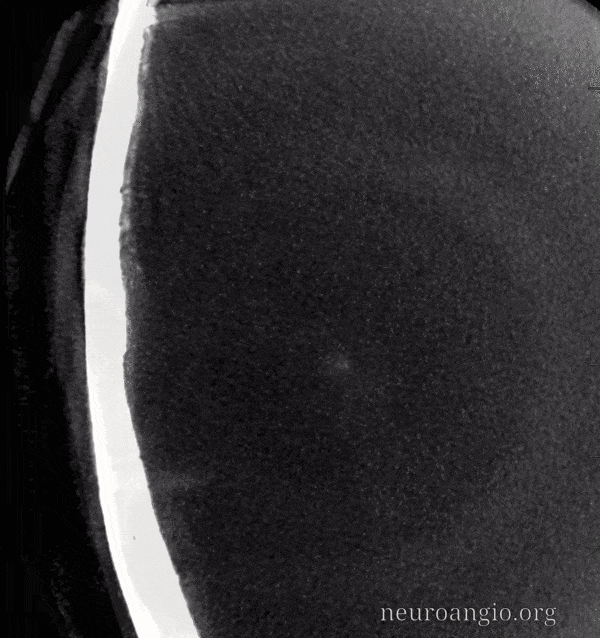
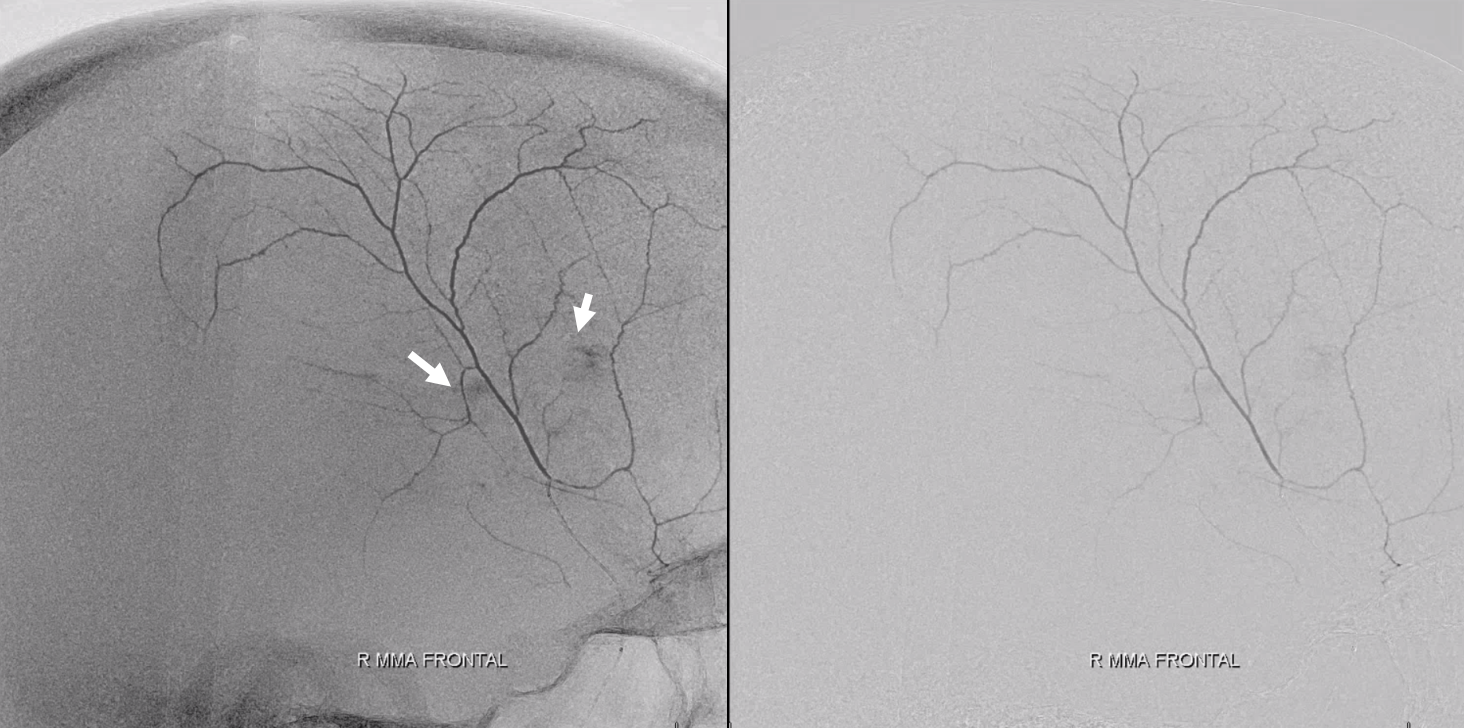
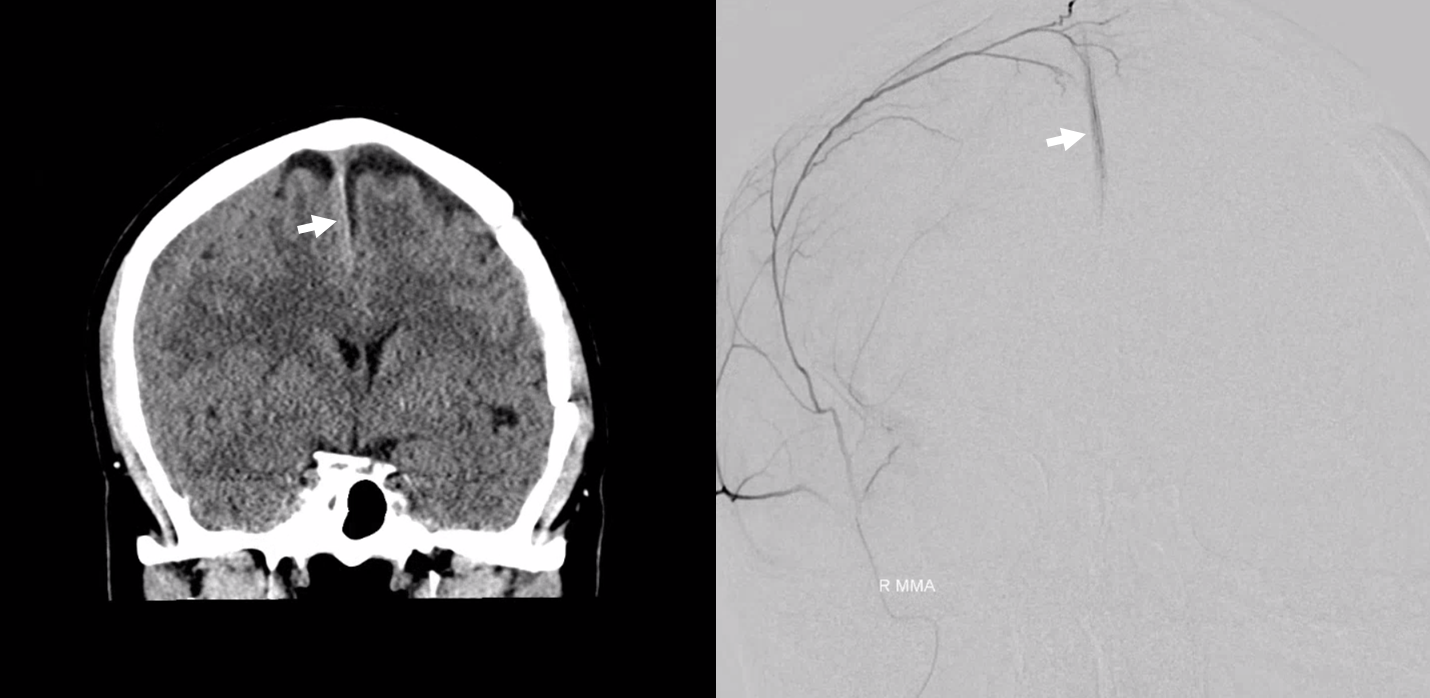
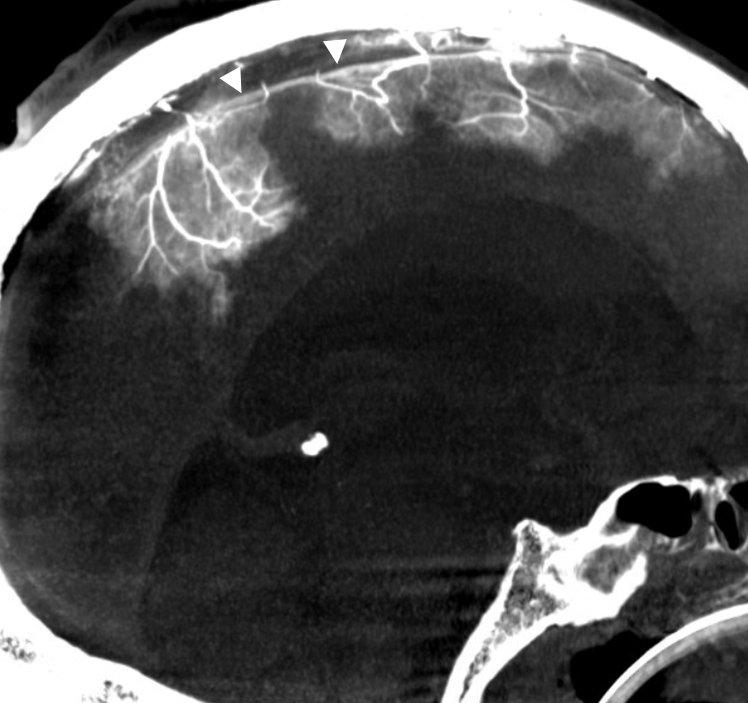
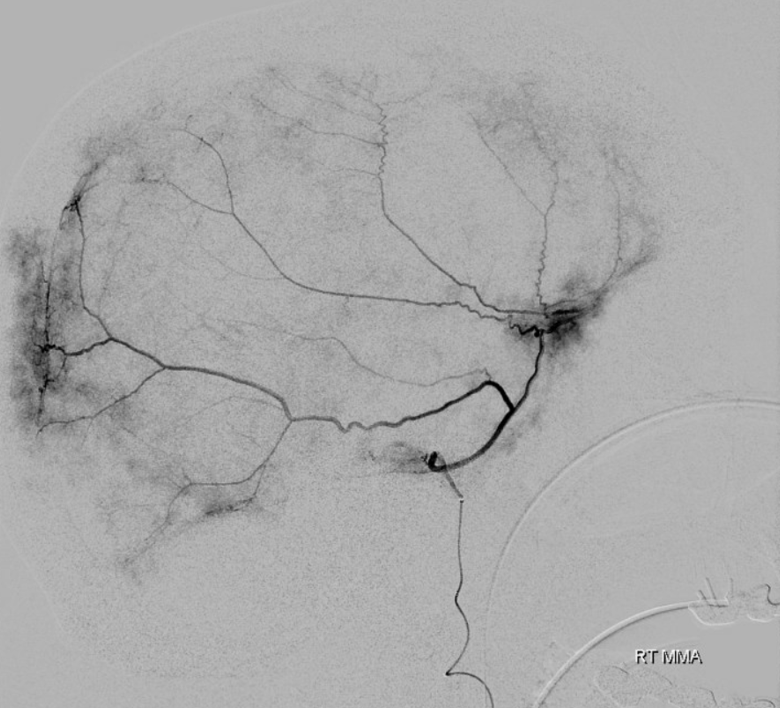
What arteries supply the skull? We really don’t talk about this a lot. The answer is arteries next to the skull. From the inside, it is meningeal arteries. From the outside, its occipital, superficial temporal, deep temporal. etc. As always, there is variation. But, in general, the meningeal arteries do a lot of skull supply. Its a bit hard to image this adequately. You can tell when there is skull supply when you see contrast in portions of the skull on tangential DSA views. However, on the same DSA view, you cant tell if the enhancement you see is dural or skull when its not tangential — basically for most of the head. Subtracted DYNA helps sometimes. Here is the same image we showed above, with supply of skull seen tangentially

