Conceptualizing the basal vein as a conduit extending from the more anterior sinuses (most commonly the cavernous sinus) to the more posterior ones (most commonly the straight sinus) is very helpful in understanding its normal variations and pathology. Most typically, the basal vein extends from the cavernous sinus or its deep sylvian tributaries posteriorly around the mesencephalon, to meet with the internal cerebral vein, thus forming the vein of Galen. Three segments are described in relation to the mesencephalon. The first or anterior segment extends from the cavernous sinus to the ambient cistern. The second middle segment curves over the mesencephalon. The third or posterior segment extends from the end of the second segment until the vein of Galen. This concept is useful as any of the three segments may be hypoplastic and drainage will be redirected accordingly.
Basal Vein (purple) relationship to the anterior choroidal artery (red) — this is a consistent relationship with both vessels running adjacent to each other. The sphenoparietal sinus is orange and cavernous sinus dark blue.
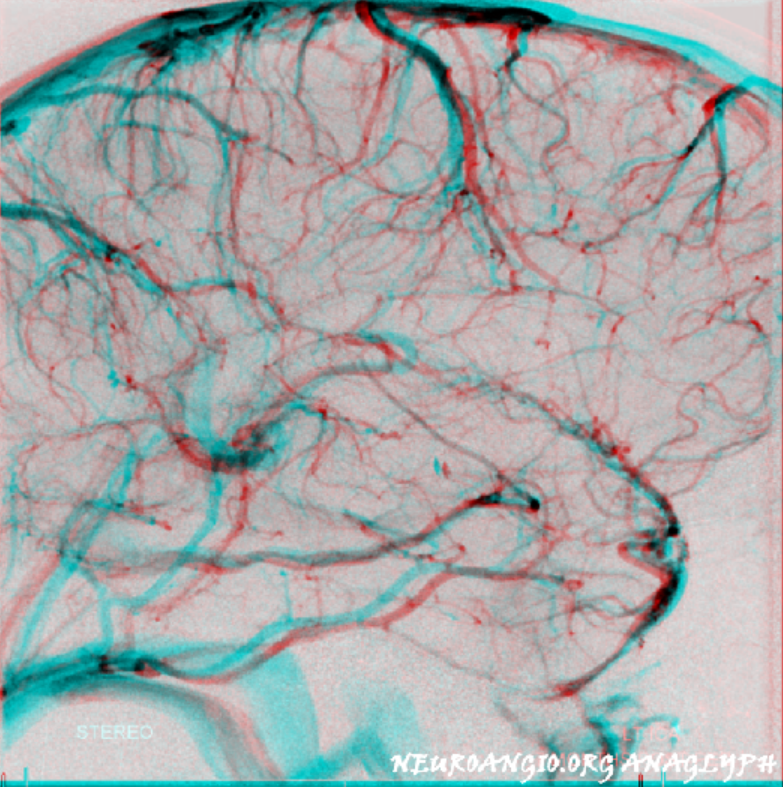
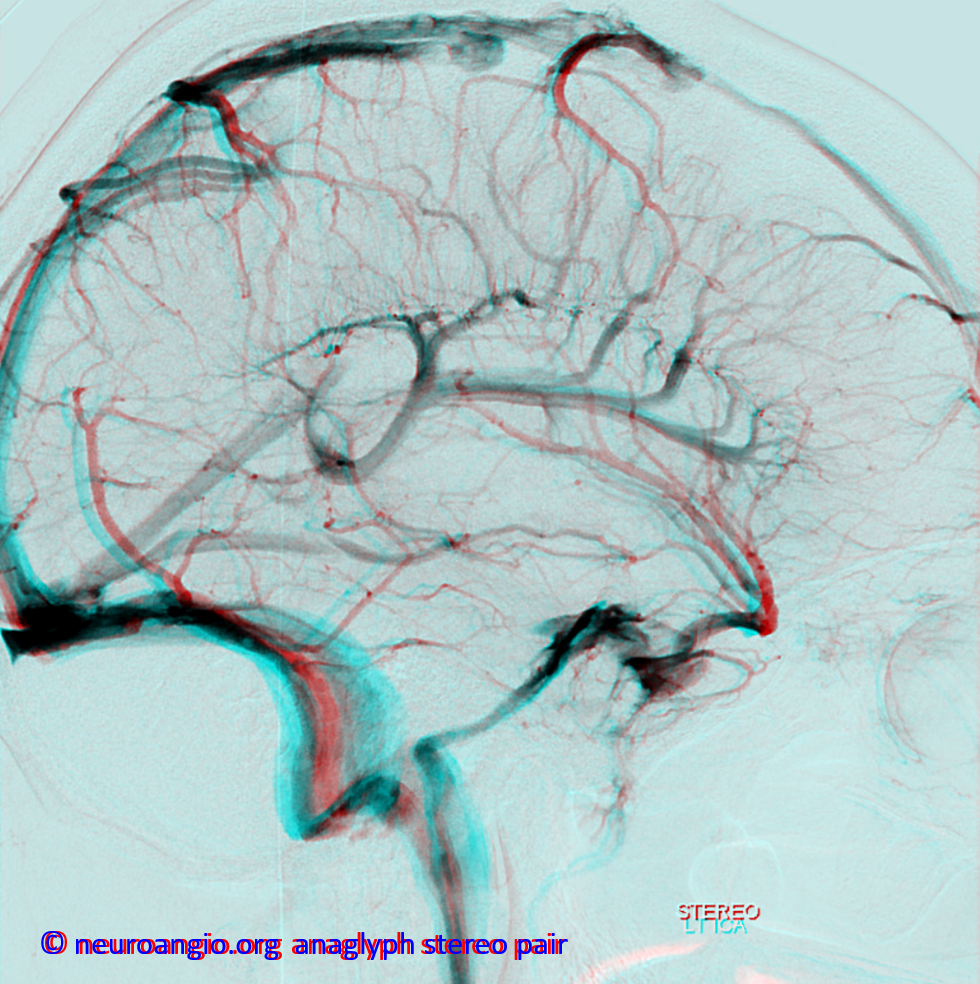
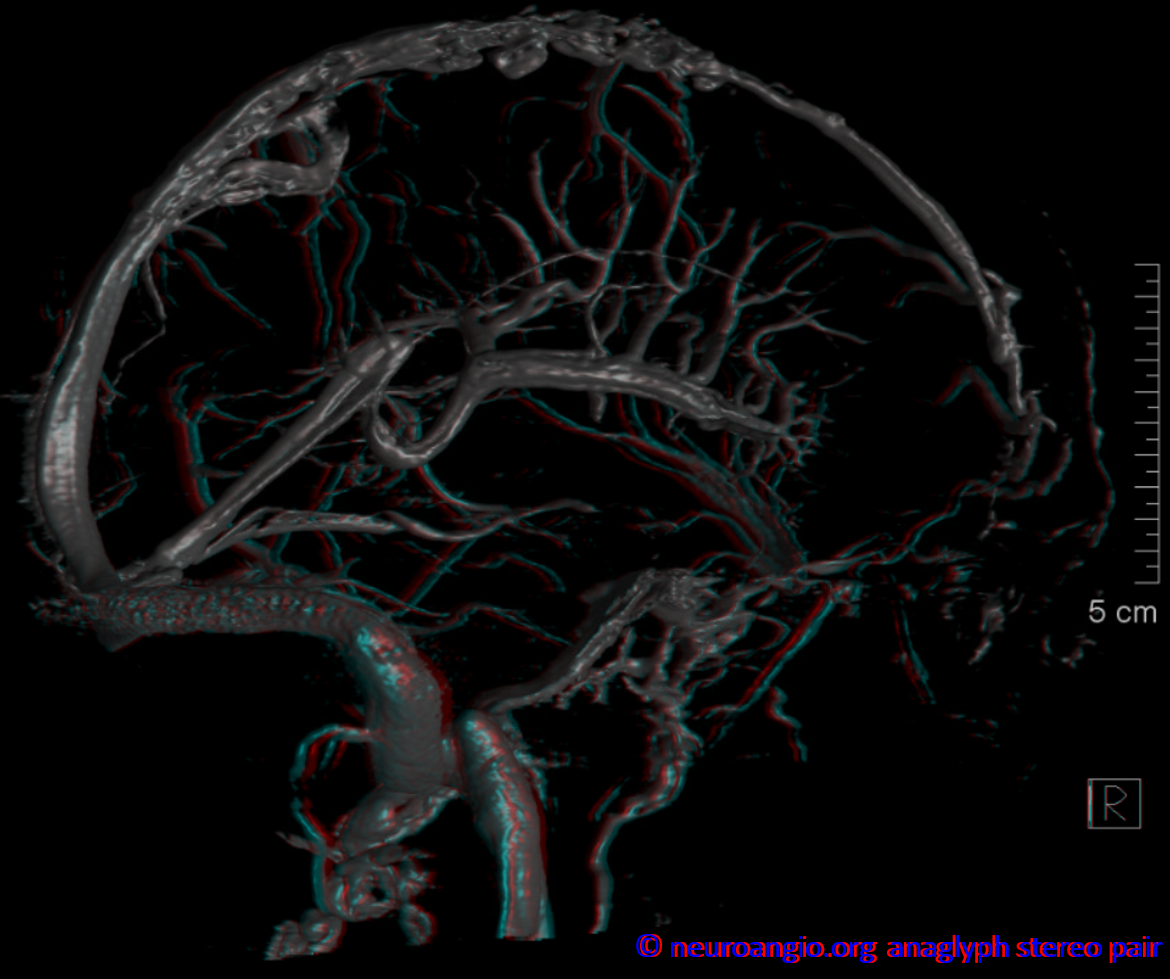
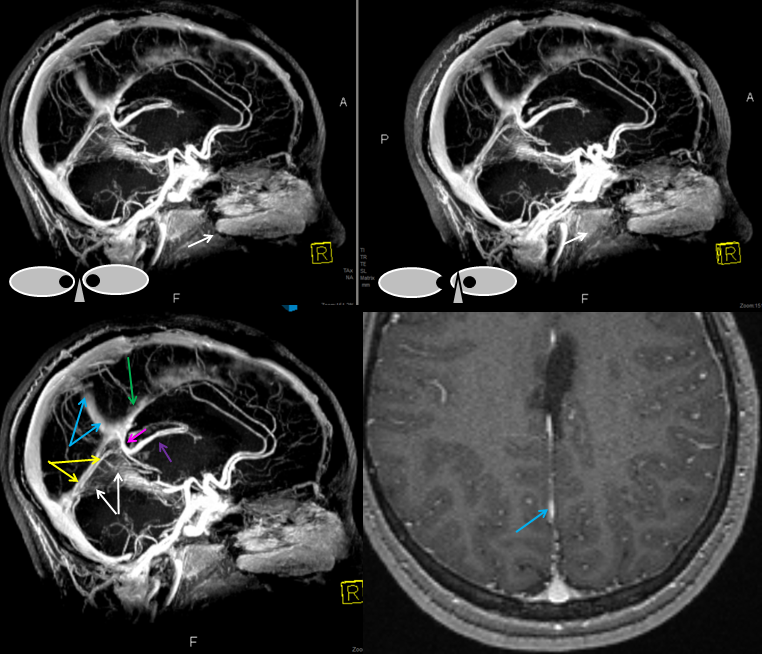
Basal vein, full extent from cavernous sinus to Vein of Galen.
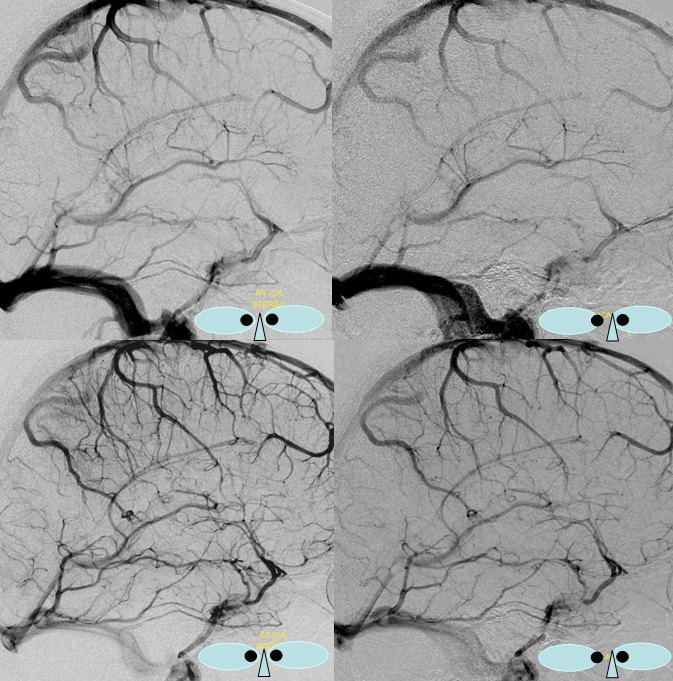
Stereo views of early (top) and late (middle row) lateral projection demonstrating a fully developed basal vein (blue), connecting the sphenoparietal sinus (brown) and cavernous sinus (blue-green) to the vein of Galen. Also seen are inferior sagittal sinus (green), the internal cerebral vein (red) with its anterior caudate (light blue), Thalamostriate (pink) and posterior atrial (black) tributaries. The vein of Trolard is in yellow.
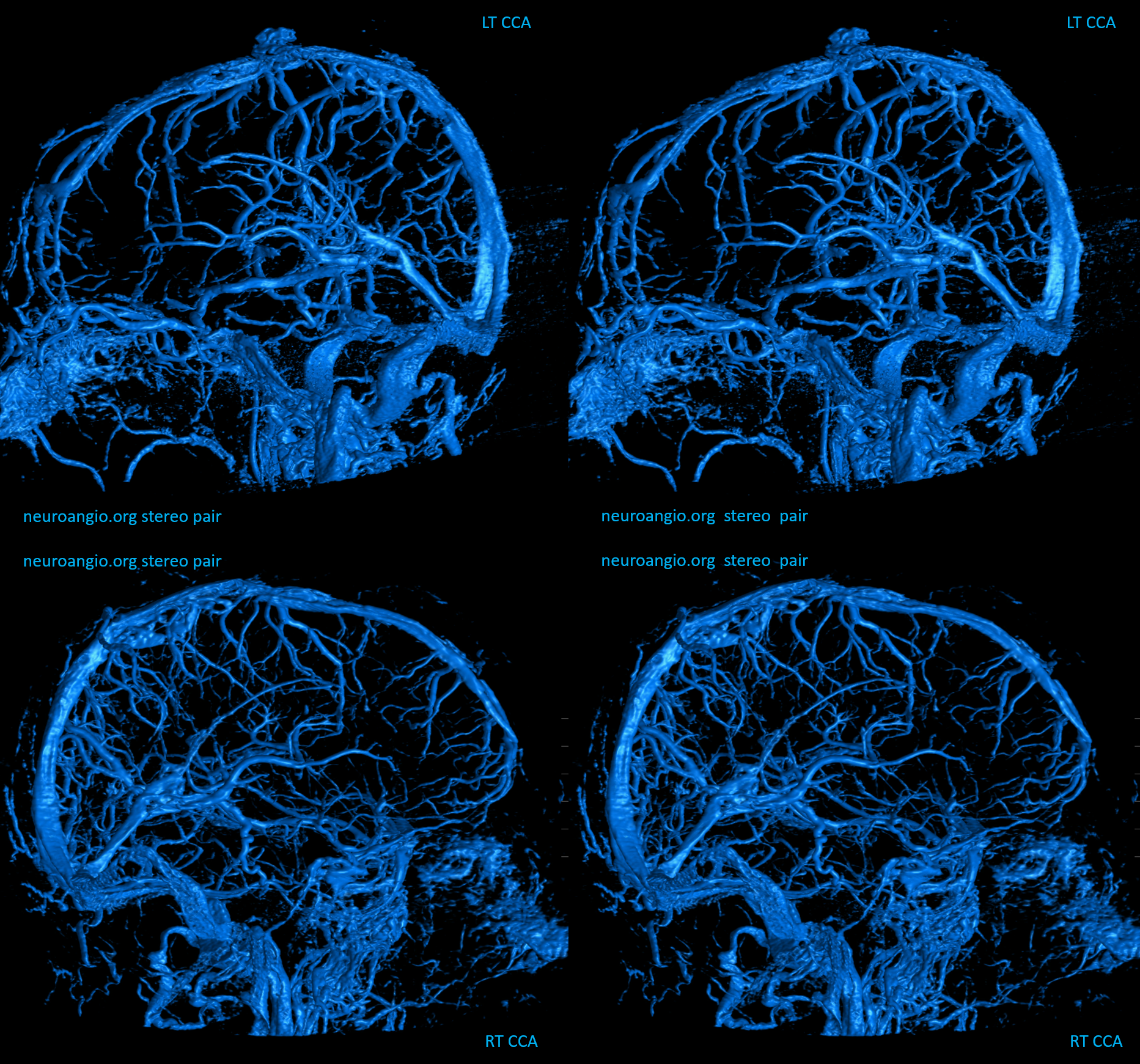
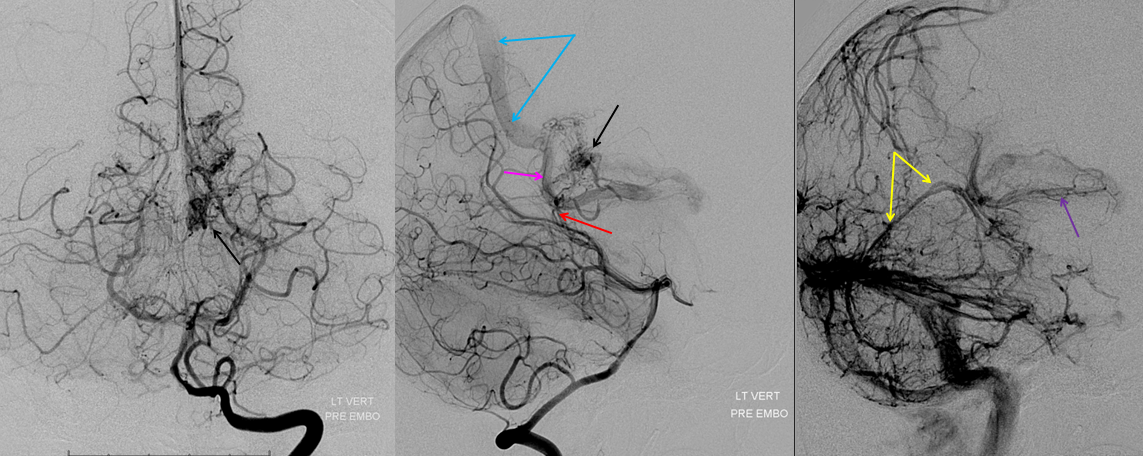
Basal vein visualization from carotid and vertebrobasilal injections
Again, remember that in angiography only veins of the arterial territory which has been injected are going to be chiefly visible. If another territory, which is unopacified by contrast, also joins a vein that is opacified, the unopacified venous inflow will dilute the contrast material from the injected territory, producing a slipstreaming effect. For example, the Galen is usually less well opacified than the internal cerebral or basal veins because of inflow from the contralateral side. Notice, in this example, the “washed out” look of the posterior 2/3 of the basal vein (blue), whereas the anterior third, which is its deep sylvian territory (pink) is fully opacified. Now you know where vertebrobasilar territory venous contributors enter the basal vein — right where it becomes diluted. So, remember to inject both carotid and vert to fully understand the basal vein
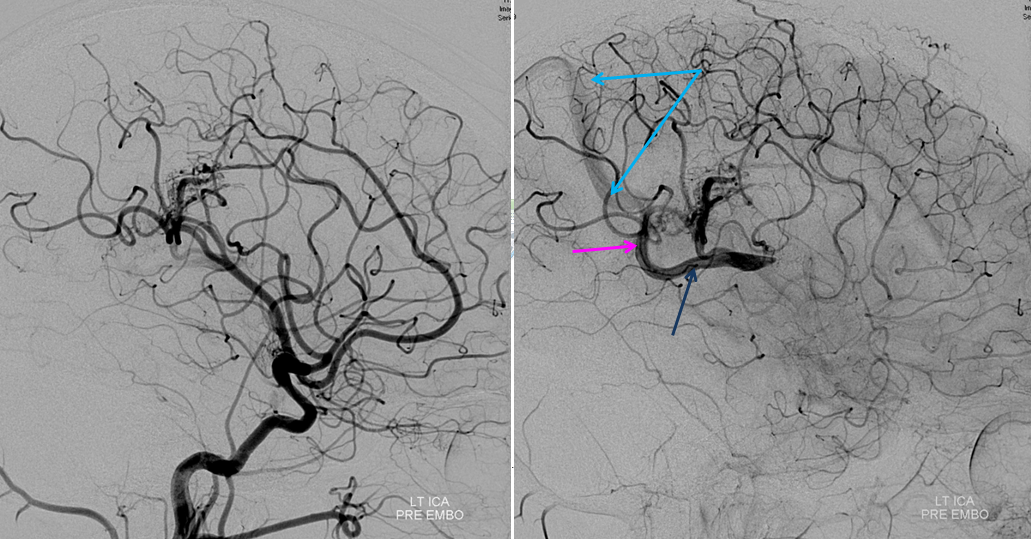
Stereo pair of the contralateral carotid injection, similar disposition except sylvian veins also drain into the cavernous sinus
Vertebral injection of the same subject, where both basal veins (blue) are washed out by the contributions from the carotid territories. Notice very nice views of the interpeduncular veins (white), the deep recess of which (purple) is located in the interpeduncular fossa.