Later phase, same patient. Look at all that “membrane enhancement” — it is not. This patient has no subdural. Its all skull
Another example in frontal view — also a venous lake in wall of SSS is shown with an open arrow (more on this in venous section)
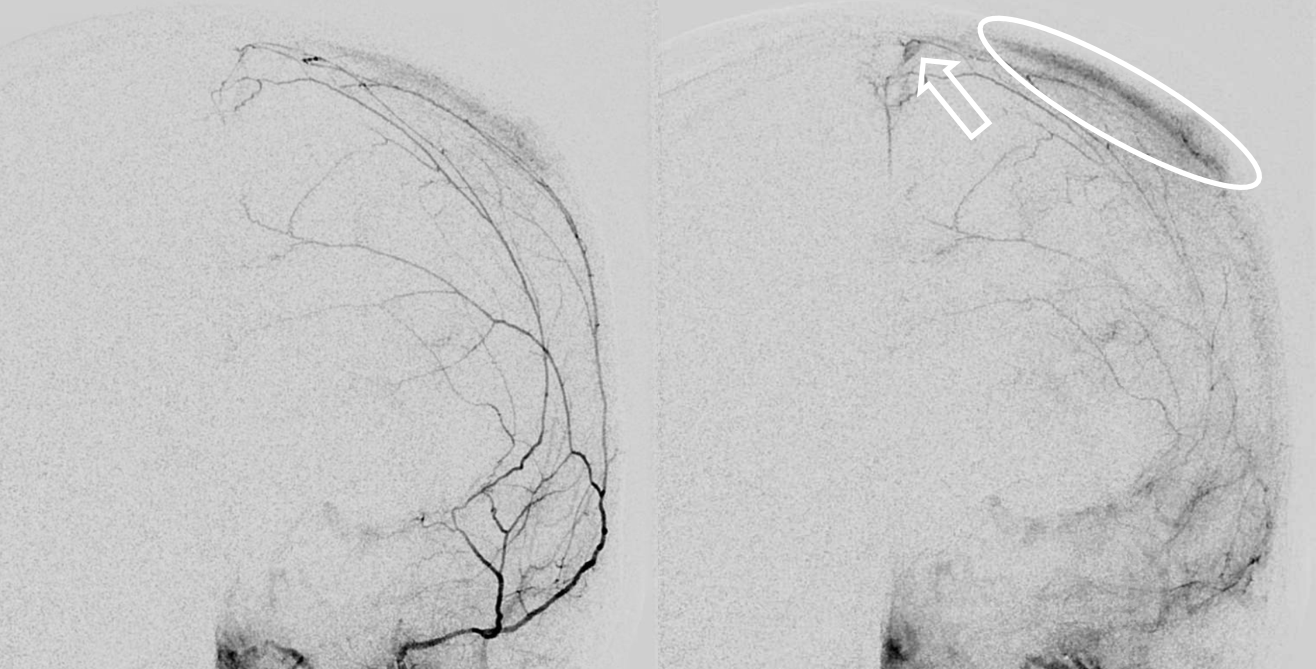
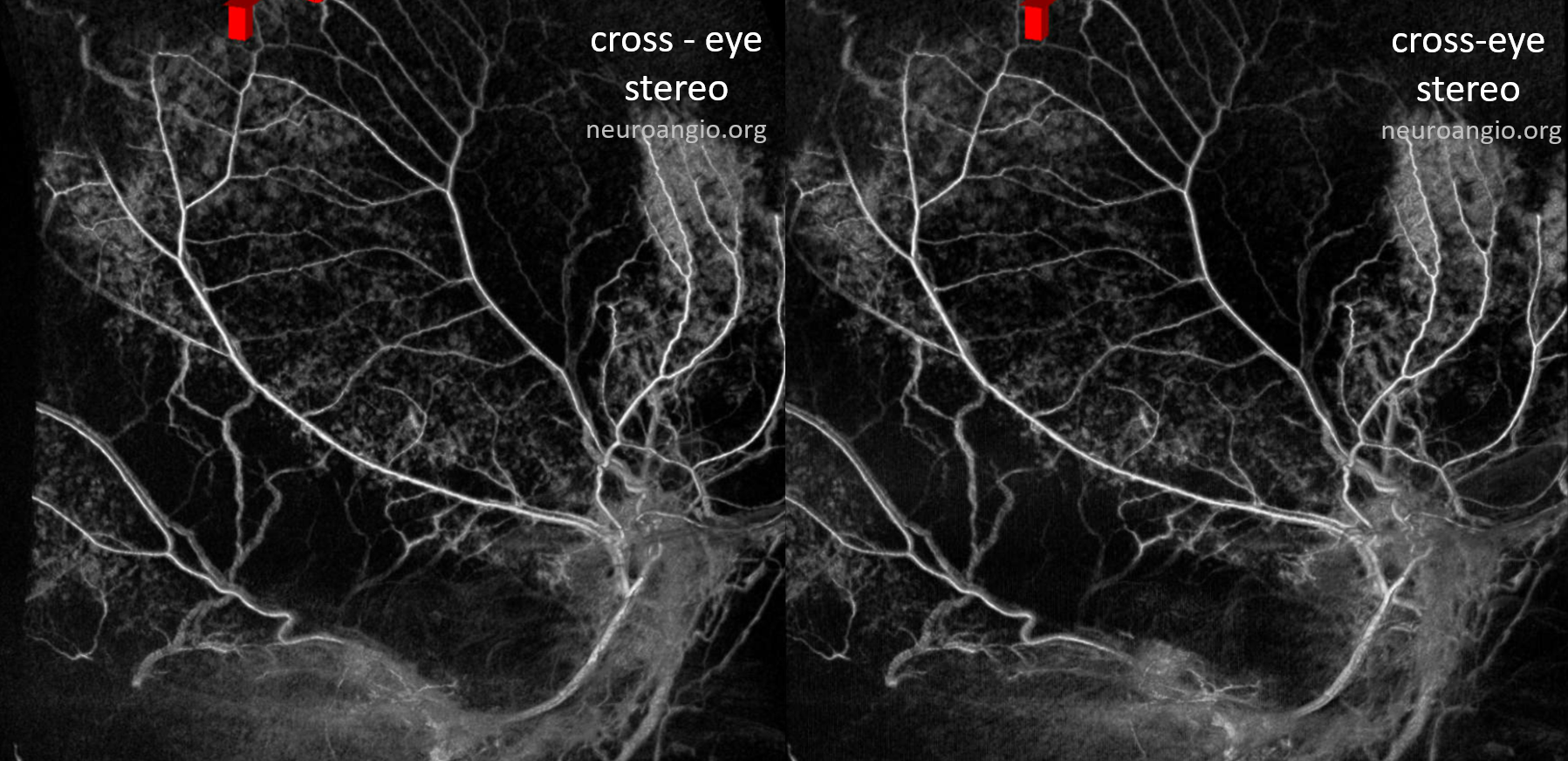
How do we study skull supply in detail — for example how do we know if what we don’t see tangentially is skull or dural enhancement. Its hard — we have not found a consistent technique yet. Subtracted DYNAs with stereo view are probably the best now. In the images below stereo view allows one to see that the patchy enhancement is superficial to the major meningeal arteries — and therefore in the skull, and not dura.
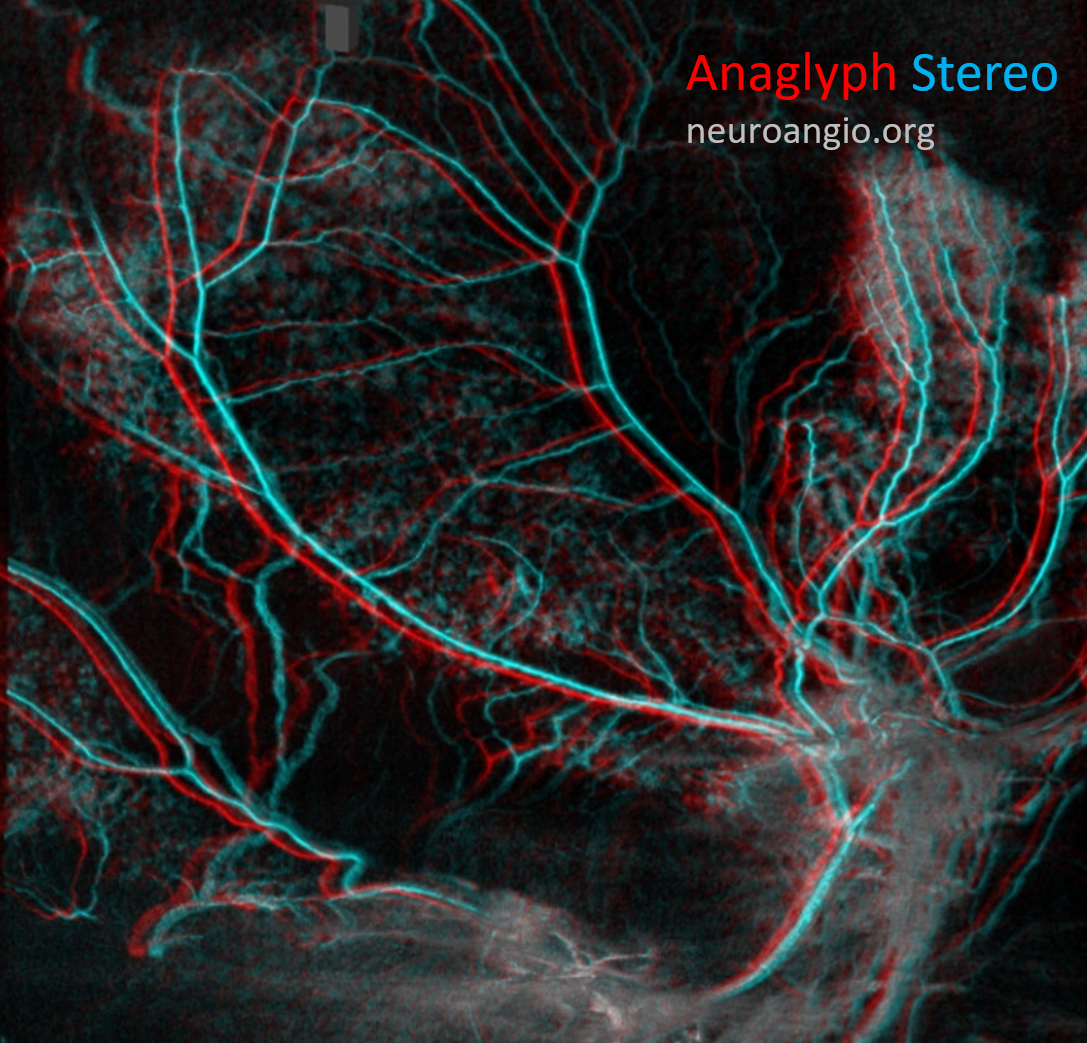
Here is an anaglyph stereo of the same
Just because dural vessels supply skull does not mean that other vessels cant return the favor. There are tons of examples of transmastoid / transosseous arteries in sigmoid region — both participating in dural fistulas (all of those occipital branches), and in supply of posterior meningeal artery. However this can happen anywhere. Look at this MMA injection in a patient with cCSD — what’s up with that parietal dura not showing up?

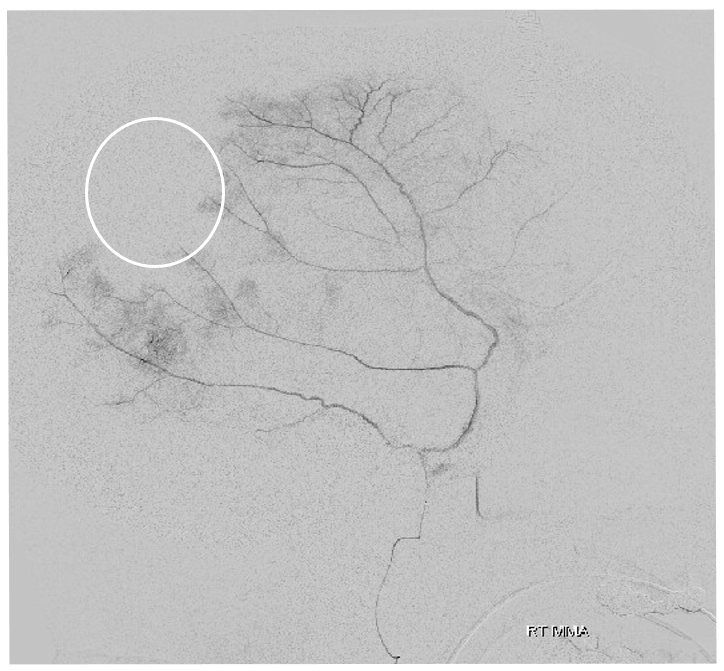
Here it is — post MMA embo, occipital artery transosseous supply to dura — parietal area seems a frequent spot for this very infrequent occurrence
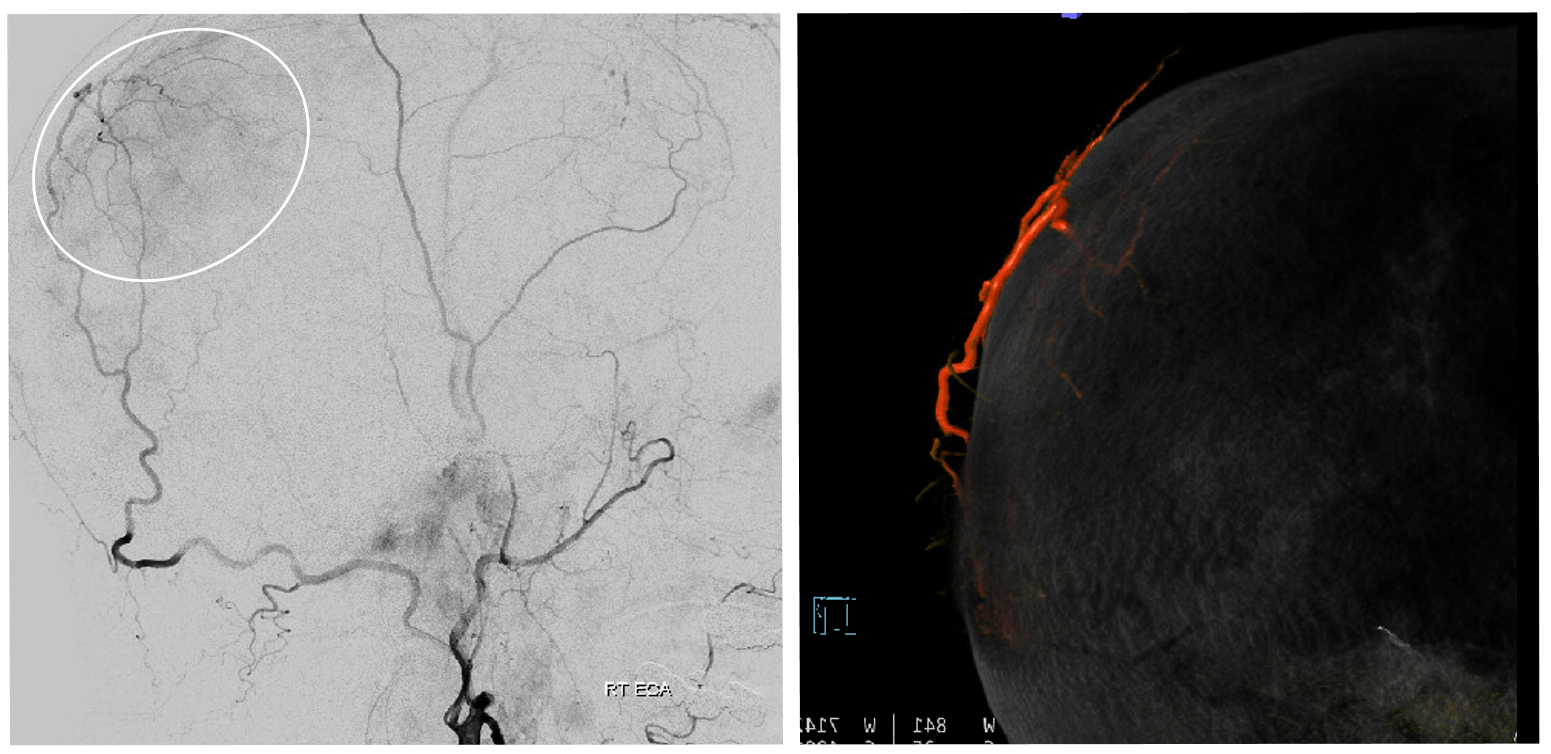
Of course, we embolized it — headway duo up to transosseous branch — with particles — who knows at this point if branches like these contribute to revascularization and failure of MMA embo
Arterial channels in walls of sinuses
We have emphasized them a lot. See “Meningeal Vessels” page. Here is our frequently used schematic, showing a network of arteries in walls of major sinuses – letters r, v, w, x, y below.