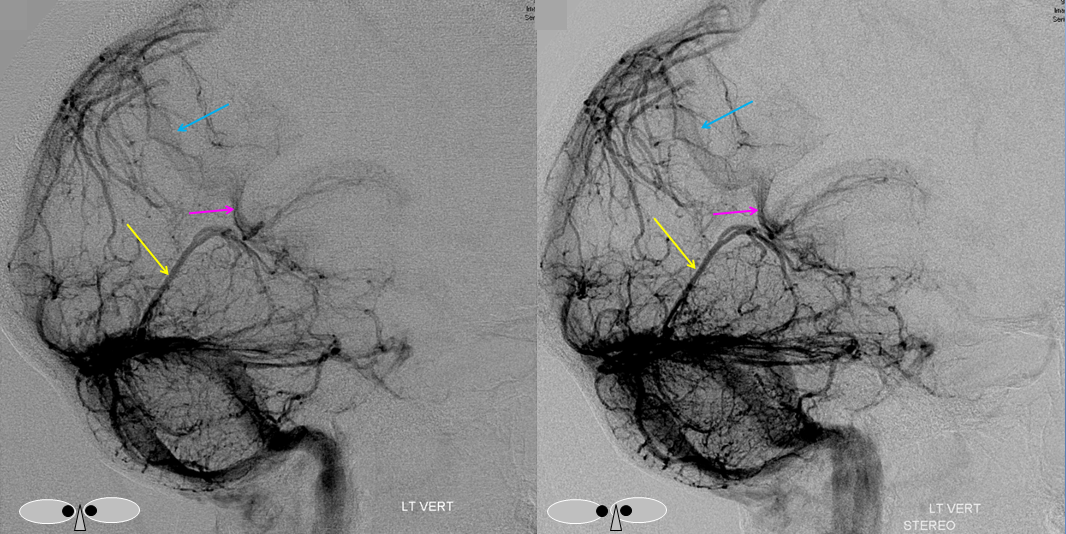
Stereo views of the same, much better seen
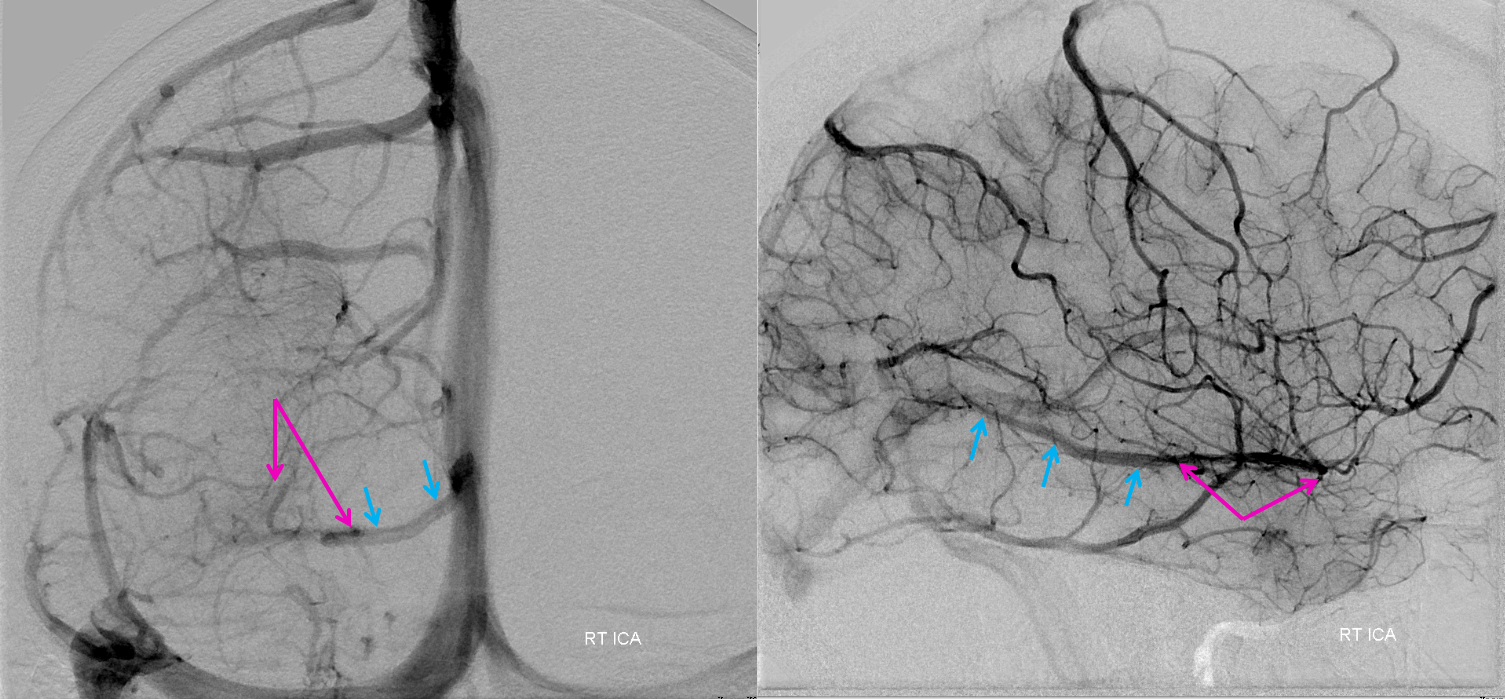
Frontal view, same subject, in stereo, perhaps the best demonstration of dilution (white arrows)
Discontinuous Basal Vein
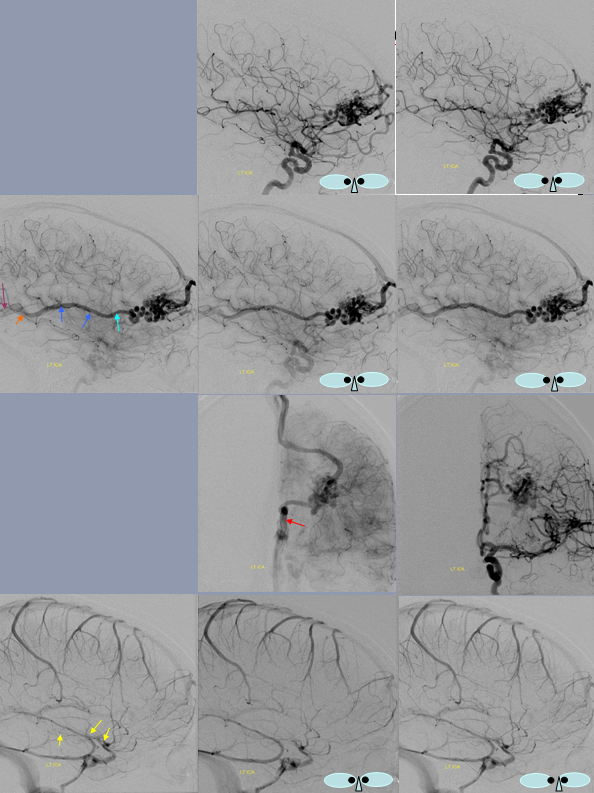
Left and Right venous phase angiograms of the same patient demonstrate a hypoplastic middle basal vein segment with anterior portion of the basal vein draining into the deep sylvian (MCA) veins and posterior 1/3 portion emptying into the vein of Galen.
Anterior segment of basal vein (blue) and posterior segment (light blue).
Three segments separate drainage
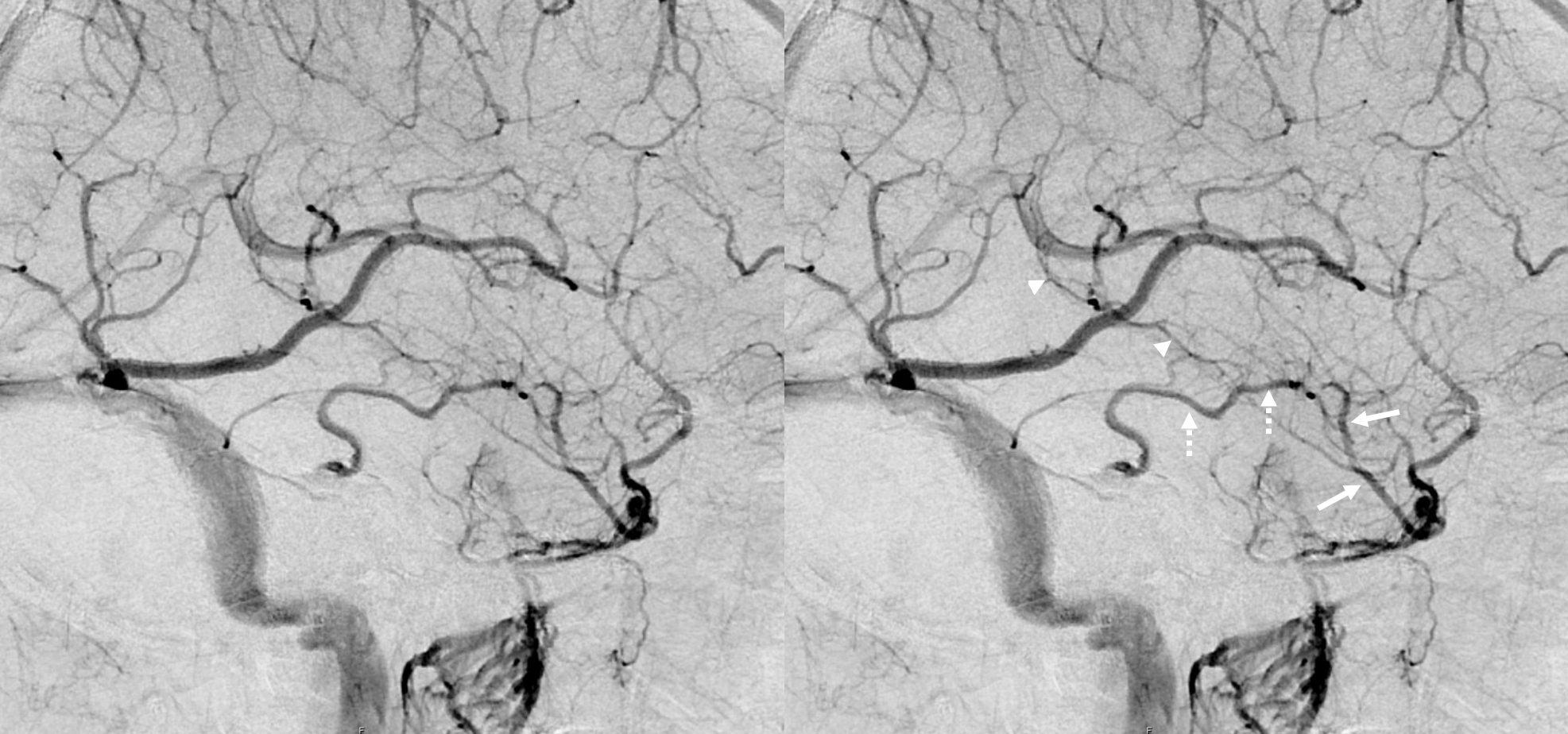
Anterior segment “telenecephalic” (arrows) into cavernous sinus, middle “mesencephalic” (dashed arrows) into the lateral mesencephalic vein, posterior (arrowheads) into the Galen
Anterior drainage of basal vein into the cavernous sinus. In this stereo example the ICA territory of the basal vein drains towards the cavernous sinus. Two possibilities exist — the posterior segment of the basal vein may be truly hypoplastic, or potentially the major inflow into an existing posterior segment comes from territory of the vertebrobasilar system.
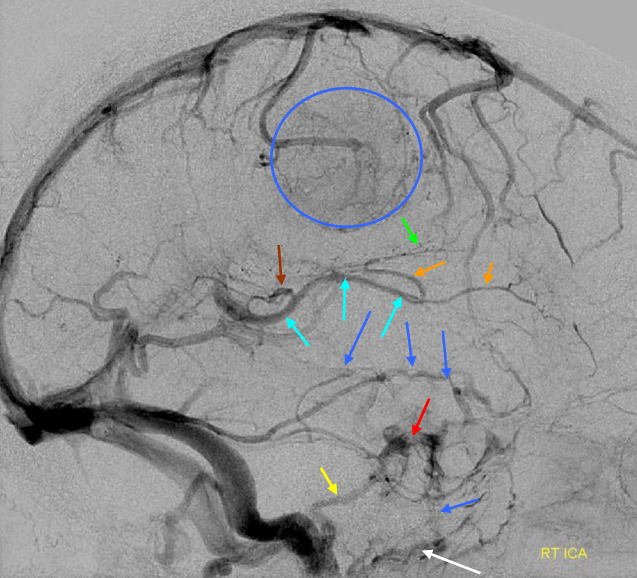
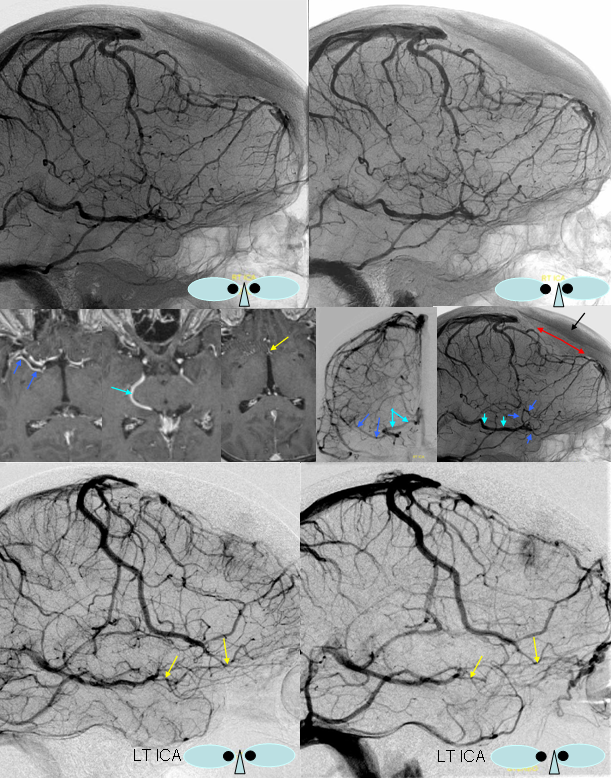
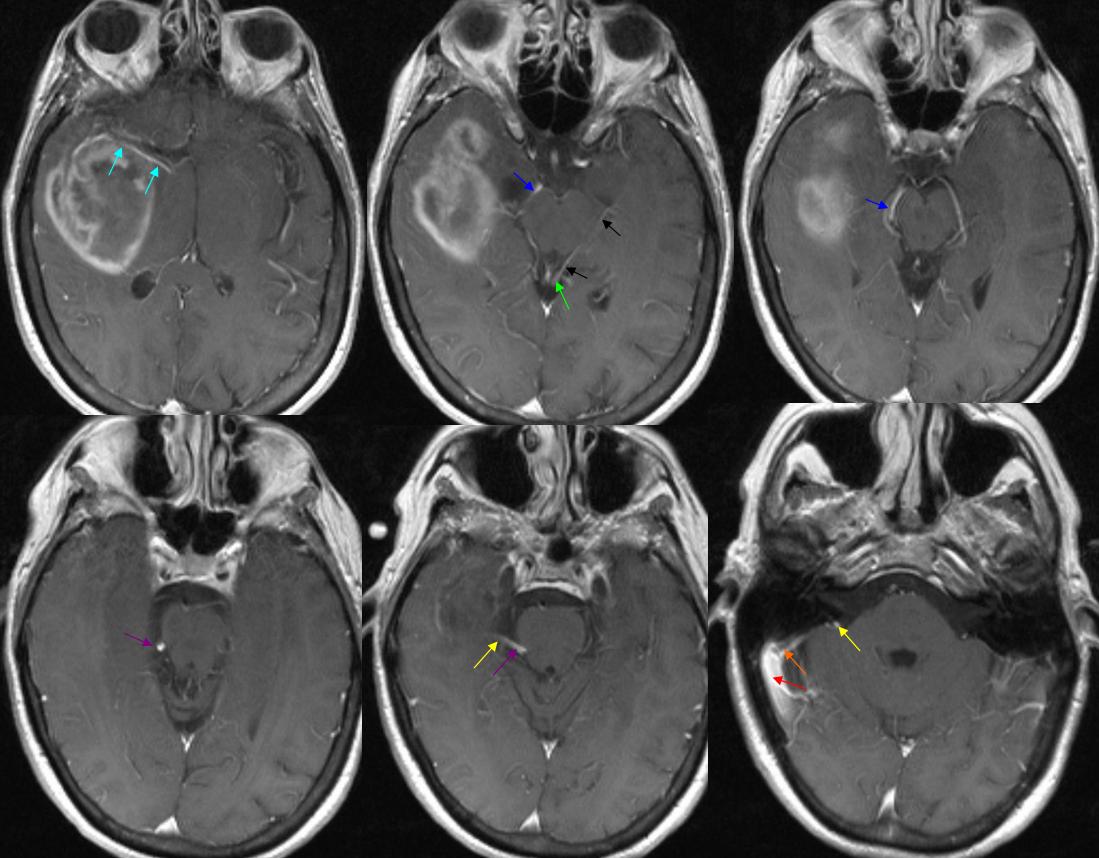
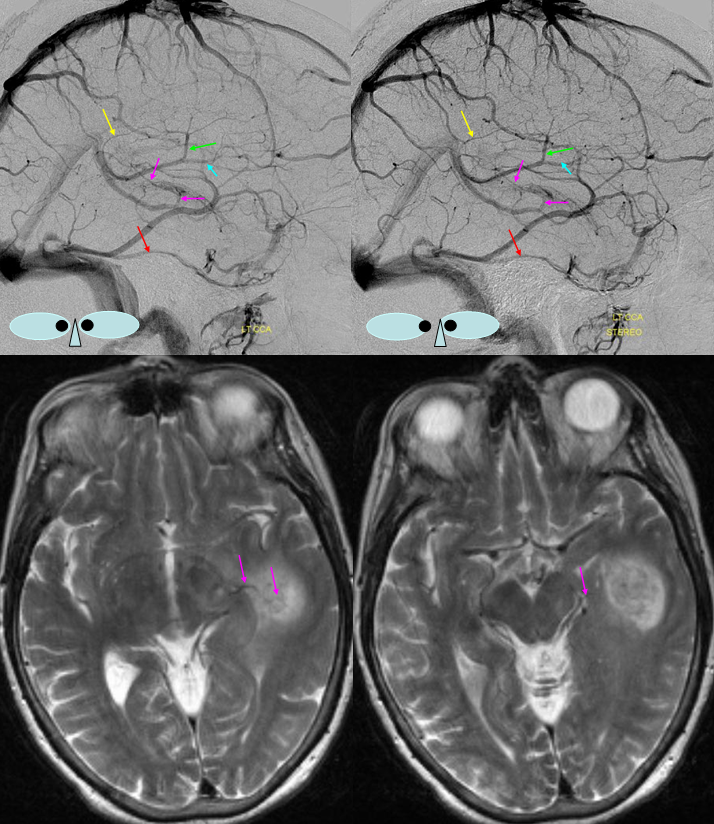
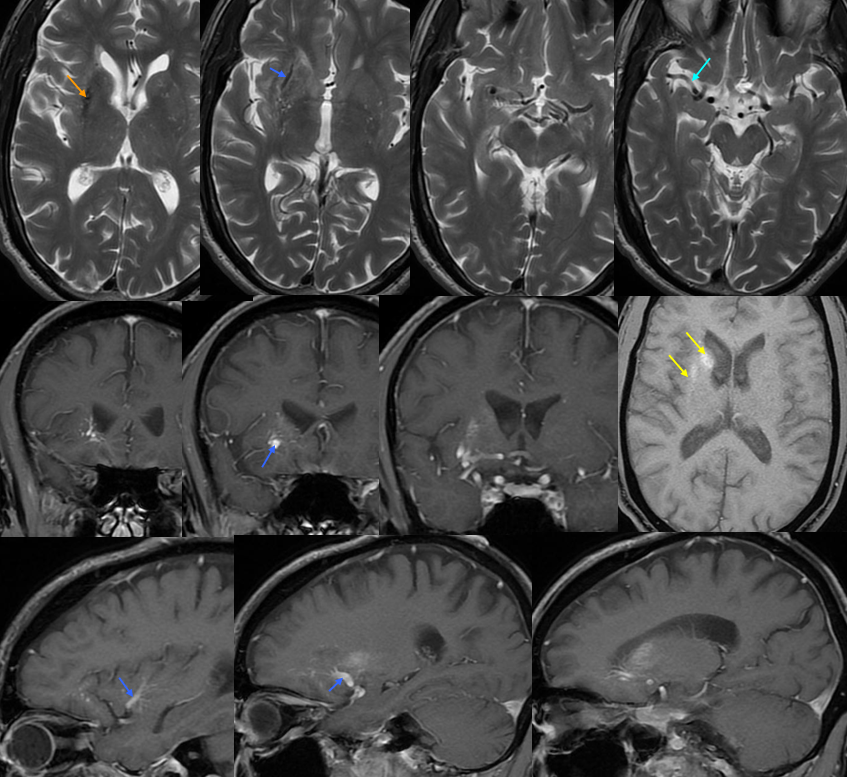
Basal vein = dark blue. Internal cerebral vein = light blue. Cavernous sinus = red. Another blue arrow points to the foramen ovale drainage of the cavernous sinus into the pterygoid plexus (white). Inferior petrosal sinus = yellow. Direct lateral vein = brown. Anterior septal vein = more anterior orange arrow. Thalamostriate vein = more posterior orange arrow. Blue circle = tumor blush (GBM). Notice mass effect of the tumor on lateral caudate tributary (green) of the Thalamostriate vein.
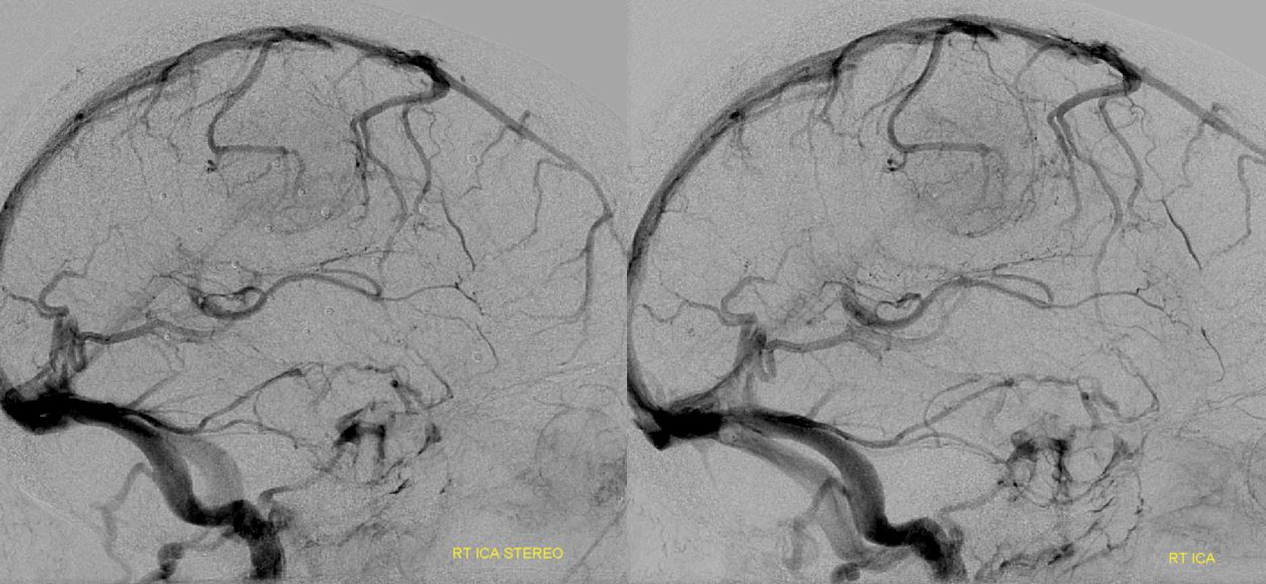
A very large basal vein in absence of pathology. The cavernous and both petrosal sinuses are hypoplastic, as is the Labbe. On both sides, the superficial Sylvian veins drain into the posteriorly draining basal vein, making it look pretty enormous.
Blue = deep sylvian veins (on MRA this is also known as the middle cerebral vein). Light blue = basal vein. Yellow = inferior frontal vein becoming anterior cerebral vein (seen on MRA) middle image posterior the the ACAs. Red=meningioma occluding SSS. Black = hyperostosis from said meningioma.
Basal vein drainage into superior petrosal sinus. Not ifrequently seen variant which illustrates the embryologic concept of a transcortical venous system which connects deep and superficial venous networks. In this patient, for some reason, basal vein drainage into the VOG did not develop. An alternative transcortical pathway persisted, emptying the basal vein territory into the superior petrosal sinus. The way to connect the basal vein to the superior petrosal sinus is via the lateral mesencephalic/petrosal veins (see Veins of Posterior Fossa section)
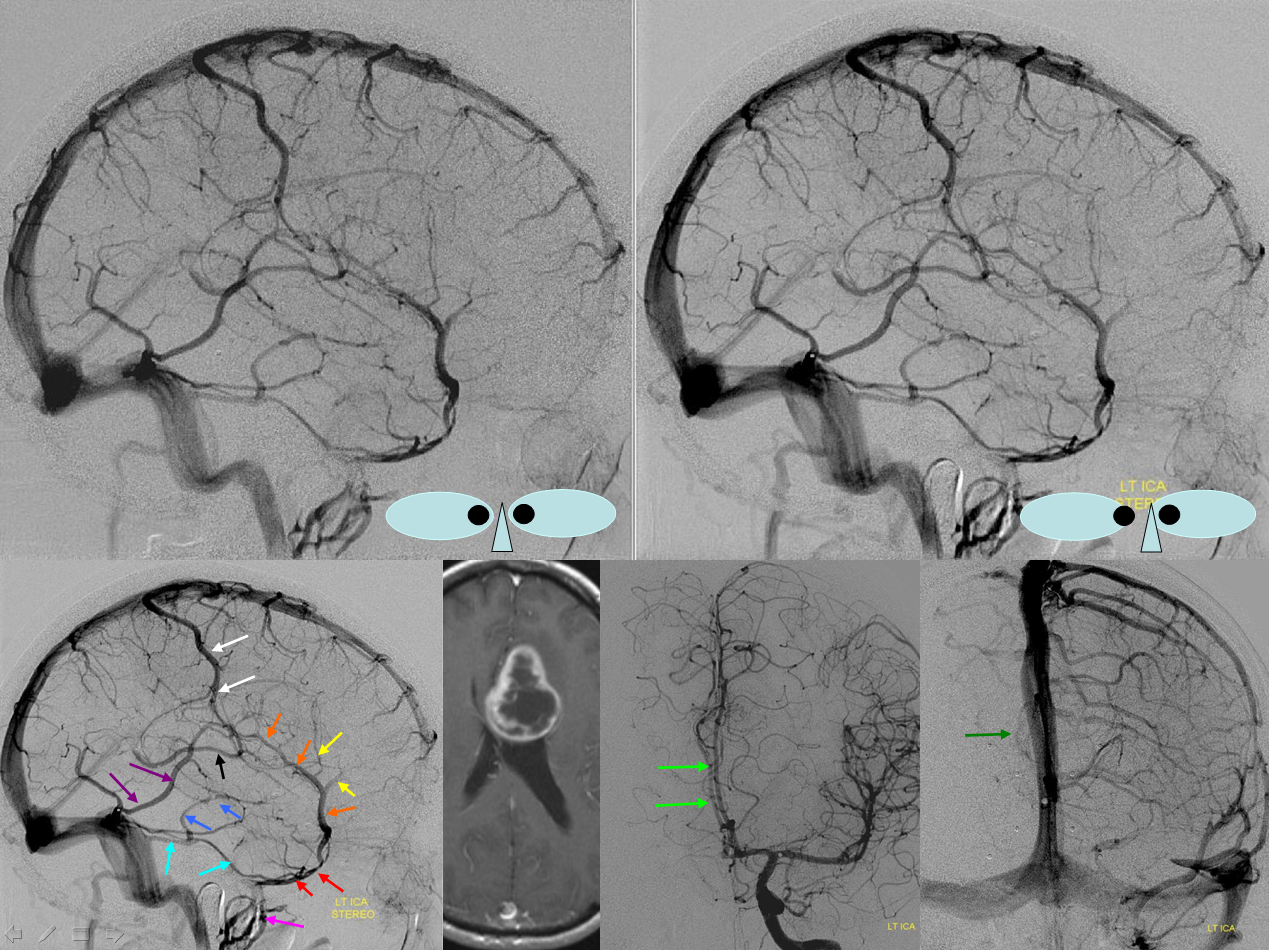
Lateral mesencephalic/petrosal veins = deep blue. Superior petrosal sinus = left light blue arrow. Right light blue arrow is on an inferior temporal superficial tributary to the sinus. Trolard = white. Labbe =purple. Superficial sylvian veins = orange. Sphenoparietal sinus = red. Cavernous sinus = pink. Notice a balanced superficial draining pattern with robust connections between Trolard, Labbe, and Sylvian networks. Mass effect is evident with midline shift of the Anterior cerebral artery (bright green). There is also venous mass effect present on what is likely a medial septal vein (dark green)
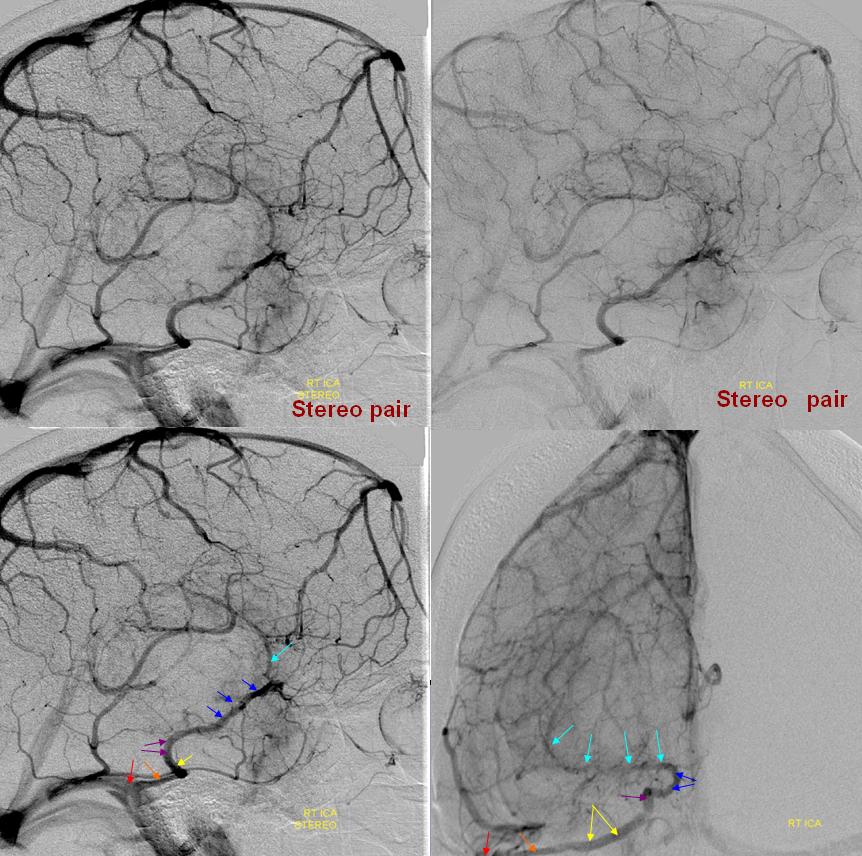
The middle segment of the basal vein is hypoplastic. The deep sylvian vein (light blue) drains into the anterior basal vein segment (dark blue), and subsequently into the lateral mesencephalic vein (purple) to petrosal vein (yellow) and petrosal sinus (orange), ultimately into the straight/sigmoid sinuses (red). The tumor serves to enlarge the vein via hyperemia and probably also via compression of the superficial sylvian system outflow. “Usual” disposition of the basal vein (black) draining into the Galen (green) is seen contralaterally.
Basal vein drainage to Superior Petrosal Sinus — via the lateral mesencephaic vein and petrosal vein == same appearance. Different case.
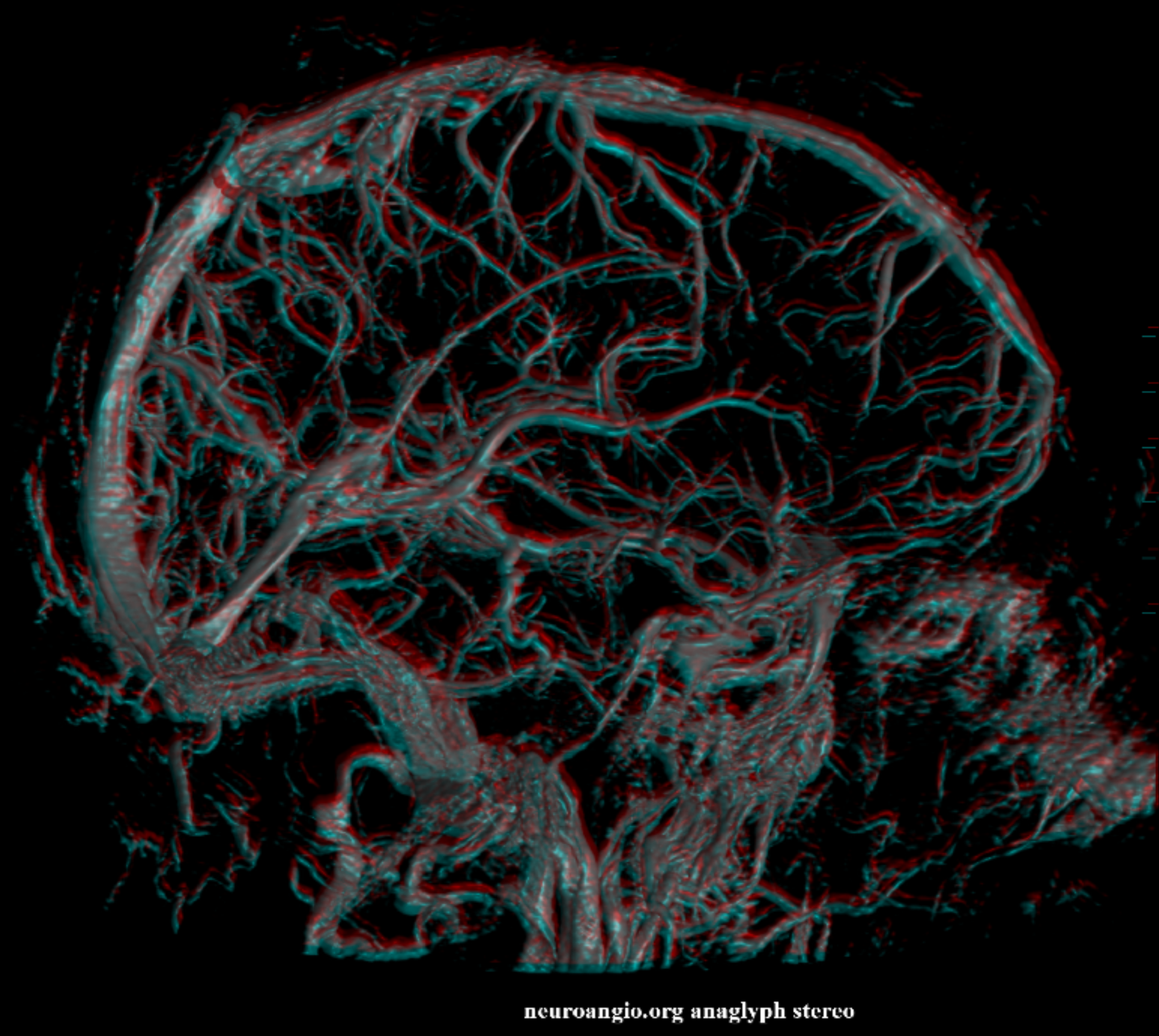
Yet another case, with anaglyph stereos
Lateral anaglyphs looking from both lateral to medial and medial to lateral
Tentorial Sinus Drainage
Very large basal vein draining into the lateral mesencephalic vein, from which it enters a large tentorial sinus, with corresponding hypoplasia of the superficial sylvian veins. The tentorial sinus is much more common than most realize. Veins don’t always get into the named sinuses right away. Sometimes, they empty into an unnamed venous channel inside the tentorium (called tentorial sinus generically) which then empties into the named venous sinus. The larger the vein, the more likely it is to have a tentorial sinus component — part of embryology. See “Dural Venous Channels” page for more info