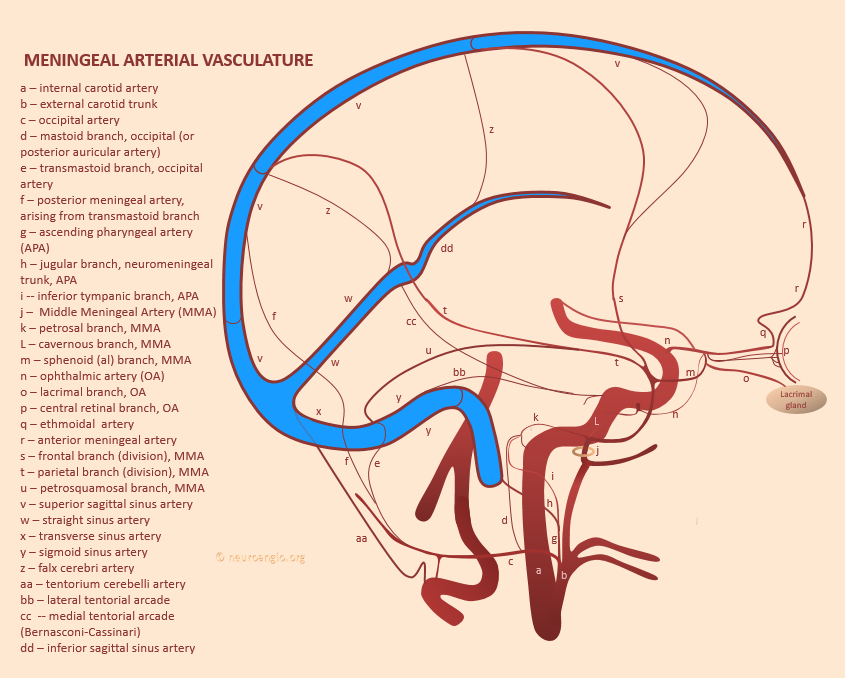
Letter “c” in figure below

These are part of the whole meningeal arterial structure of the primary outer layer network. We have lots of examples, in normal and abnormal vasculature
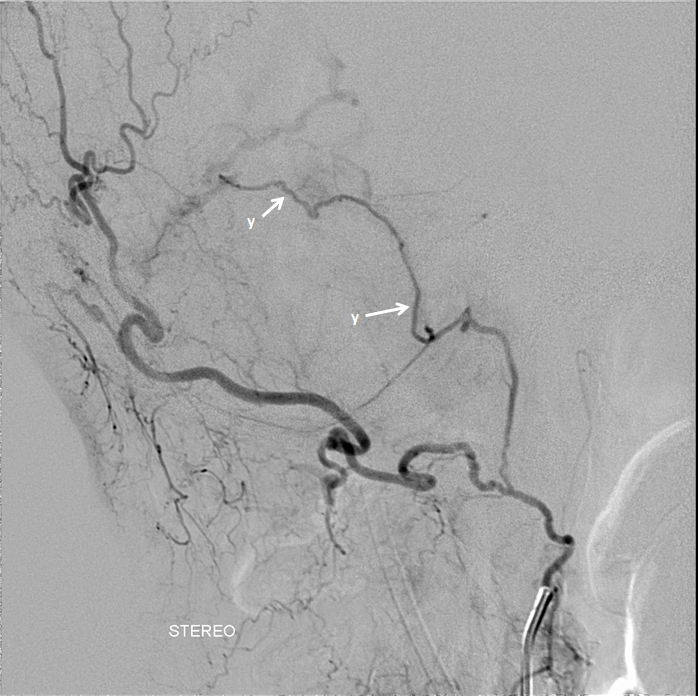
The importance of these vessels in practical applications is paramount. They frequently are the “common collector” of myriad other arteries in supply of dural fistulas. See cases 1, 2, 3. In some cases they can be used as access routes for embolization — see ethmoid fistula case here.
See Meningeal Vessels umbrella page for more examples
Meningeal Layer Network
Letter “I” in the “ML”

Connects outer and inner networks. About 40 microns in diameter. Relatively hypovascular and not visible in vivo
Inner Layer Network
Letter “J” in the BCL. Contains parallel-appearing arteriolar and capillary sized arteries. Thin but relatively dense. Located in the key “border cell layer”. These cells are technically part of the dura, but are adherent tightly to the arachnoid. Tighter in fact than to the meningeal layer of the dura. This is the layer where chronic subdural hematomas live. Whatever sets them off — microtrauma, brain atrophy, etc. — what follows is a cycle of inner layer microhemorrhage, hyperproliferation, fragility, and further microhemorrhage. Layers of inner network are found within and on the inner surface of the chronic subdural — the “membranes”. In reality, the subdural hematoma is really intradural — or within the inner layer at least. The whole notion of subdural space is really useful from a surgical perspective as space below the dura, but not yet in the arachnoid, but from a histological perspective the “subdural” space is not a good idea. This, plus the devotion to bridging vein rupture theory for chronic subdurals really held us back decades in how to manage them.
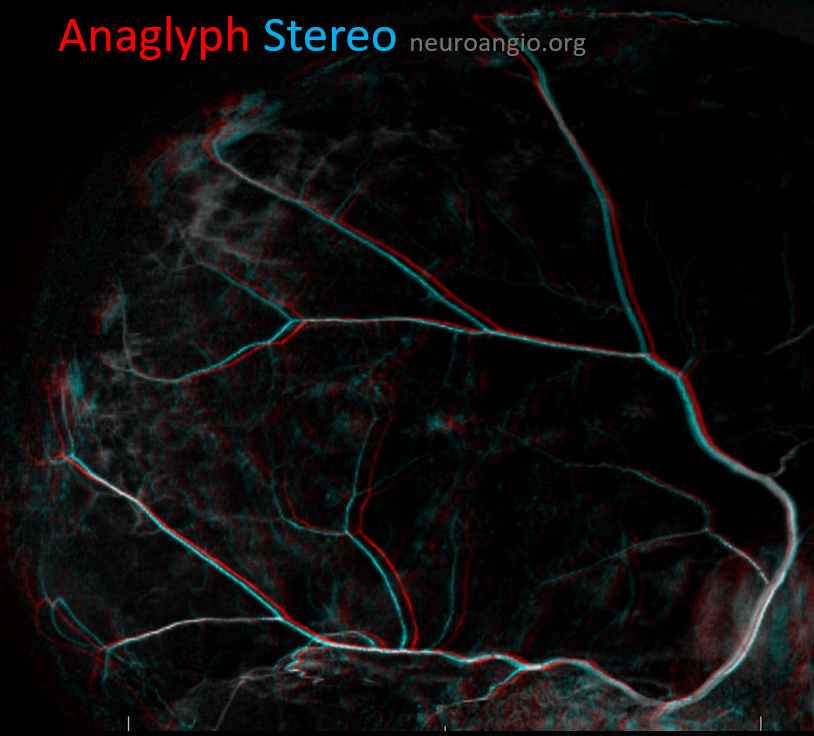
The normal inner layer is almost never visible on angio or dyna CT. As above, its hard to tell dura from skull enhancement on two-dimensional views. Subtracted DYNAs can help sometimes but not consistent yet. We can see nicely the “membranes” of thickened hypervascular dura on DYNA of chronic subdural patients. Below is a subtracted DYNA CT of a patient with a cSDH. The cross-eye stereos show patchy dural enhancement deep to the dural arteries — thus in the dura, and not in the skull.