Frontal views
Anaglyph stereo
MRI of same patient. MRI is an excellent way of looking at veins — better than angio in fact since you can see all of them at once and the nearby brain landmarks. Pink arrow shows deep sylvian component, blue the lateral mescencephalic one, and white is a tentorial sinus draining this
Even more correlation — Movies — stop and scroll thru images
This is spectacular vessel wall imaging — developed at NYU by Dr. Seena Dehkharghani
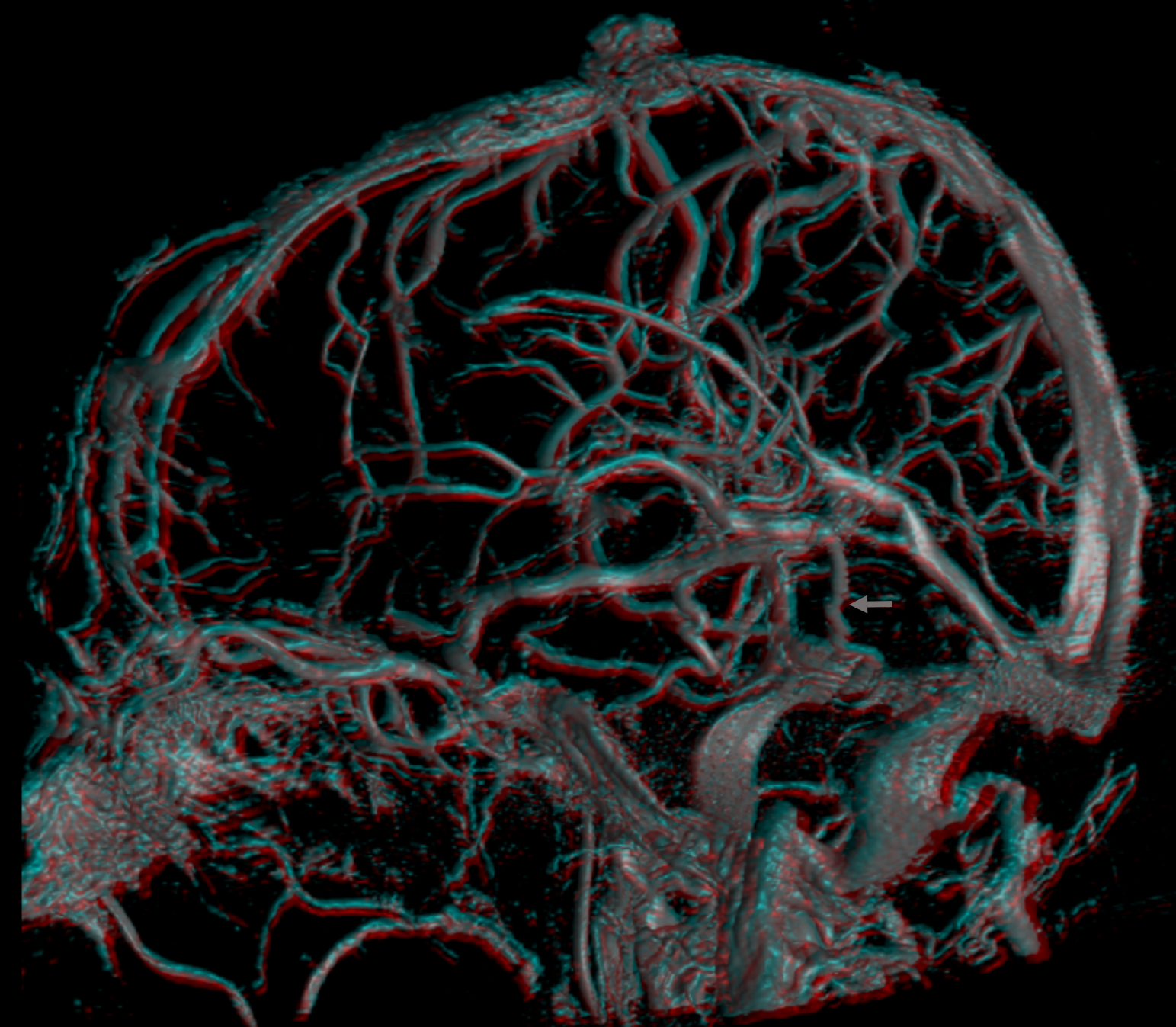
The lateral mesencephalic vein drains into the superior petrosal sinus. This is a conduit between cavernous sinus and sigmoid sinus. Of course, there is always balance. Usually flow is towards the sigmoid. Sometimes however this segment is hypoplastic and the superior petrosal sinus drains towards the CS instead. Here is one example, in stereo
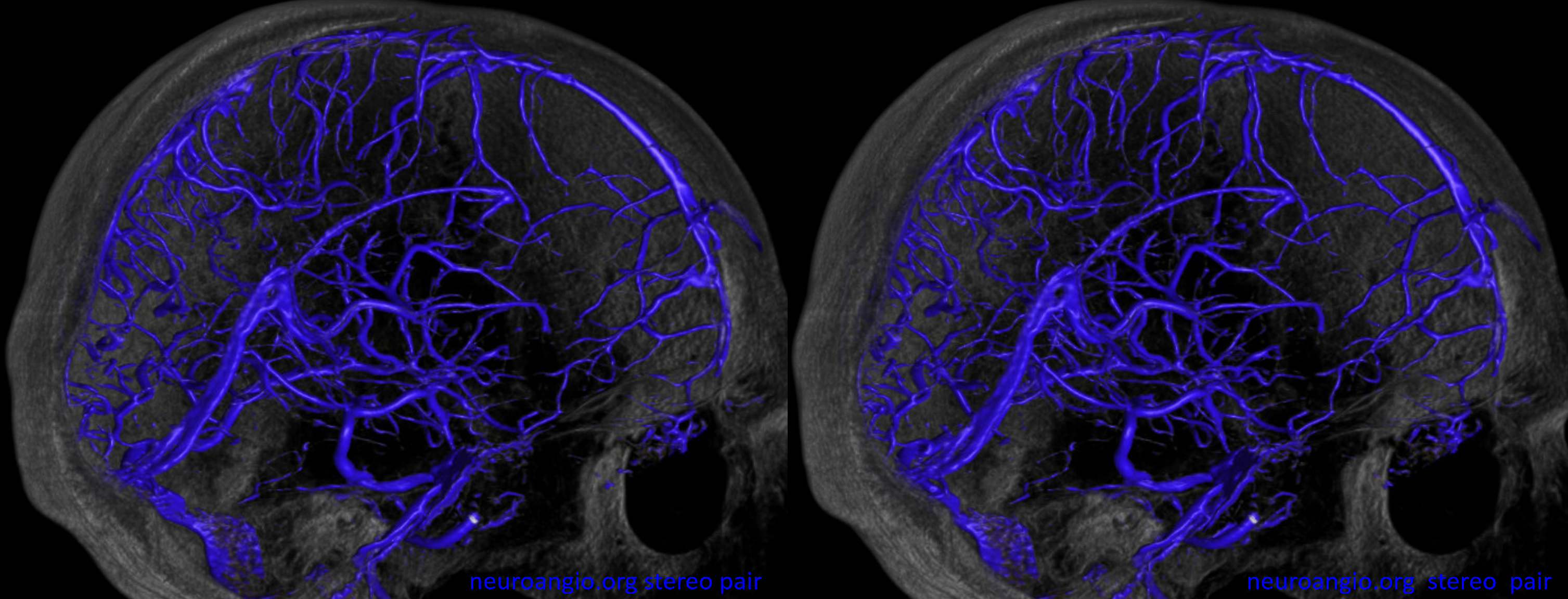
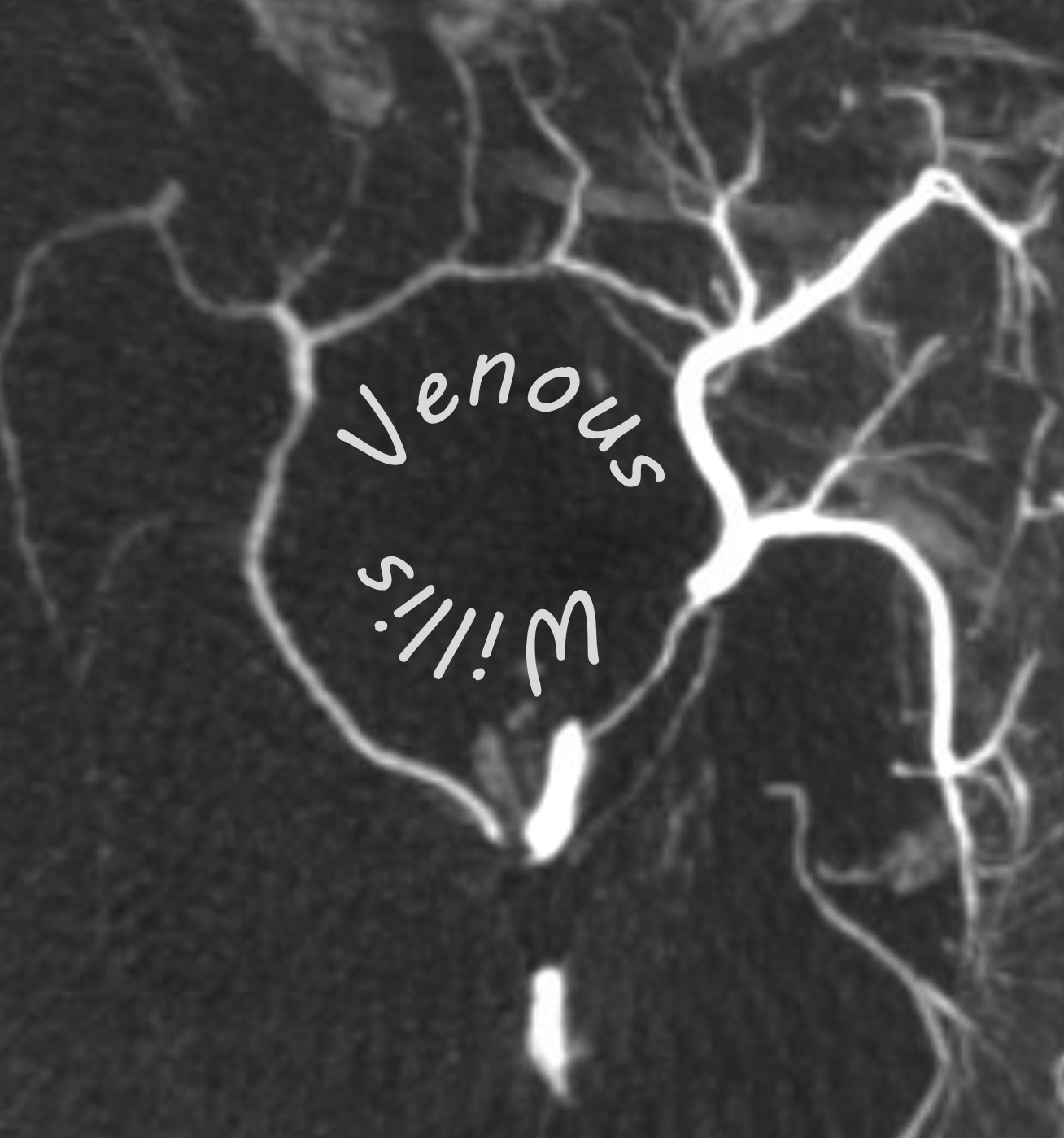
The same person — check out the venous circle of Willis — Venous Willis as it were
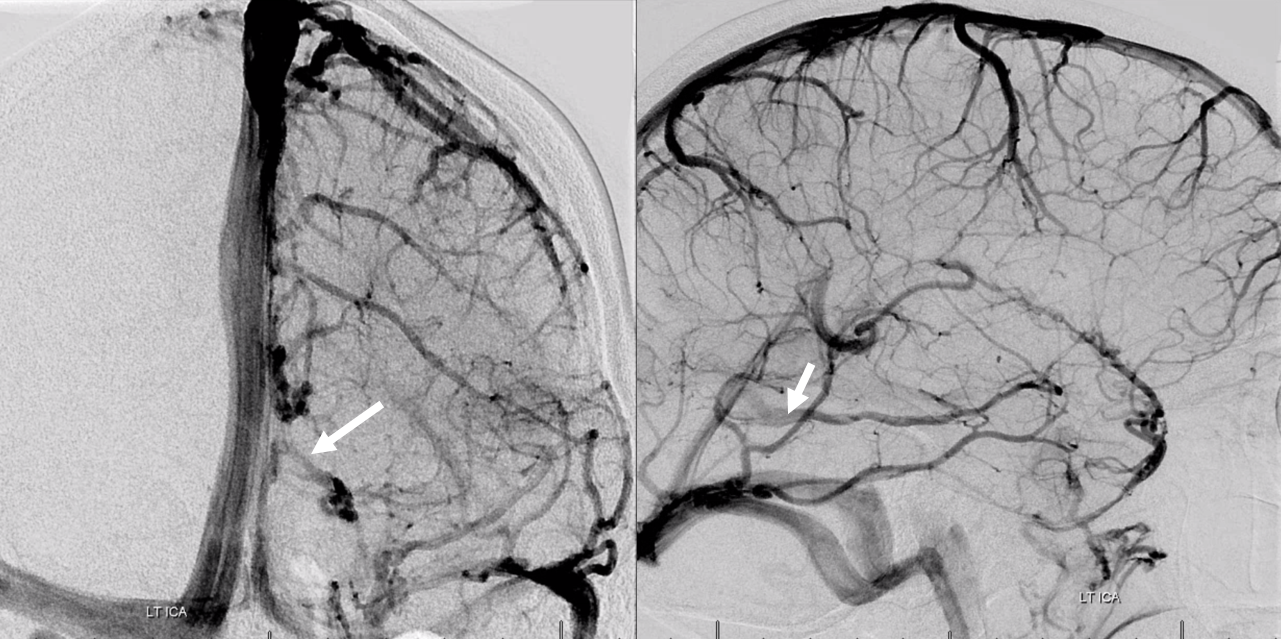
Here is another example of basal vein emptying into a tentorial sinus (white arrows). The sinus runs in the free margin of tentorium cerebelli, into the straight sinus
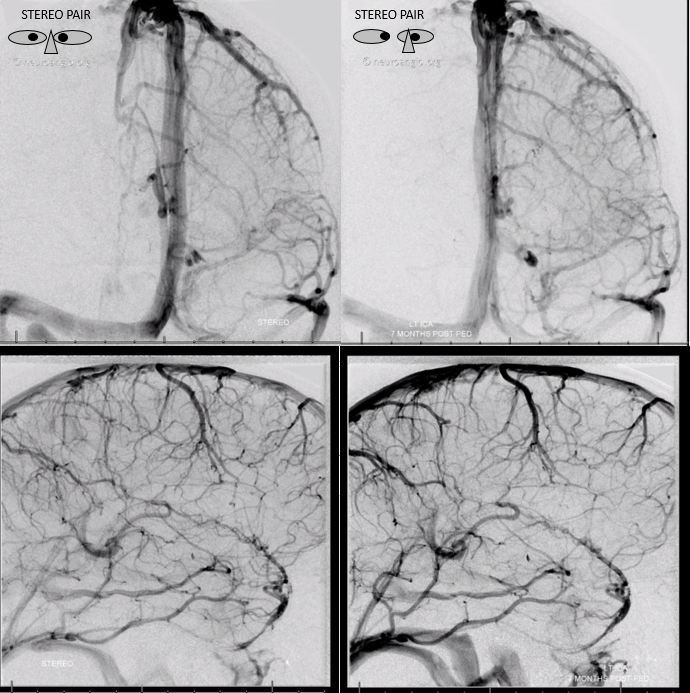
Stereos
Anaglyph stereo
MRI of the same patient; basal vein is black, tentorial sinus is white arrows. Tentorial sinuses have a flattened appearance compared to the round veins on all imaging modalities. They are easy to spot on high quality MRI once you get used to the pancaked look. The relationship between the sinus and the tentorium is easily seen
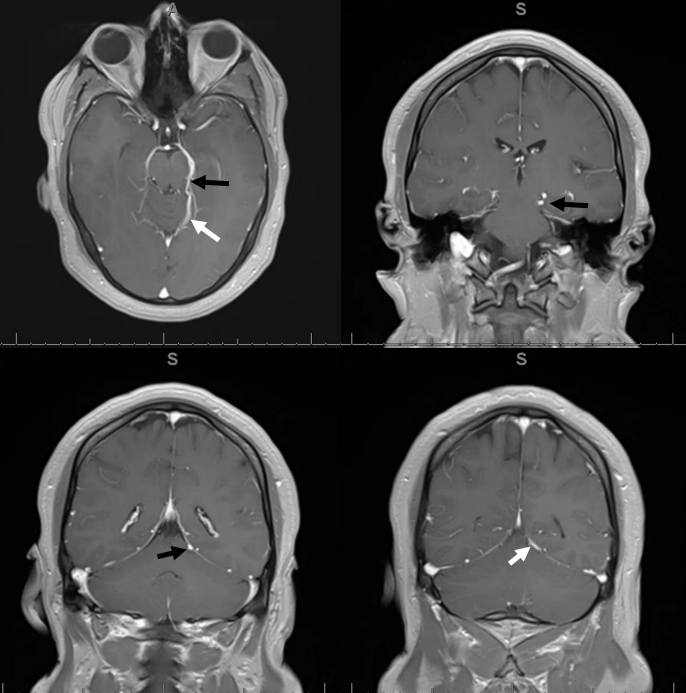
Yet another example, in a patient with a large DVA
Straight Sinus Outflow Obstruction and Internal-Cerebral-Basal Vein Anastomoses
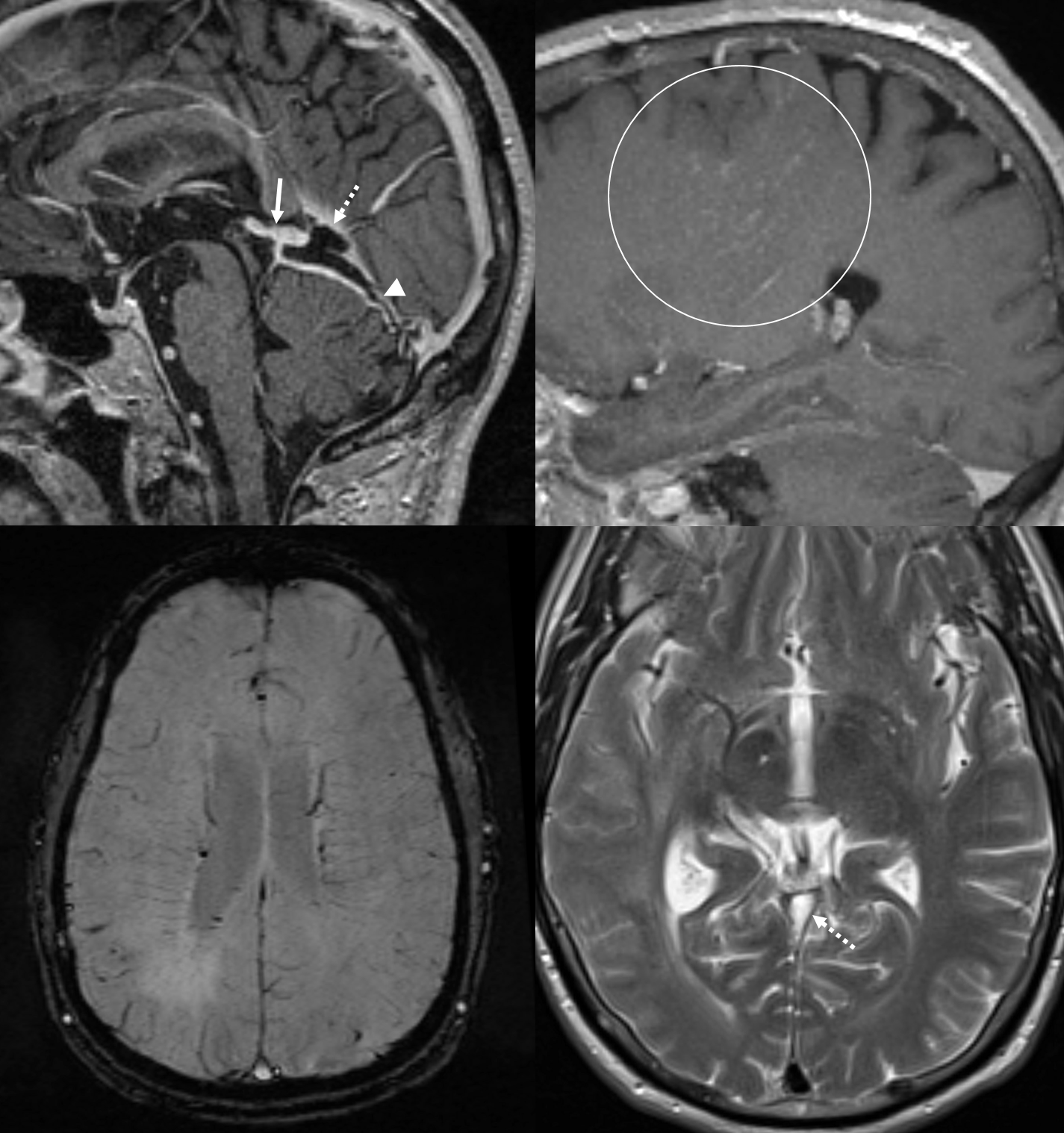
Obstruction of the Galen, especially acute (venous thrombosis) is often catastrophic, because of paucity of internal cerebral venous collaterals (see case here. Also see medullary veins page). However, obstructions of straight sinus can be very well tolerated, because of the ICV-Galen-Basal Vein connection. The basal vein has more outflow potential (into cavernous sinus or lateral mesencephalic veins for example). Below is an example of this disposition, case of Drs. Eytan Raz and Maria Borja Angulo.
MRI: prominence of medullary veins (circle), normal size Galen (arrow), hypoplastic straight sinus (arrowhead), and a granulation/nodule structure in the distal straight sinus (dashed arrow).
Angio — bilateral CCA and left vert injections. The straight sinus (open arrows) is hypoplastic. Bilateral ICVs drain via bilateral basal veins, into the lateral mesencephalic veins (arrows), into the cavernous sinus via the telencephalic / anterior segment of basal vein (dashed arrows), and on the left via a tentorial sinus (arrowhead) into the sigmoid.
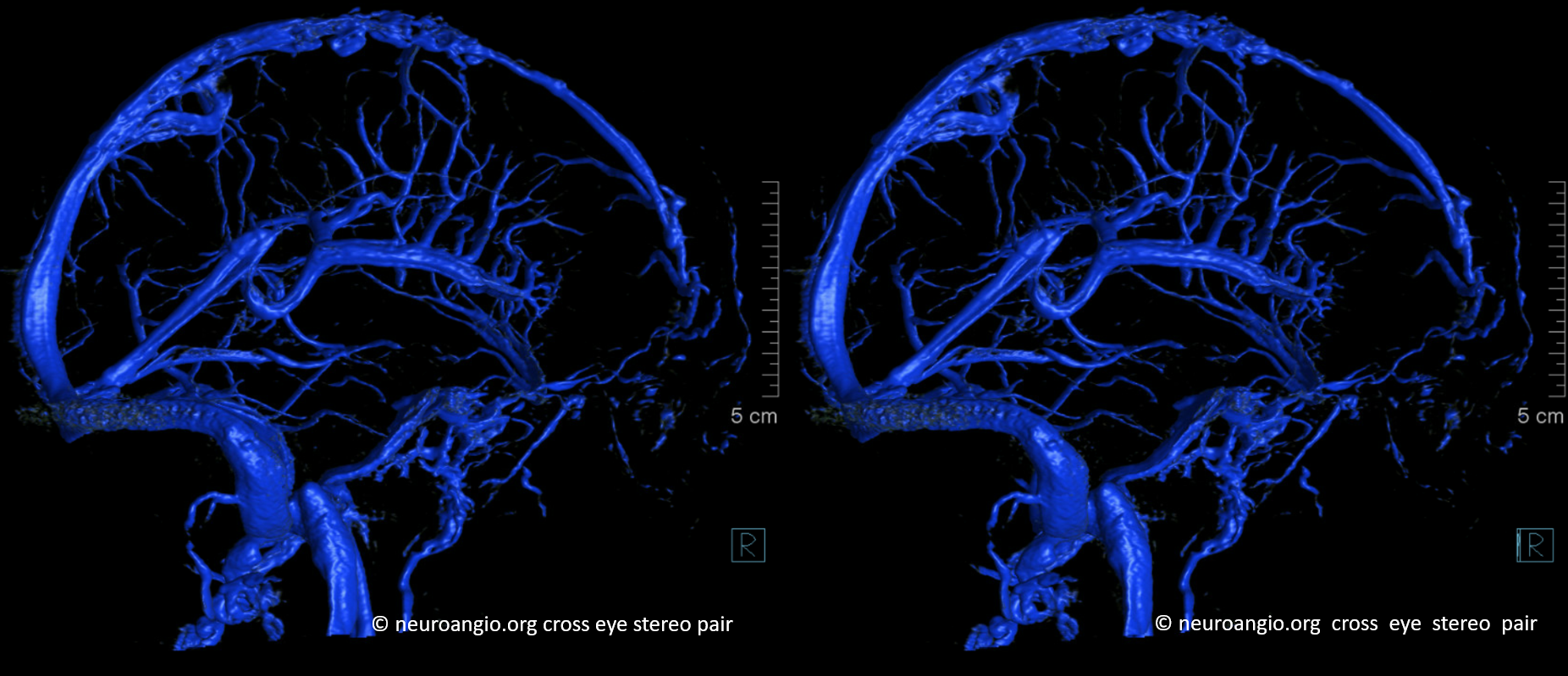
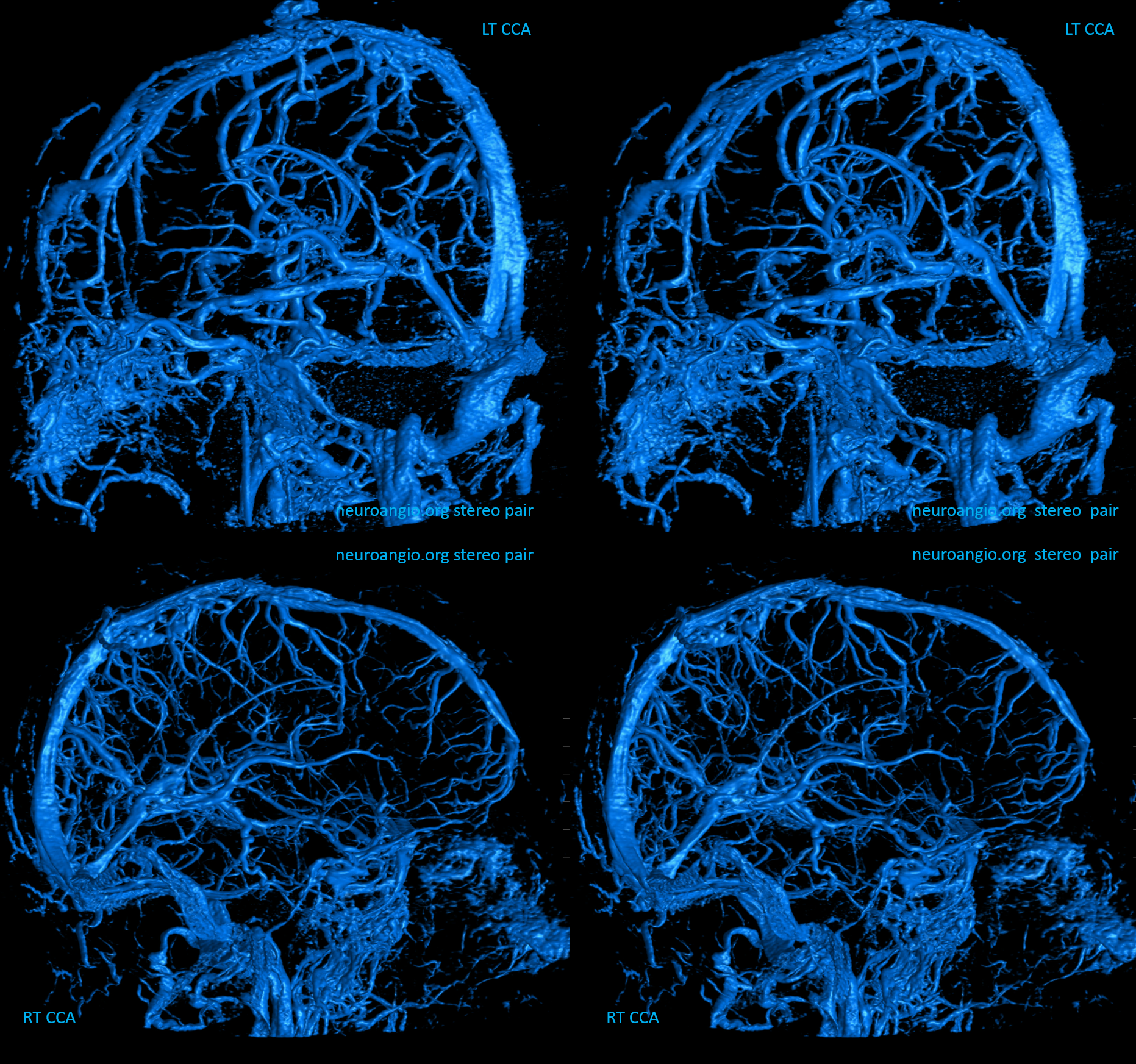
Right hemisphere. Note presence of diploic veins as well
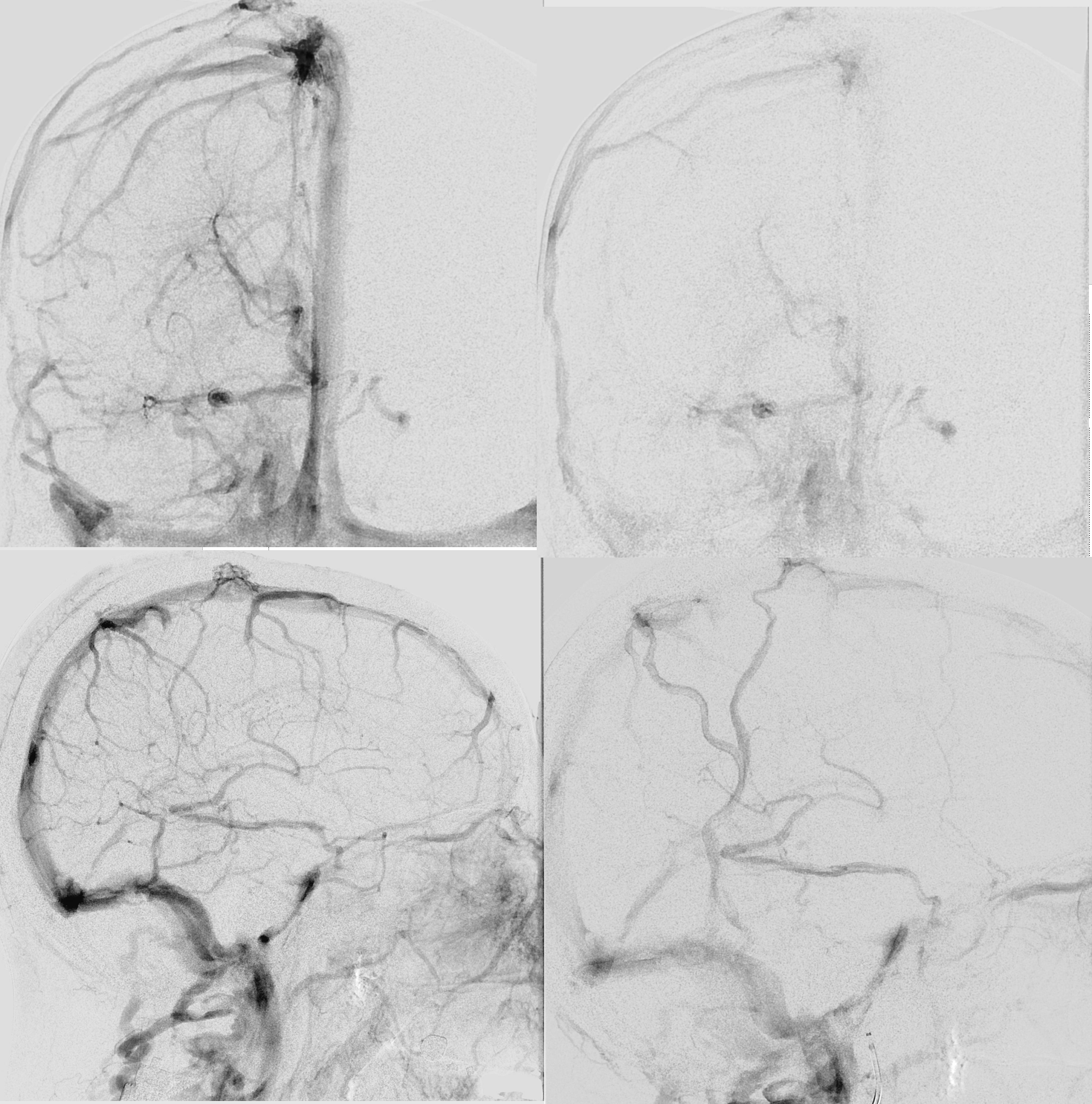
Left hemisphere
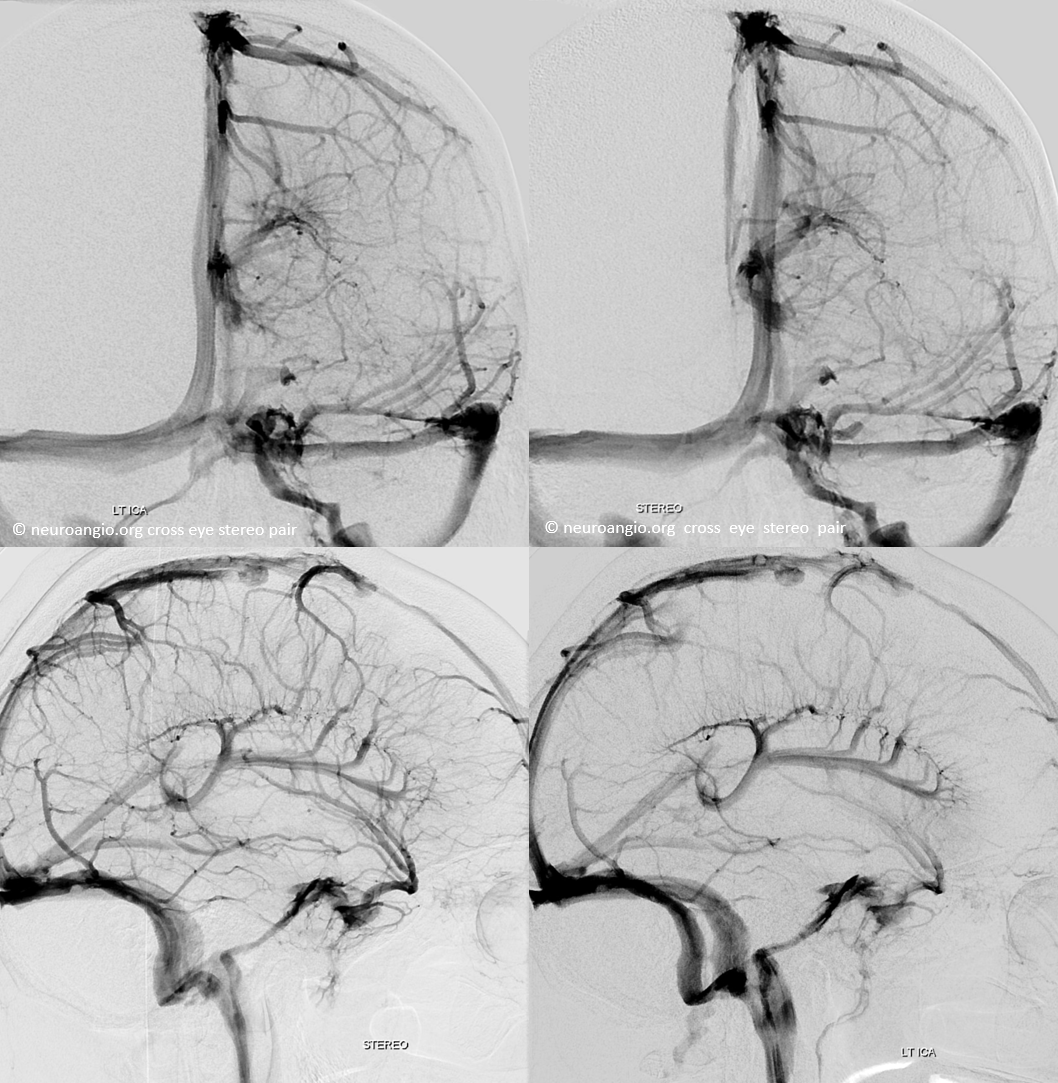
Stereo cross eye
Deeper stereos
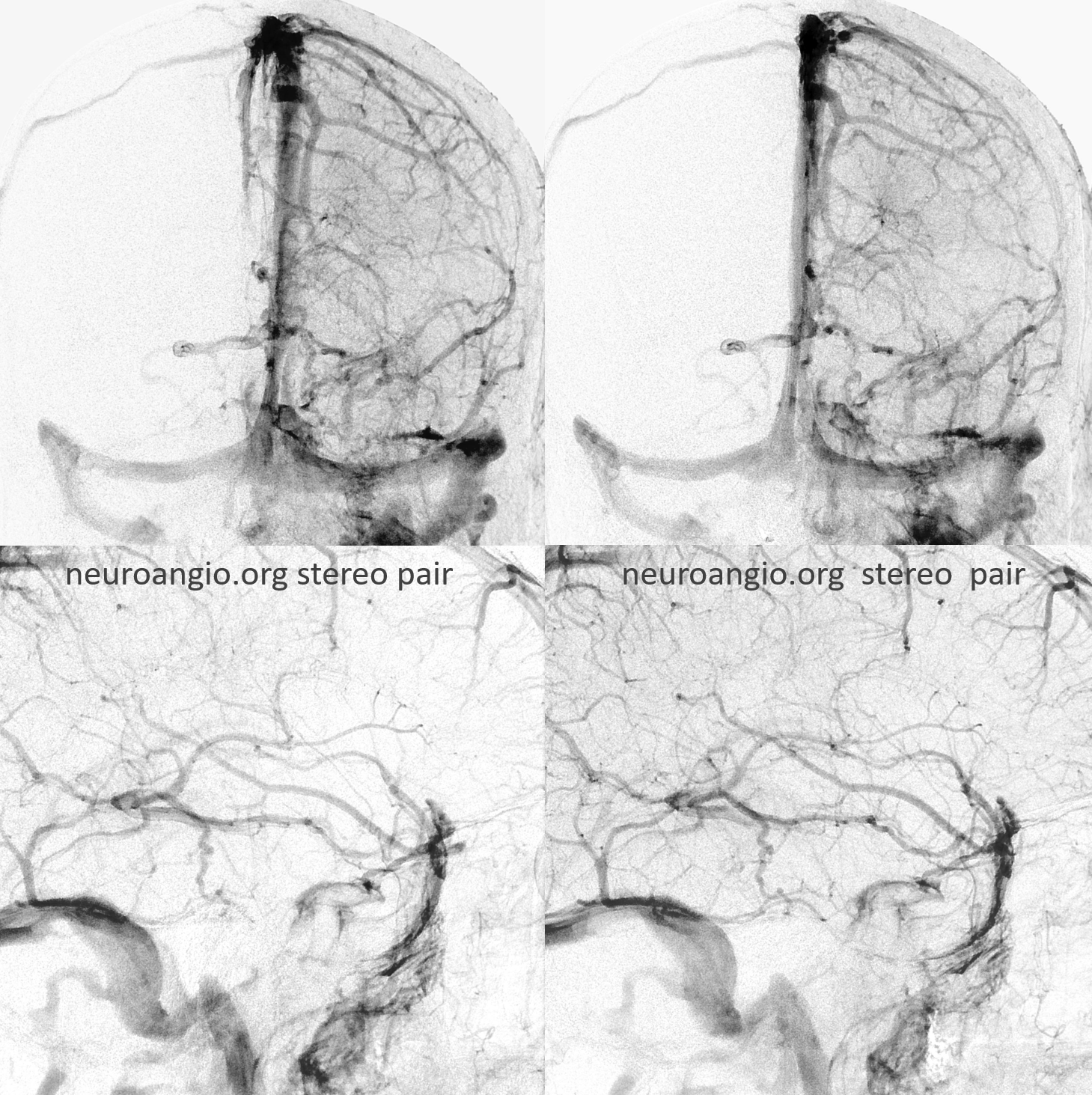
RT Anaglyph
LT Anaglyph — arrow on the tentorial sinus
AVM
Frontal AVM drainage into the internal cerebral vein — demonstrating anterior cerebral vein tributary (runs along anterior cerebral artery) into the anterior portion of the internal cerebral vein.
Light blue = septal vein. Blue = internal cerebral vein. Orange = VOG. Purple=straight sinus. Red = overlap of deep venous structures on the AP view. Yellow = deep sylvian tributaries.
Full length of basal vein in case of temporal AVM
An enlarged basal vein drains prominently into both cavernous and straight sinuses from a deep component of a right temporal AVM. The arterial phase (top) shows AVM supply from MCA, A. Choroidal, and PCA sources. The venous phase, aside from basal vein, shows drainage via enlarged Labbe and parieto-occipital veins. The venous phase of the brain shows congestion and drainage predominantly via cortical channels into the SSS as well as true internal cerebral vein deep drainage both into the VOG and via a trans-cerebral anastomosis to the convexity. The brain continues to utilize the basal vein and frontal vein as well.
Arterial phase. Yellow=Ant. Choroidal, Red=MCA, Orange=PCA
Venous Phase: Dark blue on top and green on bottom =basal vein; light blue=Labbe; red=superficial sylvian vein; yellow=parieto-occipital vein; white=posterior frontal vein; black=venous angle of the deep venous system; orange=trans-cerebral vein connecting the thalamostriate vein to the posterior frontal vein.
Basal vein to tentorial Sinus
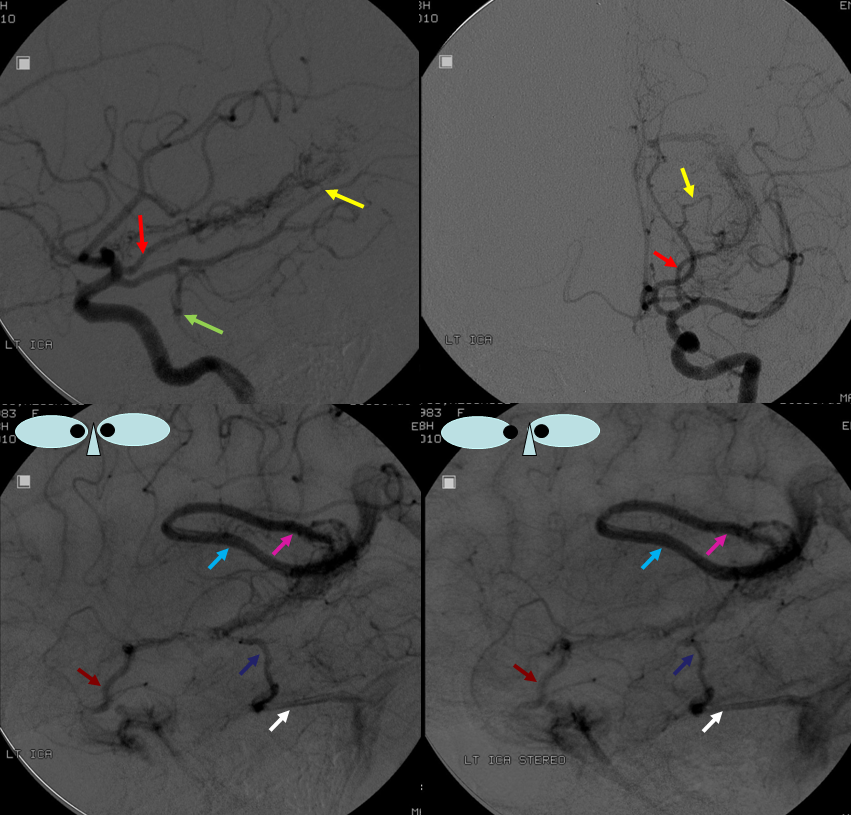
AP stereo view: Blue=thalamostriate vein; light blue=basal vein; yellow=tentorial sinus proximal; pink=tentorial sinus distal
Lateral Stereo View, same patient: dark blue=proximal basal vein; light blue=middle portion, basal vein; yellow=tentorial sinus receiving basal vein; bright green=internal cerebral vein; white=anterior caudate vein; black=direct lateral vein
Interpeduncular Vein
A connection between basal veins in front of the midbrain, also known as the “posterior communicating vein”. Can be seen angiographically from the anterior circulation in high-flow states which overwhelm the ipsilateral basal vein (as in this case), or when ipsilateral vein is diseased. It is much more consistently seen in the venous phase of vertebrobasilar injections (see Veins of the Posterior Fossa)
Interpeduncular vein is shown in white. Basal vein middle and posterior portions (dark blue arrows) outline the midbrain. Anterior portion of the basal vein (light blue arrow) is contiguous with the deep sylvian (a.k.a. middle cerebral ) vein (pink). Large left temporal lobe AVM also produces congestion of the bilateral cavernous sinuses, with good visualization of the intercavernous sinus (yellow arrows) and pterygopalatine venous plexi (green arrows). The carotid artery flow voids are inside blue circle/oval.