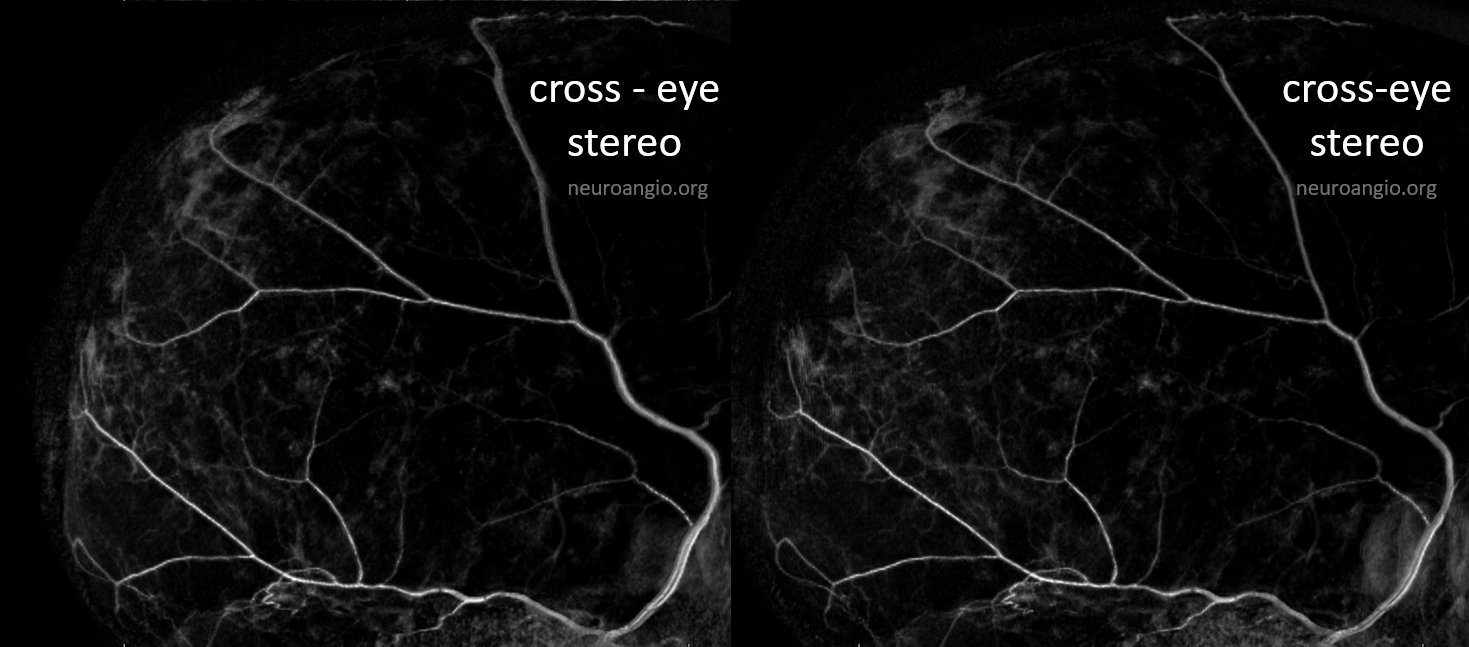
Anaglyph stereo of the same patient
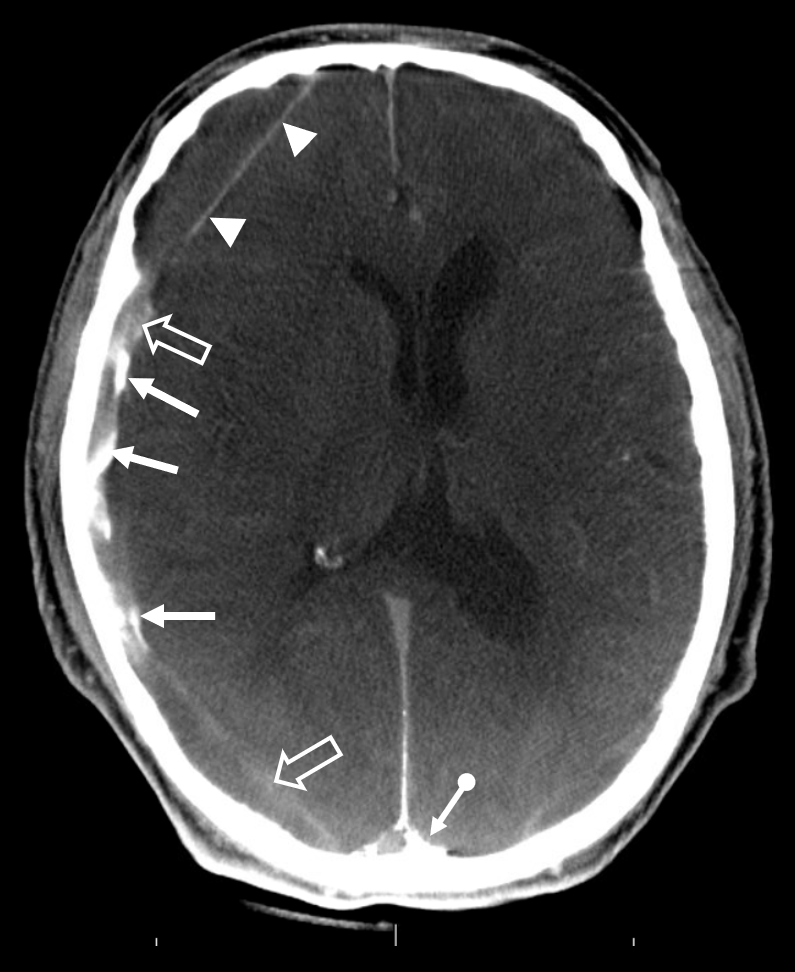
This is a nice CT cross-sectional look after embo — all the pieces are in place. Arrows point to particularly thickened membranes filled with contrast after particle embo. Arrowheads point to membrane at the inner aspect of this “subdural” hematoma — letter “M” in the Figure above. Open arrows point to contrast diffusing into the “subdural” fluid from the embolized membranes. Finally the ball arrow points to contrast in venous pouch adjacent to the SSS (more on that on the dural venous system page)
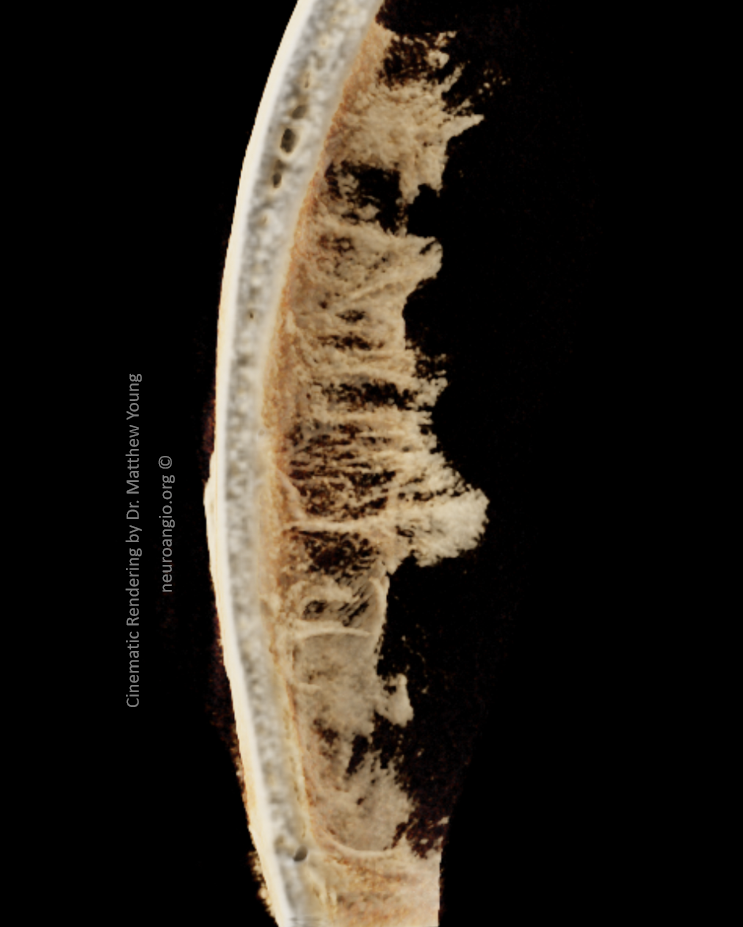
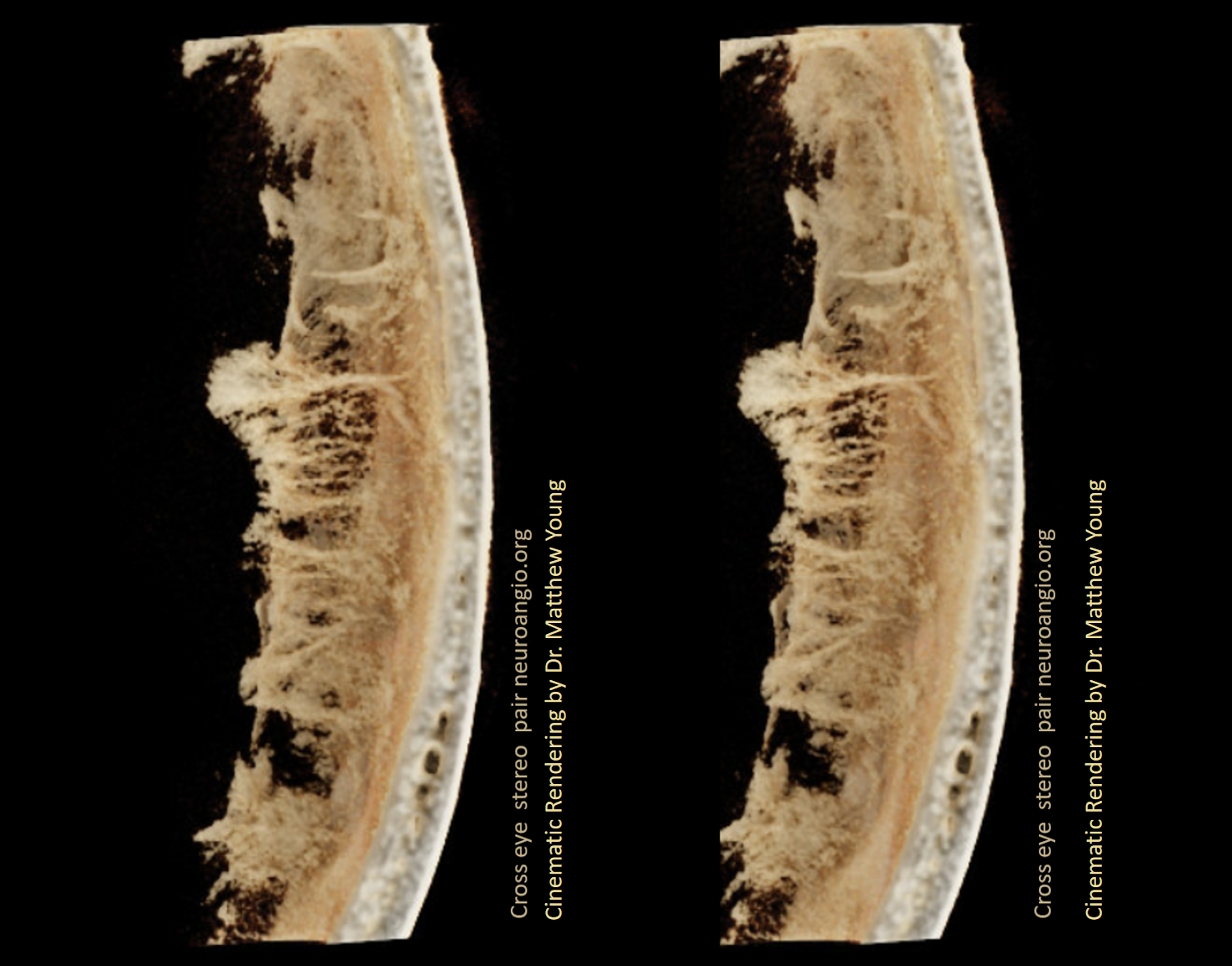
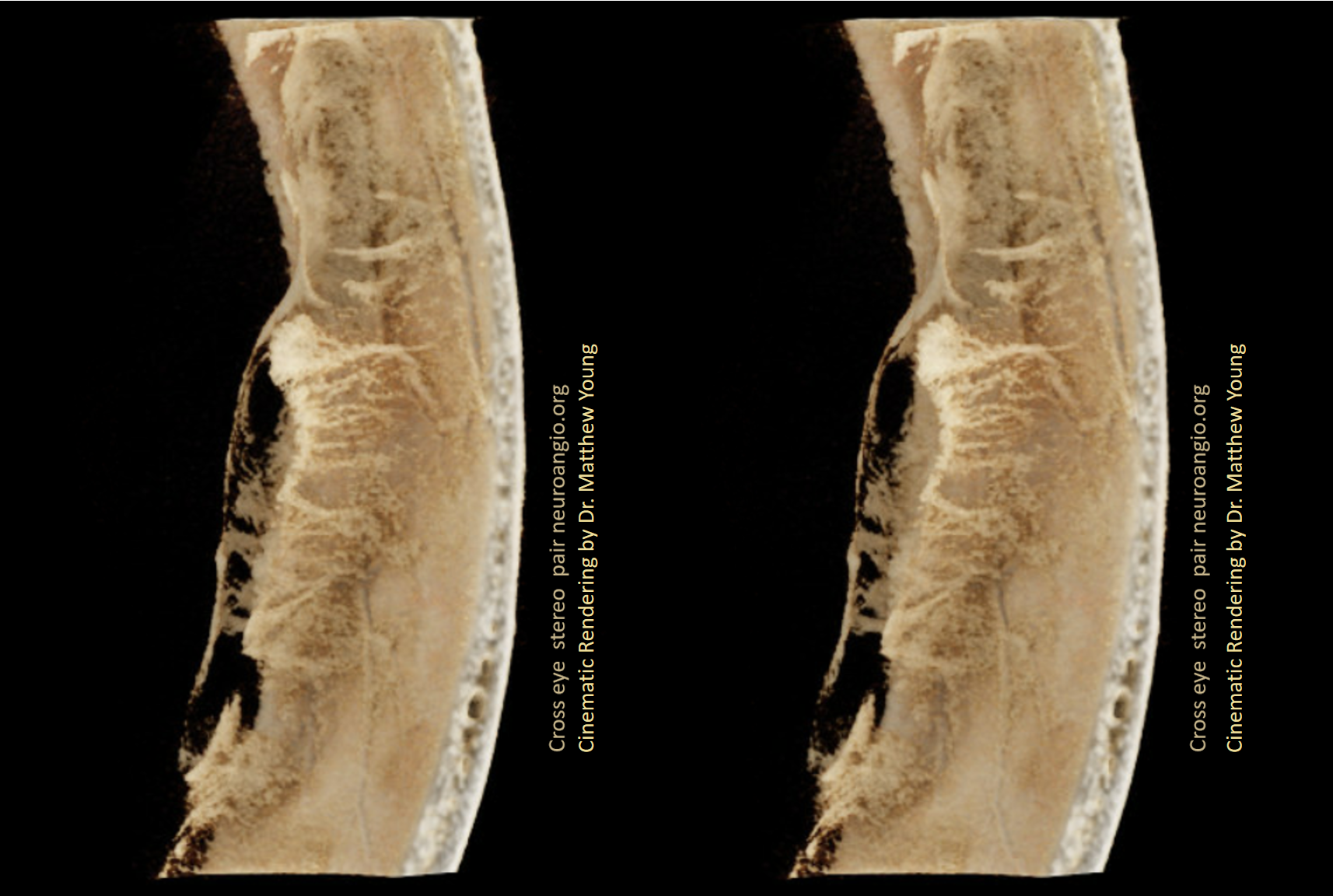
Subdural Hematoma Membranes — the best demonstration of neovascularity in subdural membranes we have come upon yet — courtesy Dr. Eytan Raz. Sinematic Rendering Courtesy Dr. Matthew Young