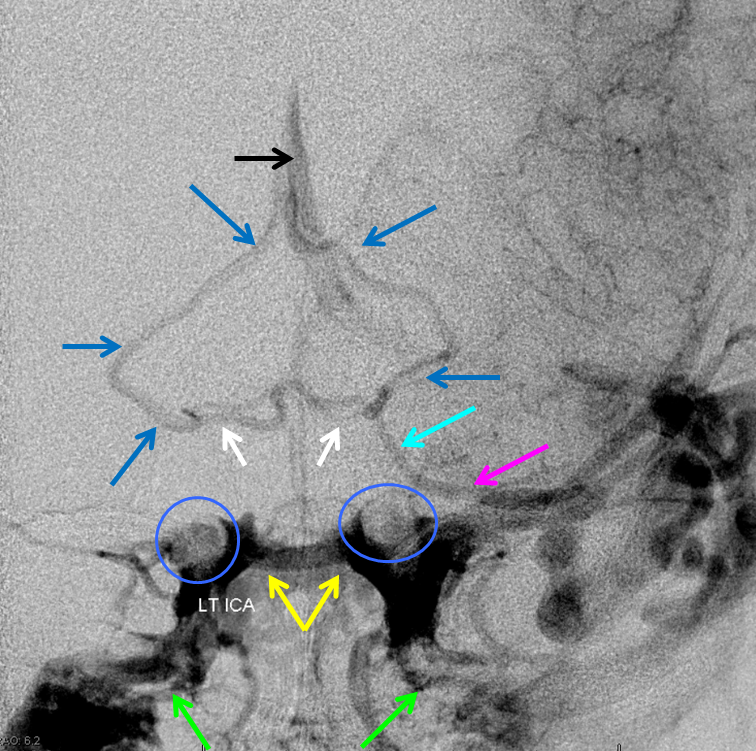
The same case, with arterial phase mask to show relationship with the carotid artery.
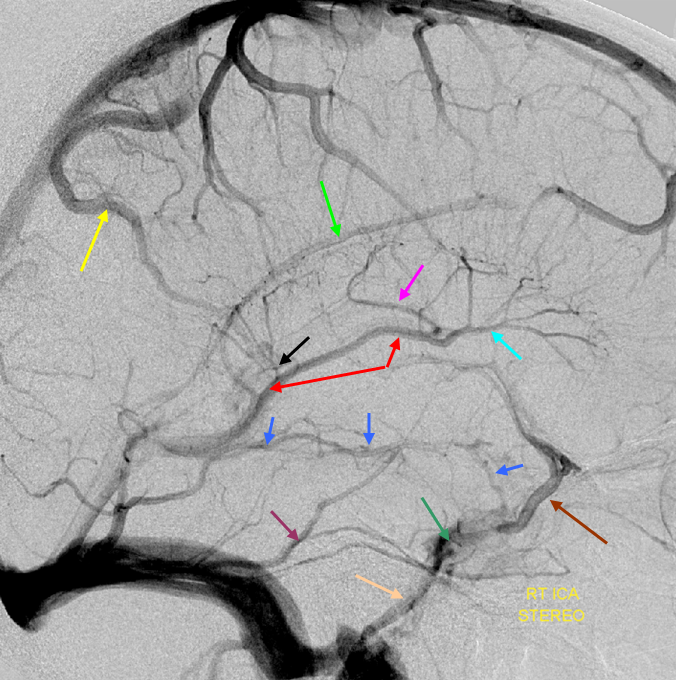
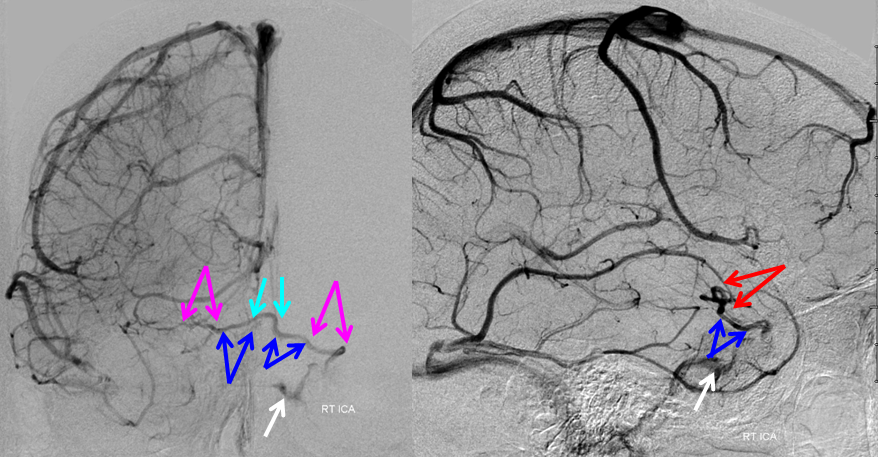
Tributaries of the basal vein
For the most part, this is a straightforward excersize. Whatever brain is adjacent to the basal vein is likely to be drained by it. The anterior portion of the basal vein collects frontal lobe base (olfactory, anterior cerebral, and orbitofrontal veins), inferior parts of the basal ganglia, hypothalamus, optic tracts, uncus and various more or less extensive territories of the temporal lobe base (depending on hemodynamic balance with the superficial sylvian network), such as the uncal and hippocampal veins. The veins which drain the basal ganglia are often referred to as striate veins (corresponding to medial and lateral striate perforators). They look like a candelabra, with the base formed by the anterior segment of the basal vein.
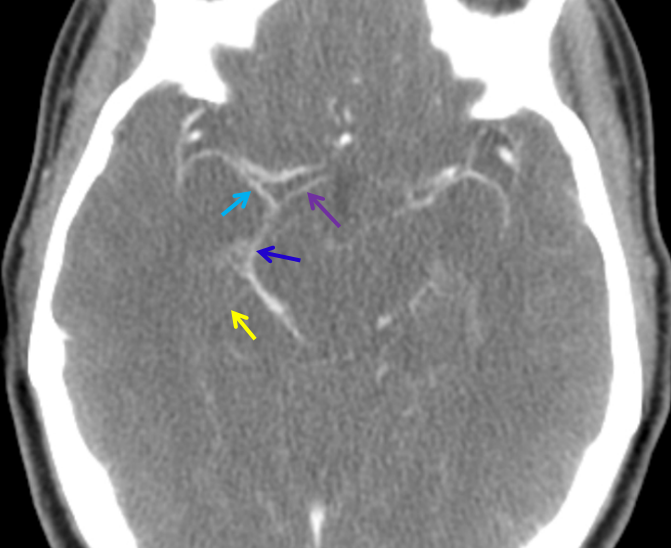
Late phase CT angiogram with anterior (purple) and middle cerebral (a.k.a. deep Sylvian, light blue) vein forming the basal vein (dark blue). A small uncal vein is in yellow.
As above, alternative routes of drainage for these structures include cavernous sinus (via basal vein), or sometimes superficial sylvian network when basal vein is hypoplastic. The middle and posterior portions of the basal vein can collect the inferior ventricular vein which drains the temporal choroid plexus, and portions of the temporal lobe as well as territory of the cerebral peduncles, hypothalamus, collicular plate, and sometimes receives veins of the cerebellum (which more commonly will drain into the Galen).
The two basal veins are usually interconnected via a bridging vein located anterior to the midbrain, within the interpeduncular cistern, and therefore called interpeduncular vein (a.k.a. posterior communicating vein). The anterior pontomesencephalic vein is connected to this vein from below (see Veins of Posterior Fossa section)
Basal vein involvment in high-flow pathologies such as AVMs or CC fistulas can have profound consequences. Venous hypertension in its drainage territory affects important brain structures (such as temporal lobe) and may lead to seizures or signficiant focal deficit, apart from risk of rupture.
THE Internal Cerebral Vein
This is truly the deep venous system. Located in the velum interpositum (right above the third ventricle) this vein collects a whole bunch of territory, including basal ganglia, thalamus, and lots of deep white matter. As always, a bit of embryology: in the very early embryo the brain drains “centrifugally”, or from inside out to the surface, like the spinal cord. Somewhat later, as the choroid plexus grows (week 5 gestational age or so), a new system develops to drain the plexus. It is still not the definitive arrangement, consisting of a single midline vein collecting tributaties of the rudimentary lateral and third ventricle plexus. Most of this vein regresses, except for the most caudal portion which some (Paget, Lasjaunias) believe to persist as the Vein of Galen. This theory allows one to state that pathologic persistence of this midline vein to a greater extent than normal results in the vein of Galen malformation, as is supported by absence of internal cerebral veins (and of straight sinus) in this infamous condition.
The adult internal cerebral vein collects a very large amount of territory. Classically its tributaries are divided into medial and lateral groups. The smaller medial group drains septum pellucidum (and some mesial frontal regions) and fornices (not much). The lateral group is much more impressive. Anteriorly there is the lateral caudate vein, followed more posteriorly by the thalamostriate vein. This vein (which does not drain the thalamus, but certainly supports the corpus striatum) is in itself an important collector. The longitudinal caudate veins are small veins on the lateral aspect of caudate body that collect tributaries from the deep white matter. Only 1-2 cm of cerebral white matter is drained by cortical veins, so that most of centrum semi-ovale and corona radiata is collected into tributaries of the internal cerebral vein. Frontal matter collects usually into longitudinal caudate veins (see below) which then form the thalamostriate vein. Posterior white matter tends to drain into the back part of the vein near the atrium of lateral ventricles via “lateral atrial veins.” The direct lateral vein (next in line posteriorly from the thalamostriate vein) drains parts of the thalamus. This is followed by the above-mentioned lateral atrial veins. Variation is the rule. Just look at what’s coming into the internal cerebral vein and you can tell what it’s draining. Remeber the classic “venous angle” — the thalamostriate vein and anterior septal vein come together near the foramen of Monro (except when they don’t, and the thalamostriate vein empties posterior to Monro, forming the “false venous angle”). Just looking at the deep venous structures on every angio (especially in stereo) will go a long way towards getting you comfortable with the anatomy, uncertain or not. Below I will be putting some examples soon…
Relatively prominent posterior septal vein (yellow). The thalamostriate vein is also on the larger side (green) with a hypoplastic inferior sagittal sinus. Notice a larger size inferior ventricular vein (pink) emptying into the basal vein, draining a posterior temporal lobe tumor. Red=superior petrosal sinus.
Periatrial Vein Draining an AVM
Red=PCA; Light blue=periatrial vein; Dark blue=internal cerebral vein; Orange=VOG; Yellow=Thalamostriate Vein; White=Straight Sinus. Four images on the bottom are from a dynamic MRA with 2 second temporal resolution — TWIST (Siemens acronym for this sequence in this case; TRICKS is a similar sequence from GE)
Venous drainage of the lateral choroidal plexus. Illustrated beautifully by an AVM confined to the lateral choroidal plexus, the drainage is shared by internal cerebral and basal vein tributaries. The arterial supply is via posterior lateral and anterior choroidal arteries.
Anterior Choroidal – Posterior Lateral Choroidal anastomosis — the anterior choroidal beyond the plexal point supplies the plexus of the temporal horn, where it is in balance with the posterolateral choroidal going to the atrium region. This is elegantly shown in the following case of left choroidal plexus AVM, supplied by both vessels with beautiful illustration of draining veins.
The Vein of Galen — images of the classic Galen can be found elsewhere… no time for now.
Transcerebral (medullary) Venous network
The white mater of the cerebrum drains primarily into the deep venous system — mainly longitudinal caudate veins and into the internal cerebral vein. Parietal white matter collects usually towards the lateral atrial veins and into the back of internal cerebral veins. These veins are best seen on susceptibility-weighted MRI imaging and not that consistently on angiography. This system illustrates important embryologic concepts and is useful in understanding of the developmental venous anomalies — which essentially represent enlargement of one of these transcerebral veins into a larger than normal collector (caput medusa) of white matter drainage.
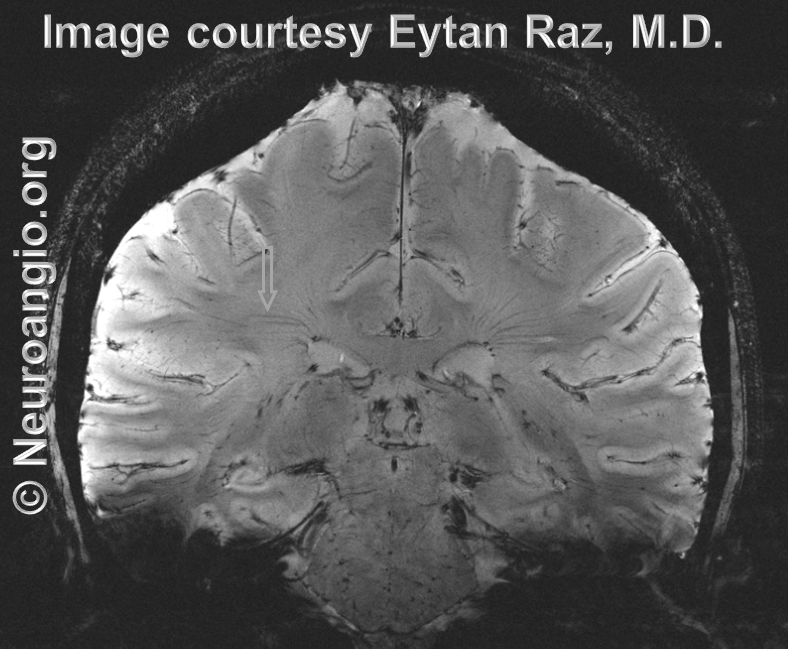
Normal transmedullary veins can be seen exceptionally well in high-field MRI, especially 7T, where images are truly stunning:.
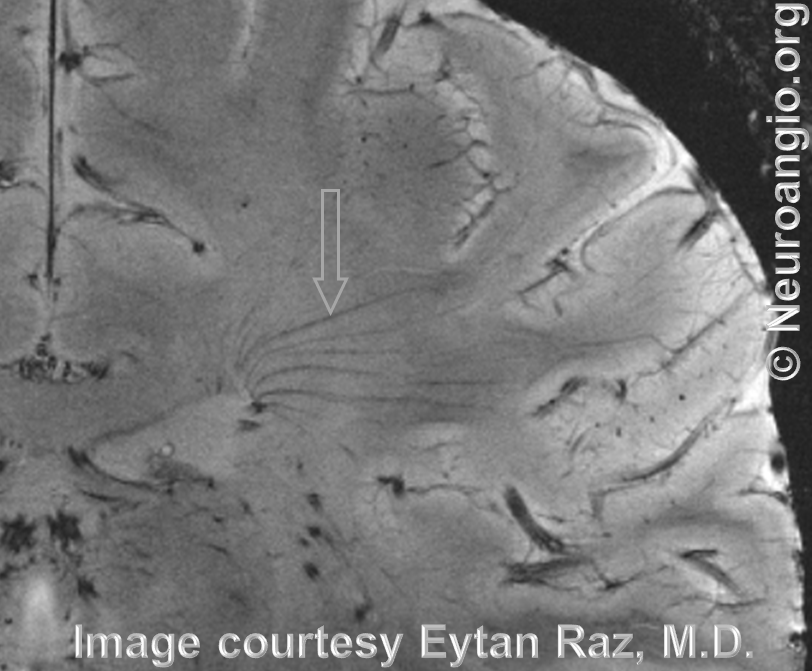
How amazing are things we can see now. And it will only get better.
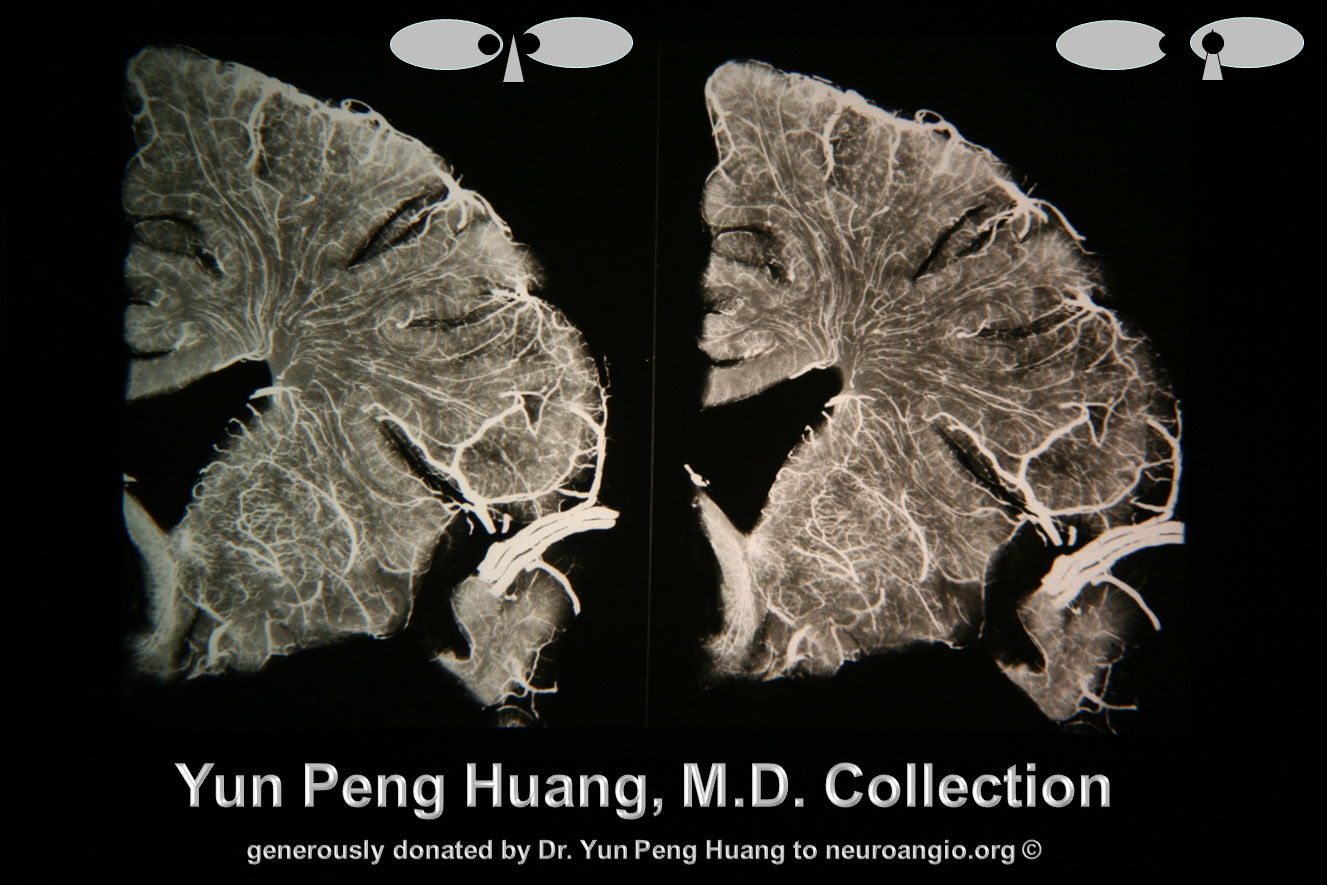
This quality of imaging is starting to rival anatomical specimens; from the collection of the great Yun Peng Huang, M.D., the first neuroangiographer to systematically study the venous system, particularly the medullary veins and their relationship to what was then generally called the “medullary venous malformation”, today better known as the Developmental Venous Anomaly, or DVA. Here are two specimens from his extensive collection, generously donated to neuroangio.org.