Super GIF/MP4s
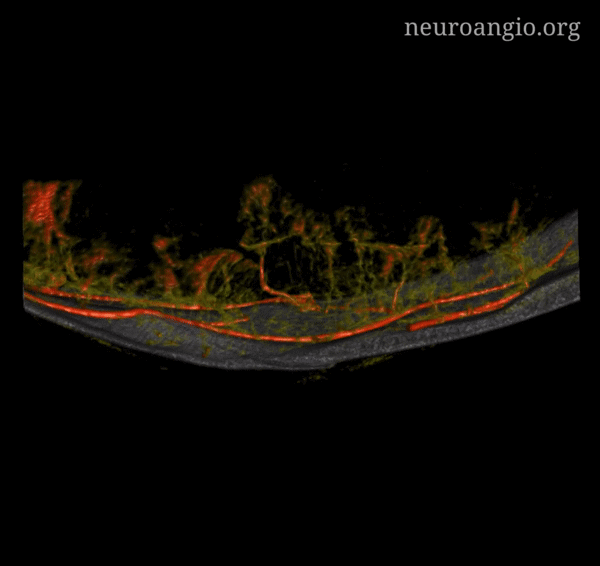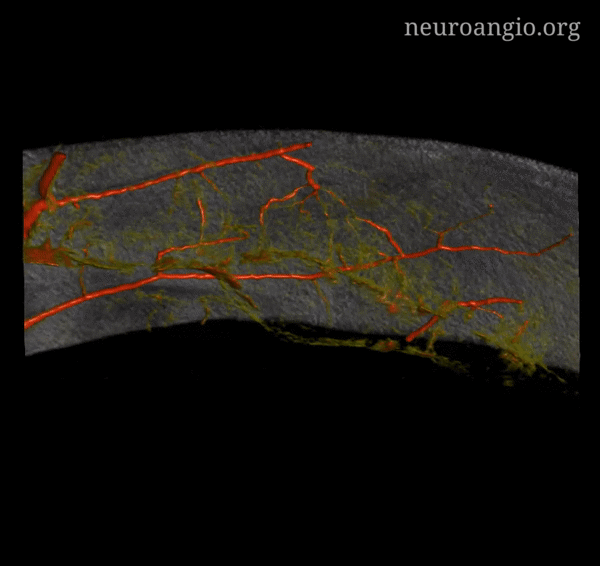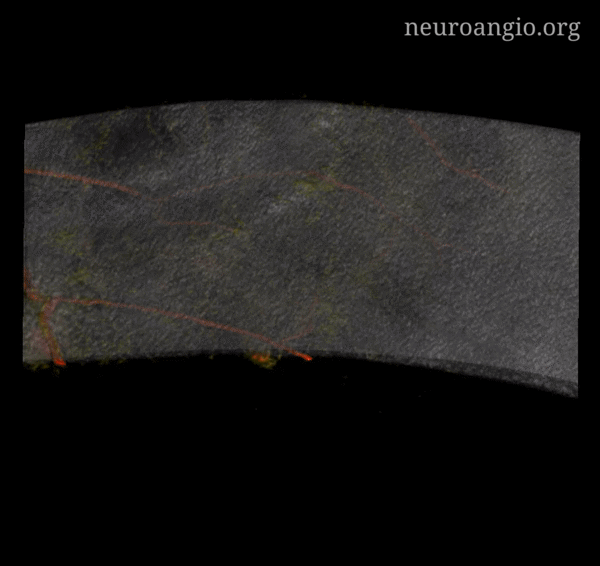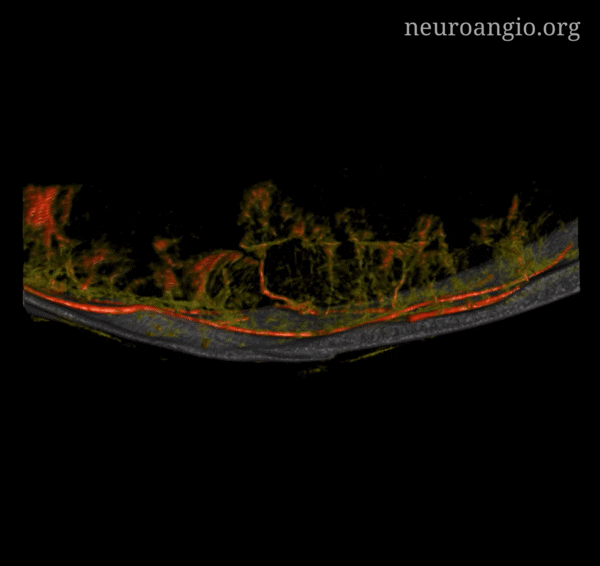
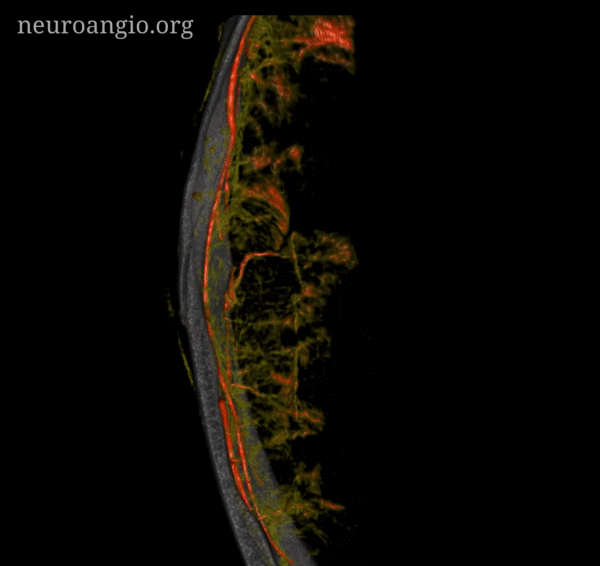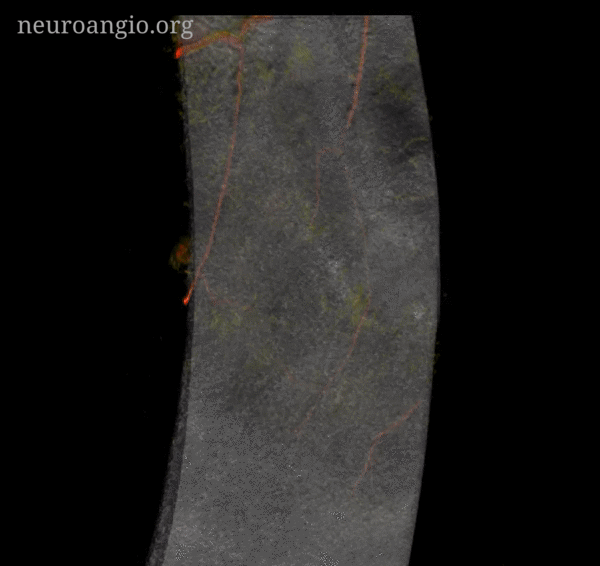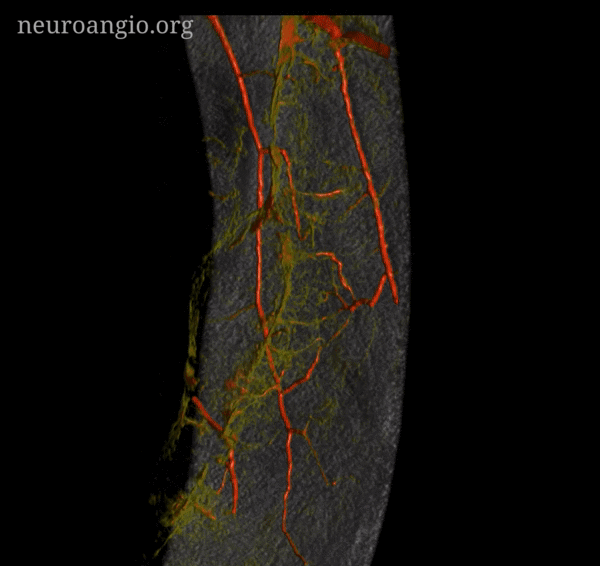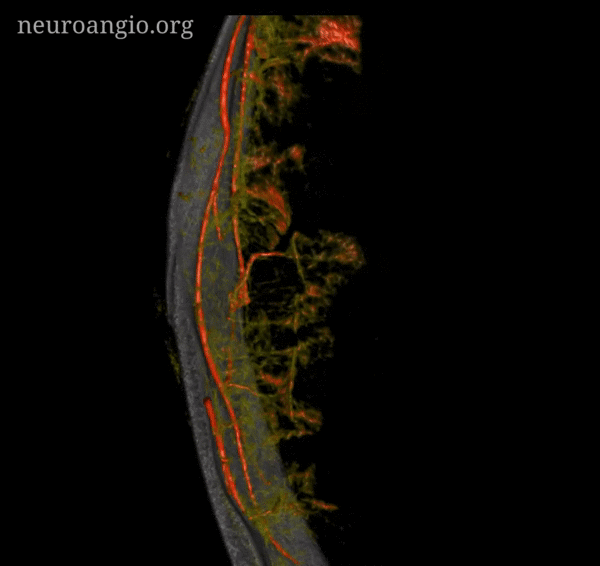
Incredible Cinematic Rendering Images

Anaglyph stereos
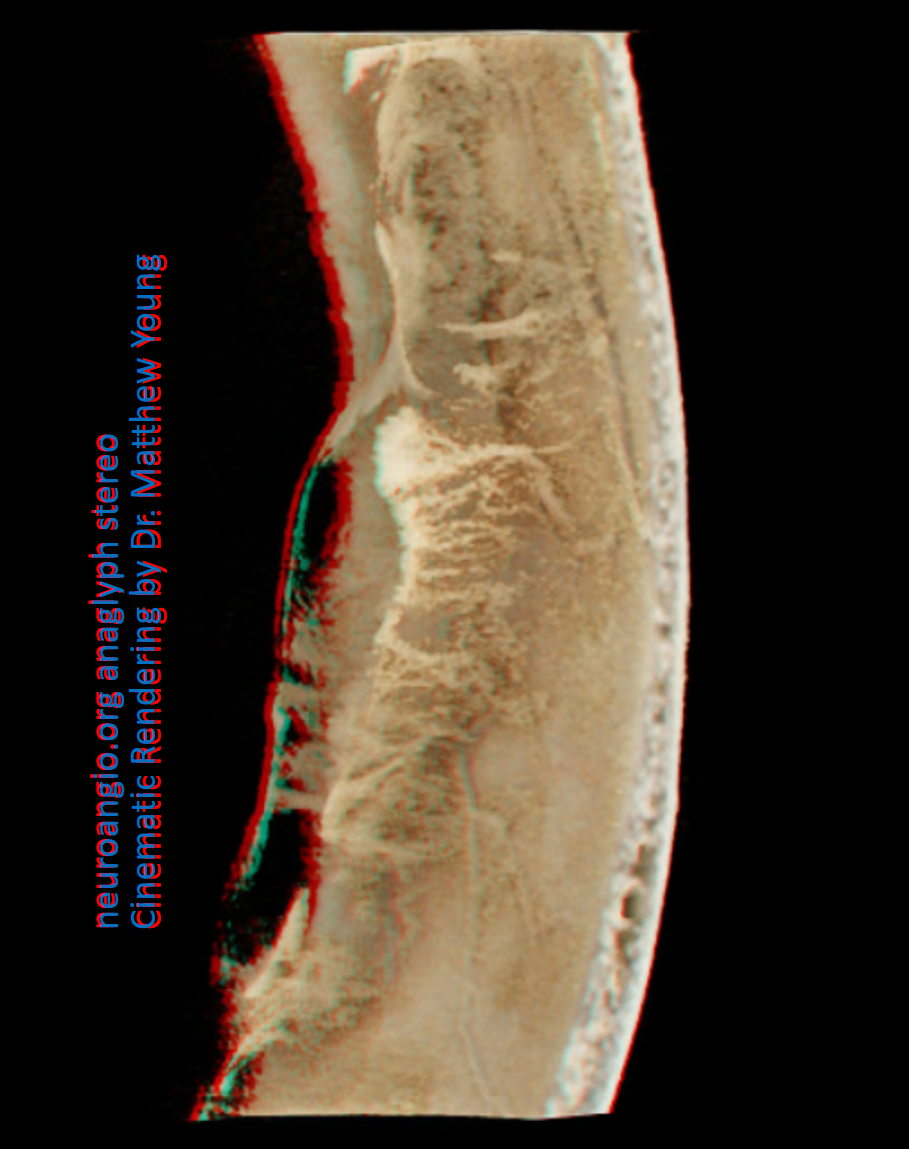
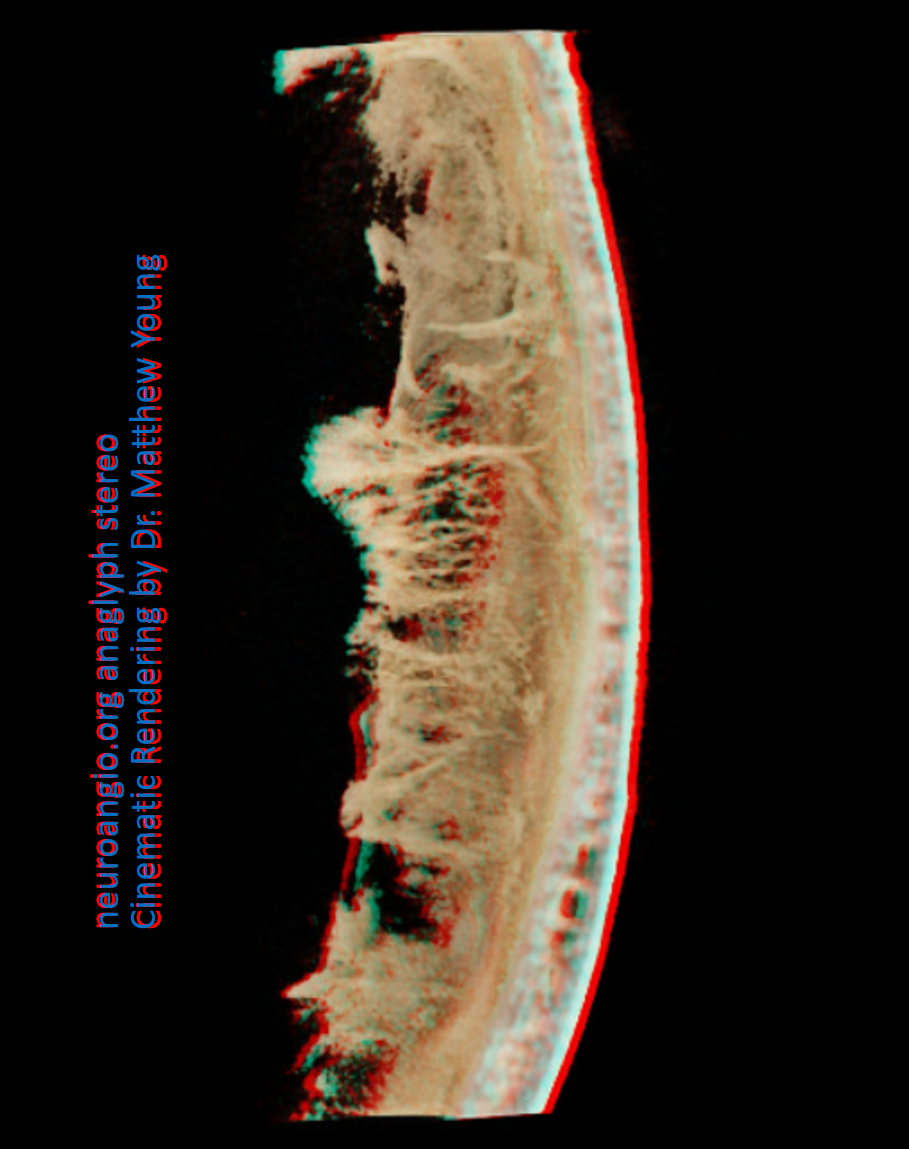
Cross-eye stereo pairs
Another series of great hematoma membrane vessels by Dr. Daniel Sahlein
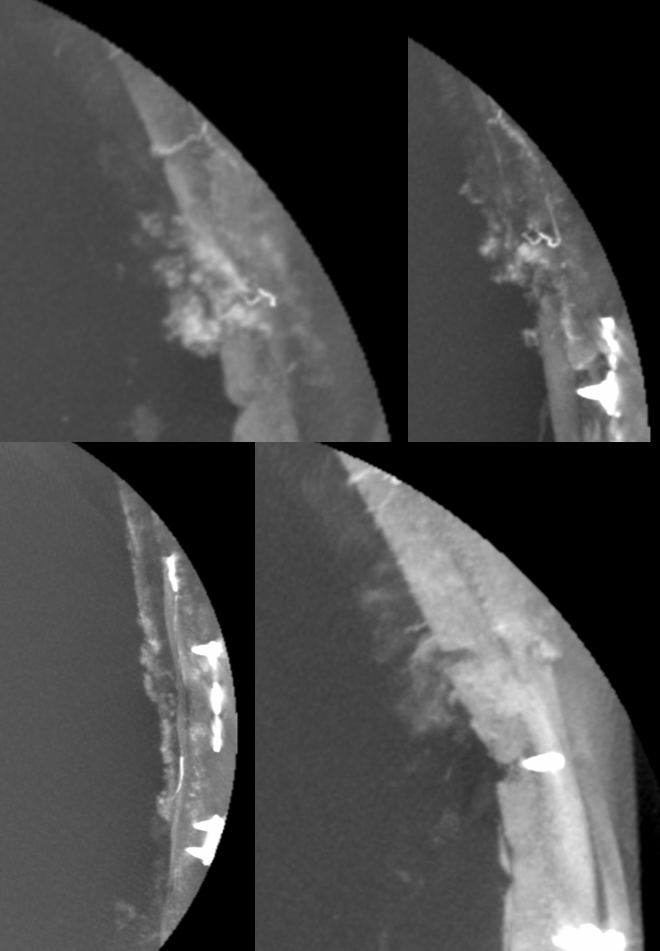
How about ONYX penetration into subdural membranes? Onyx rupture into dural / diploic/ emissary veins or venous sinuses is common. However, casting of subdural