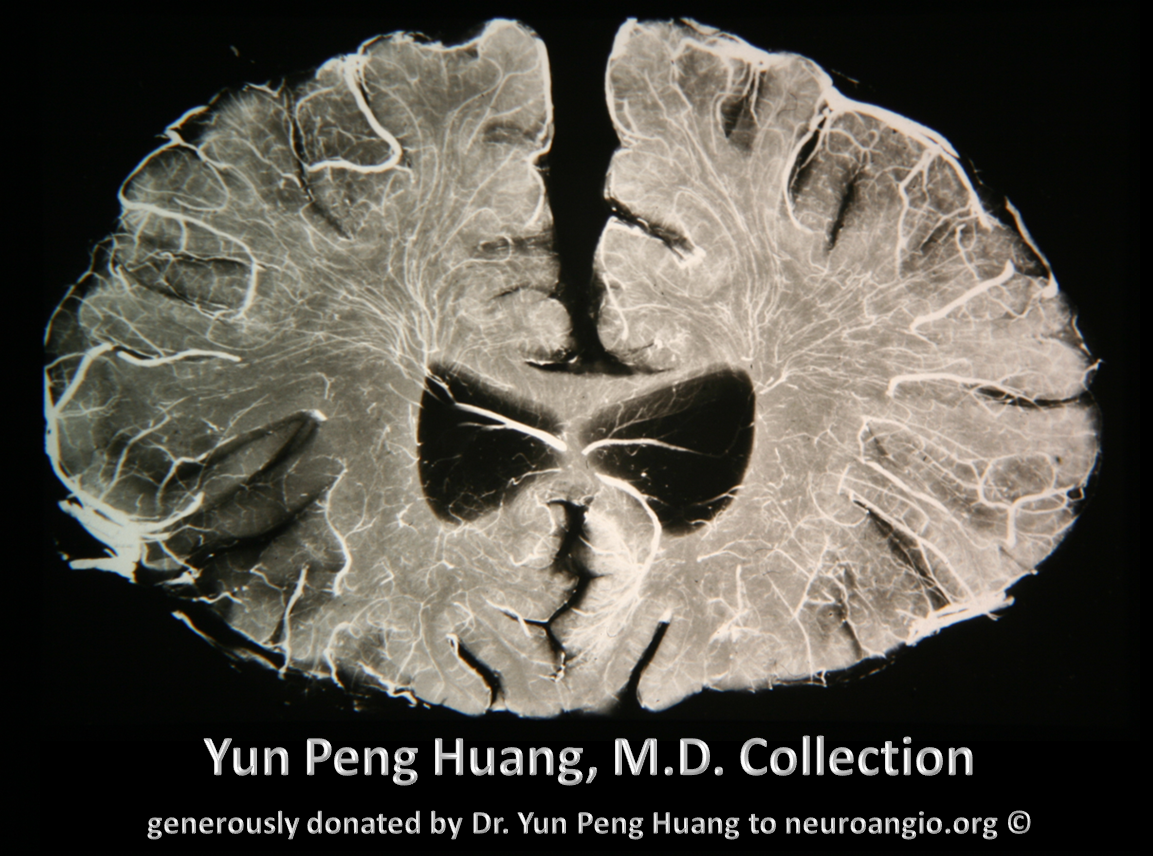
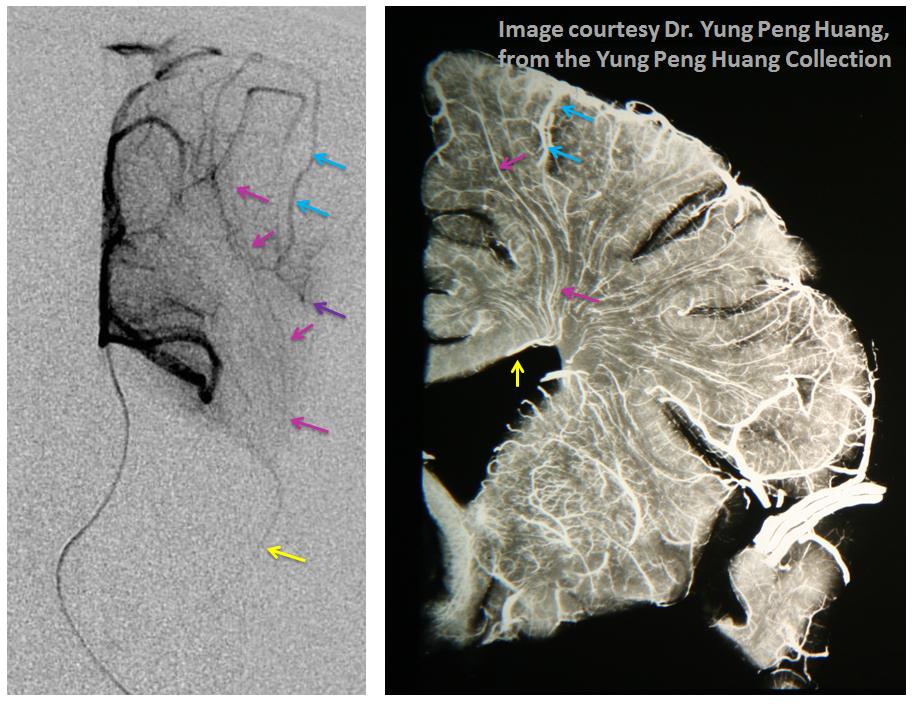
Here is the angiographic correlate to Dr. Huang’s vision, with a microcatheter injection of the distal pericalossal artery (left) and a specimen (different brain, right). The medullary veins (pink) are collected into the thalamostriate vein (yellow). The larger sulcal veins (blue) collect the territory of the cortex (purple)
Developmental Venous Anomaly
DVAs are relatively common — their incidence is directly proportional to the quality of your MRI machine and thinness of your sequence slices. 3T MRI detects DVAs in at least 25% of people. They are almost never seen on CT, unless huge enough to produce a detectable density difference with the surrounding brain. They will be as dense as venous sinuses. DVAs illustrate the above-described embryologic concept of the trans-cerebral venous sytem. In early embryology our brains drained centrifugally, that is from inside out. As the choroid plexus grew, a new central or deep venous system developed which eventually matures to drain into the vein of Galen. This system remains connected to brain surface veins by small trans-cerebral veins which run through the white matter and used to drain the entire deep brain. In the adult they drain the deep white matter. In a DVA situation, instead of small wispy veins collecting cerebral white matter blood towards the deep venous system, the DVA itself becomes a collector of neighboring venous territory and empties either into superficial or deep venous networks. It drains normal brain and represents a “do not touch” lesion. Mistakenly removing one will lead to venous infarction. If associated with AVMs, the DVA can make surgical approach impossibly risky. On MRI, they have a characteristic “caput medusa” apperance of its collectors fusing into the DVA. Although usually incidental, there are many documented instances of hemorrhages and venous infarctions associated with DVAs, not all of which can be waived away by assumption that a neighboring associated AVM or cavernous angioma was responsible for hemorrhage.
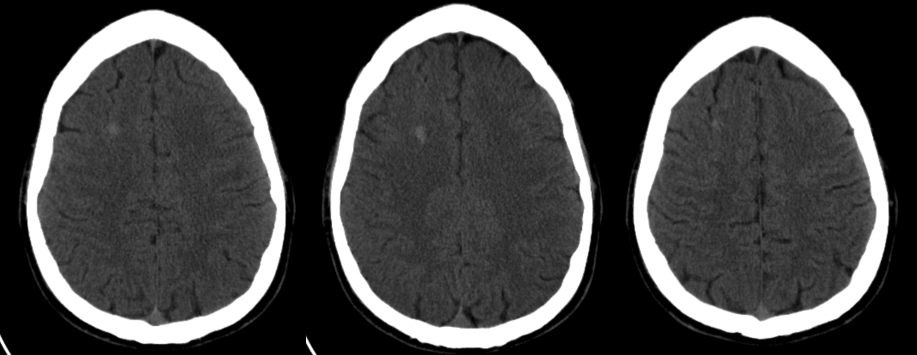
CT demonstration of a large frontal AVM in a diabetic patient presenting with hypoglycemic seizure but with right-sided brain semiology.
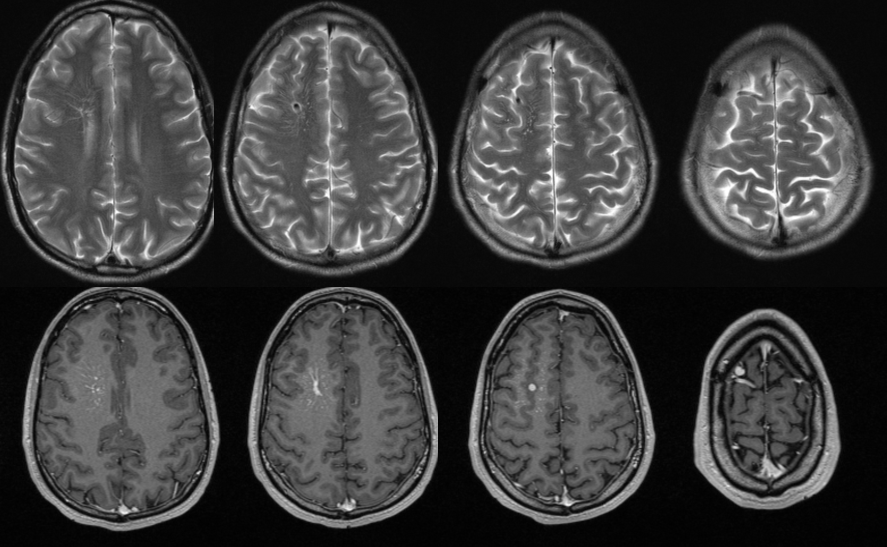
Same DVA on MRI. The surrounding brain is MRI-normal. Caput medusa nicely seen on bottom left T1-weighted postcontral images.
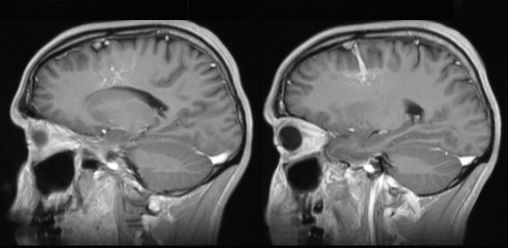
Same DVA on reformatted sagittal MRI.

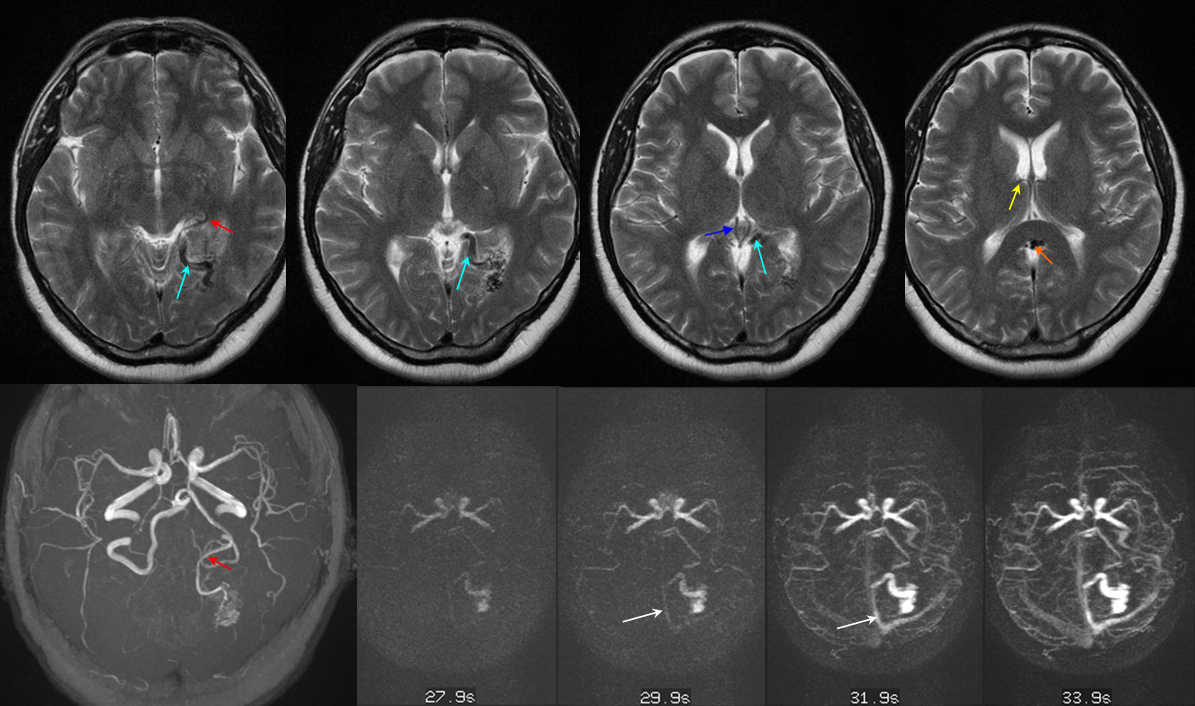
DVA presenting with pseudo-venous infarction in its drainage territory
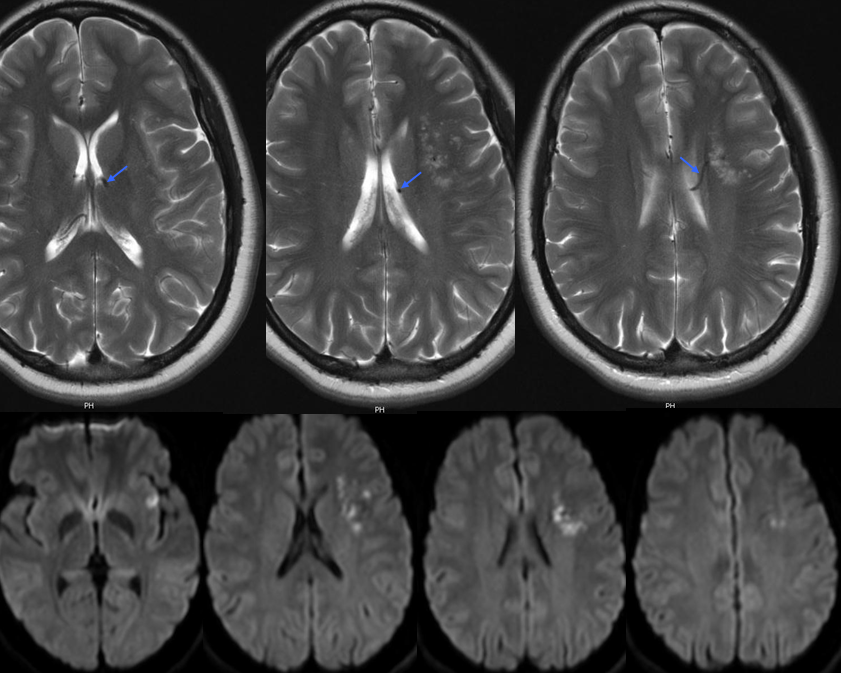
DVA in a patient with hemiparkinsonism — again demonstrating that DVAs are not always benign, this patient presented with unilateral Parkinsonian symptoms that never progressed to involve the right side.
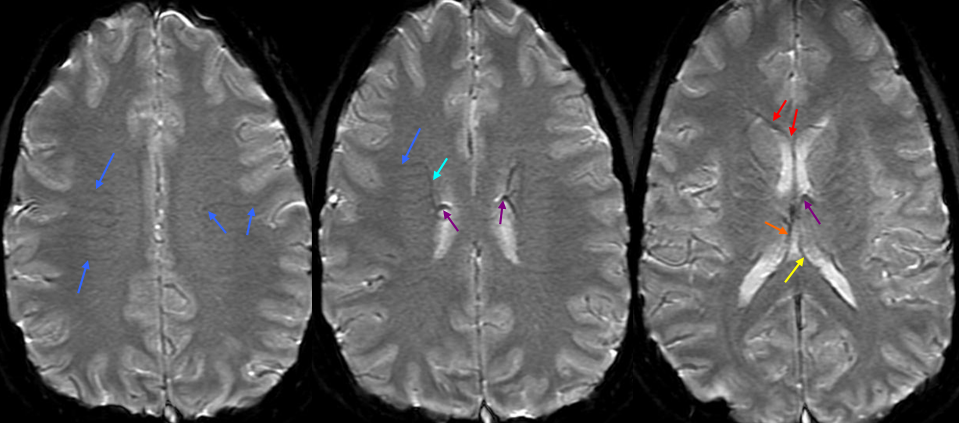
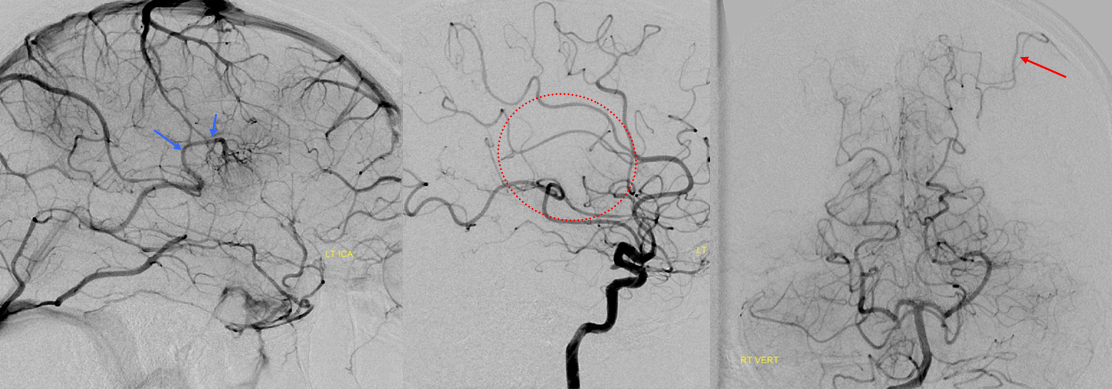
2-D TOF (top left). Sagittal post-contrast MRI (top middle and right). Late (bottom right) and super-late (bottom left) venous phase images of a catheter angiogram. MRI demonstrates a parietal hemorrhage (green, additional sequences confirm hemorrhagic nature). Notice that while the anterior frontal and occipital lobes of the bottom right angiogram are in venous phase, the posterior frontal and parietal lobe drainage is delayed (green arrows). No SSS is visible. Numerous trans-cerebral veins (dark blue), normally invisible, have enlarged to provide alternative dranage of cortex into the internal cerebral vein (purple). The Trolard (brown), previously draining towards the thrombosed sagittal sinus (because it is larger in caliber near the top) now drains inferiorly into a parietooccipital vein and into the sigmoid sinus. The sylvian network (light blue) is prominent, maximizing its cortical territory. Notice also transosseosus emissary veins (red) draining the brain into scalp veins (orange) on the delayed venous phase bottom left image
Applications:
Sturge-Weber Syndrome, a.k.a. Encephalotrigeminal Angiomatosis
SWS is part of a family of neurocutaneous disorders, characterized by abnormalities of ecto- and endoderm. The cutaneous part is a port wine stain (in CN V distribution). The brain part is described below. About 1/3 of patients only have the skin part, 1/3 have only brain, and 1/3 both — no rocket science here. The cause is unknown. There are many sources of excellent information on the Web, but I am afraid that most individuals do not appreciate the underlying vascular issues of SWS in the brain, which is illustrated angiographically below.
Cerebral manifestations of SWS appear to stem from abnormal development of the venous system — particularly surface veins. The halmark of venous imaging is absence of superficial veins (including basal vein, which is only deep as far as the surgeon is concerned, being in fact a superficial vein on the undersurface). As a result, venous drainage is impaired, with secondary tremendous venous congestion. The congestion leads to engorgement of countless small veins thoroughout the parenchyma, and particularly on the gyral surface. This produces the characteristic gyral enhancement seen on MRI, and venous congestion that is better appreciated angiographically. The characteristic gyral calcifications probably result from chronic venous congestion.