membranes is not — check out this case of Dr. Eytan Raz — arteries are red, veins (diploic — see frontal view) are blue, and onyx inside membranes is black oval.
Detail
References / Sources
There are a few excellent older sources for ex-vivo visualization of intrinsic dural arterial anatomy and pathology, particularly related to subdural hematoma embolization
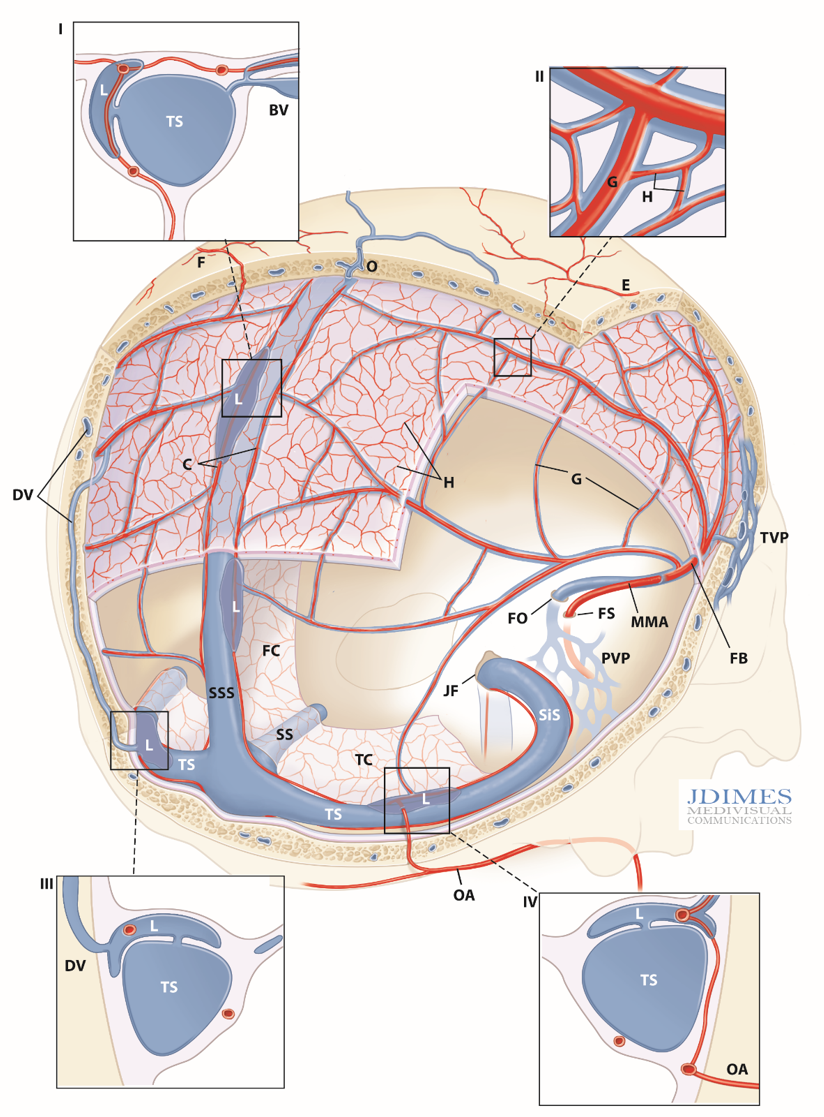
Schematic of intrinsic dural venous system and its drainage. Artwork by Jonathan Dimes of JDIMES Medivisual Communications
I-IV: detail inserts; BV – Bridging Vein (cortical); C – arterial network in sinus walls; DV – Diploic Vein, parietosquamosal region; E – cutaneous artery; F – superficial artery participating in dural supply; FB – Frontal Branch, MMA; FC – Falx Cerebri; FO – Foramen Ovale, FS – Foramen Spinosum; G – primary arterial anastomotic network of the outer layer (100-300 um diameter), H – secondary arterial anastomotic network of the outer layer (50-90 um diameter); JF – Jugular Foramen; L- venous lakes / pouches in walls of major sinuses; MMA– Middle Meningeal Artery; PVP – Pterygopalatine Venous Plexus; SiS – Sigmoid Sinus; SS – Straight Sinus; SSS – Superior Sagittal Sinus; TC – Tentorium Cerebelli; TVP – Temporalis Venous Plexus
This is totally new. No one has published on intrinsic dural venous system in vivo visualization at all as of 2021. There are very view ex-vivo studies (see bibliography below).
As we worked on the above arterial project, we got interested in angiographic visualization of dural veins. This led to the first in vivo publication of this type of imaging in JNIS in March 2021. The dedicated neuroangio venous dural vasculature page is here.