This is a young patient with intractable seizures.
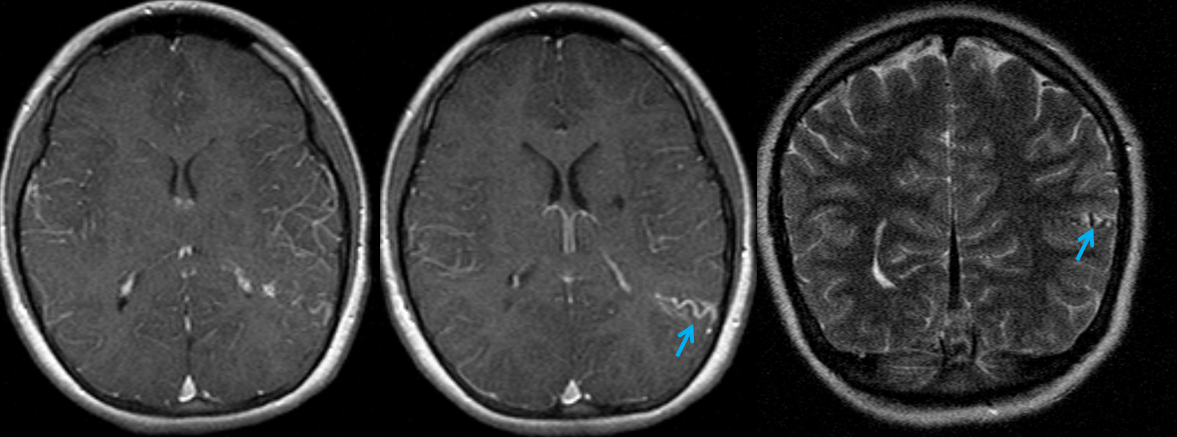
x-ray views of the skull, showing extensive gyral calcifications on background of cererbral atrophy.
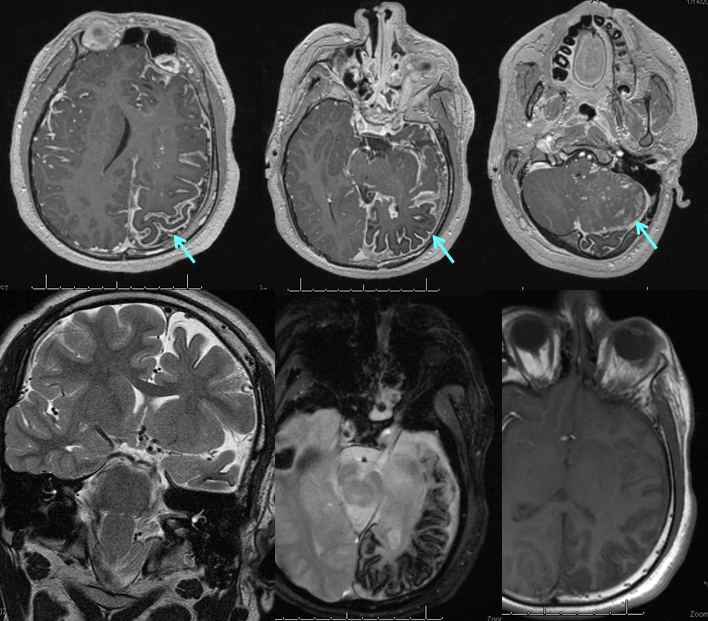
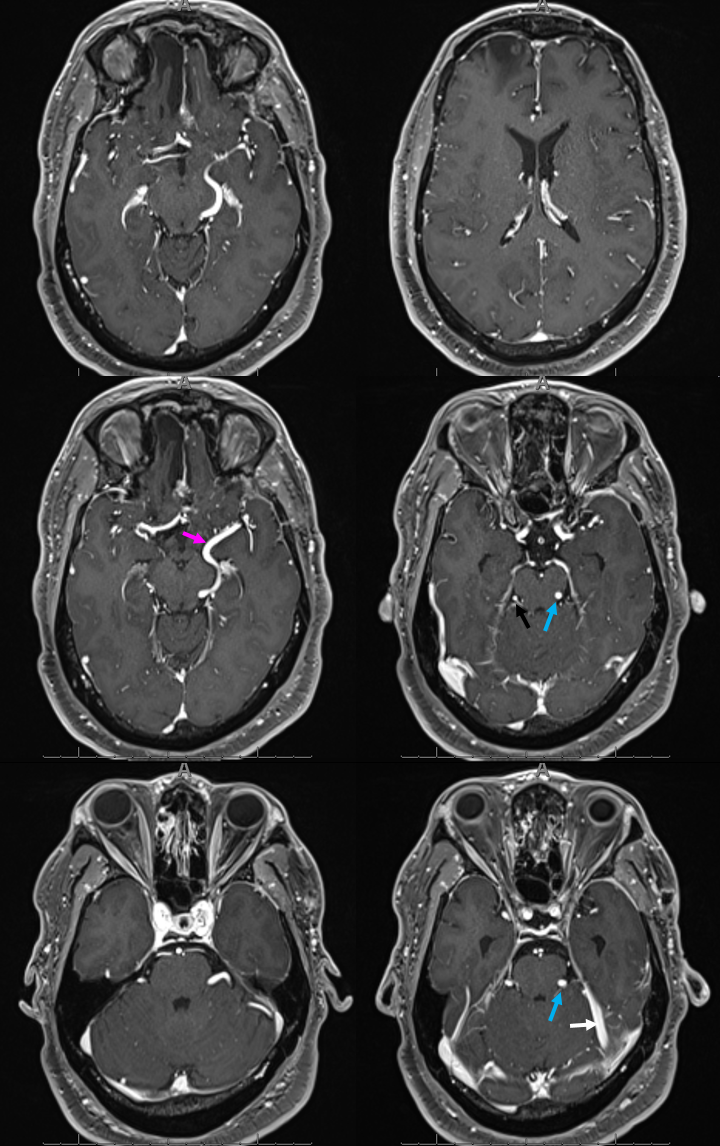
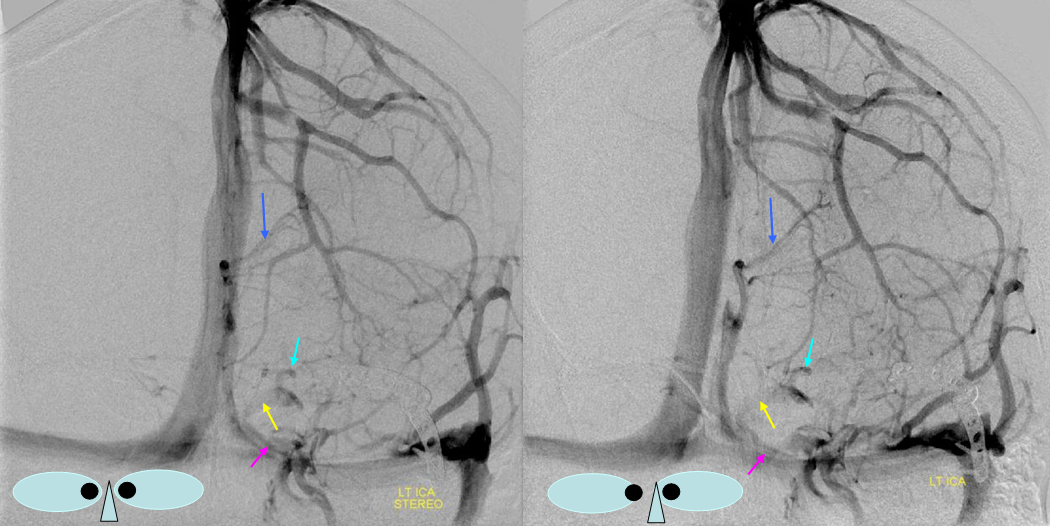
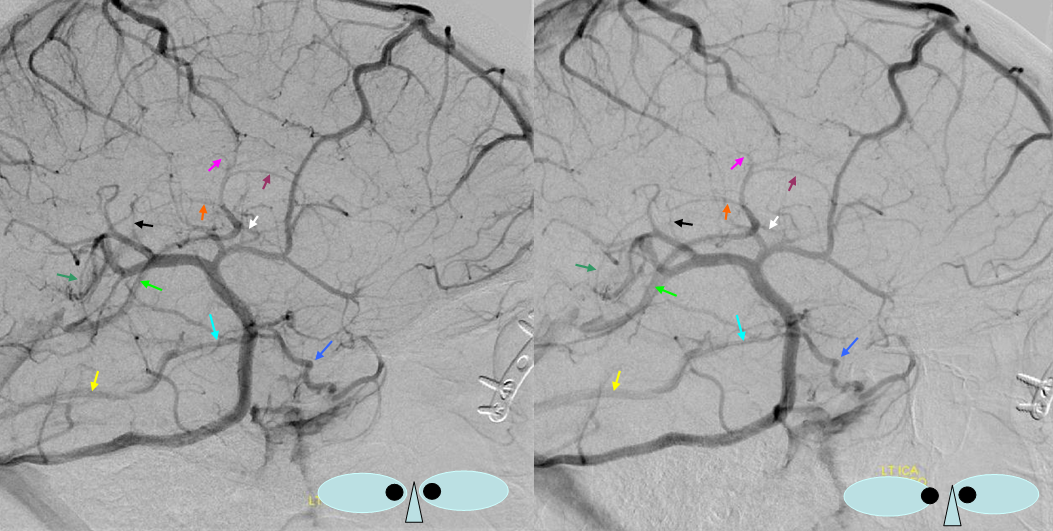
Various MRI sequences highlighting atrophy, calcifications, and gyral pattern of enhancement (top row). Notice absence of left basal vein, and most surface convexity veins.
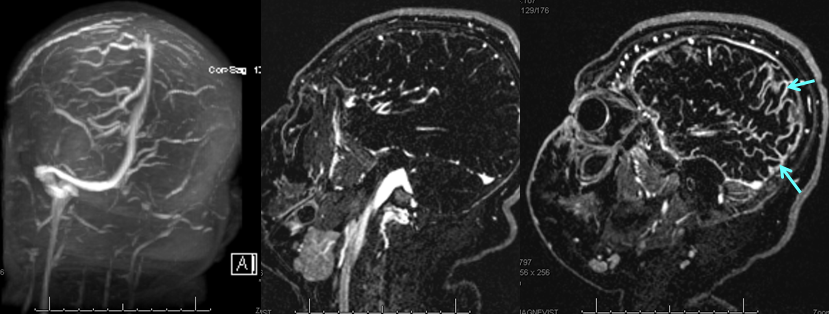
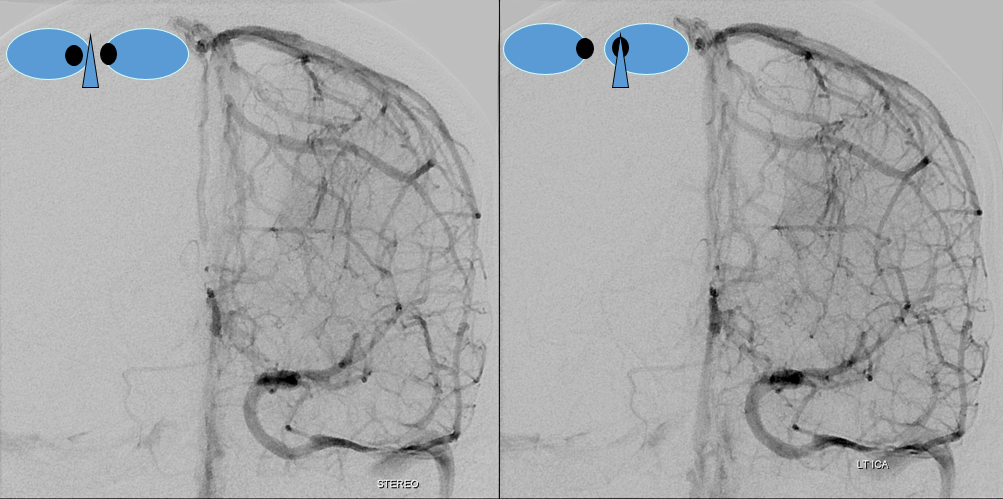
TOF and venovibe images; almost normal venous pattern on the right, and gyral enhancement (arrows) as well as transosseous venous channels on the left.
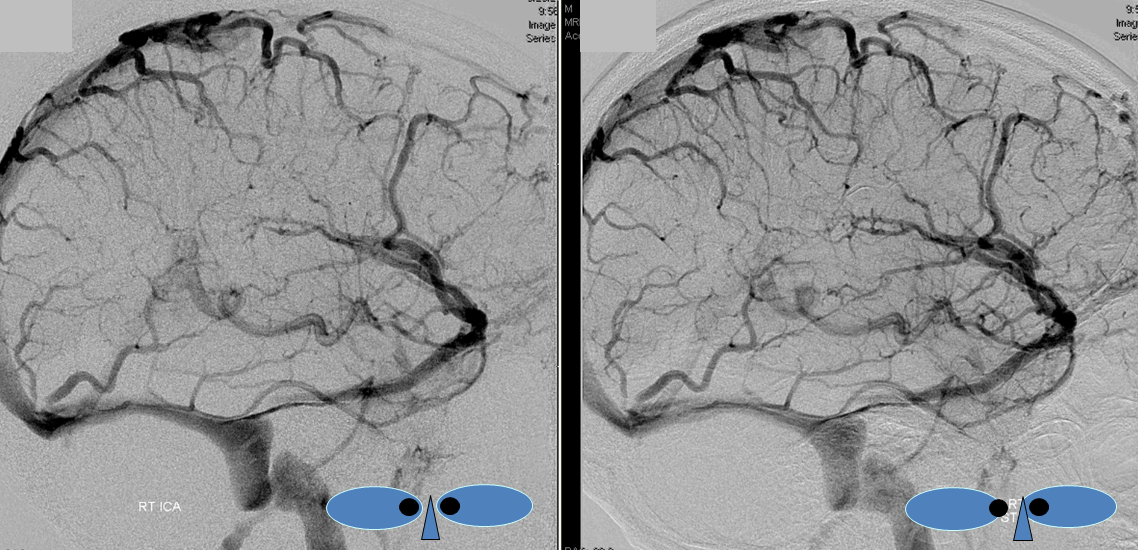
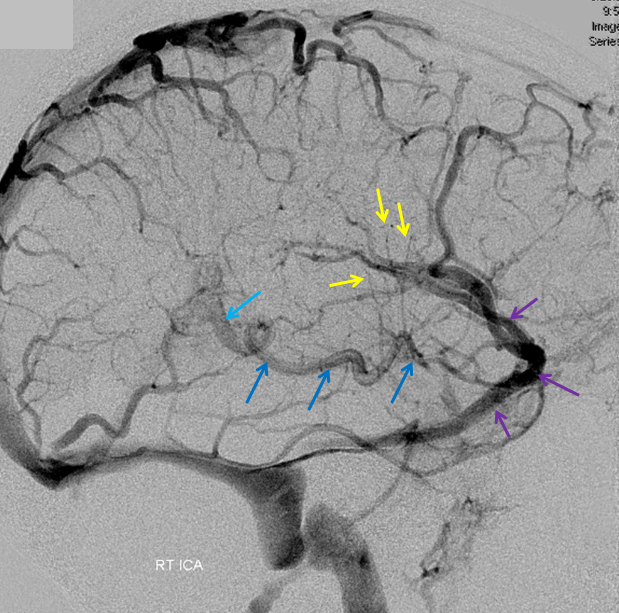
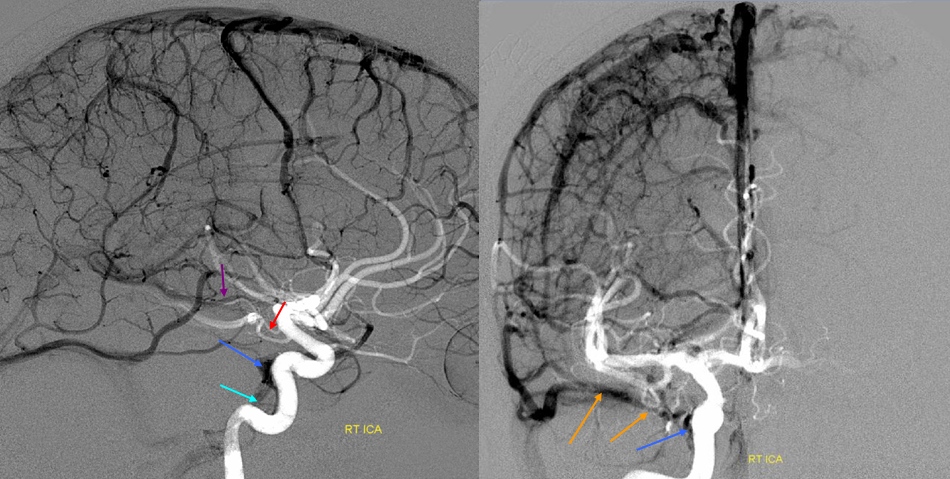
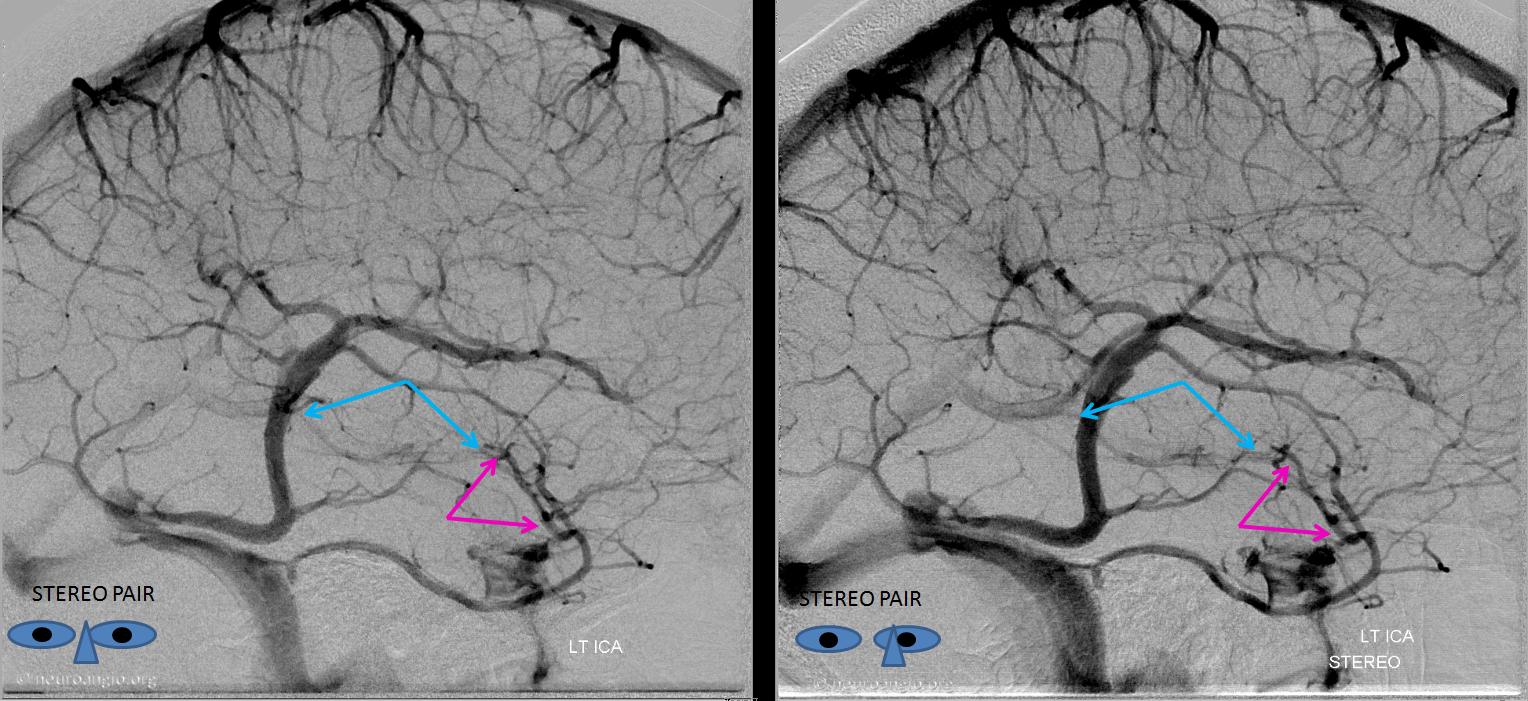
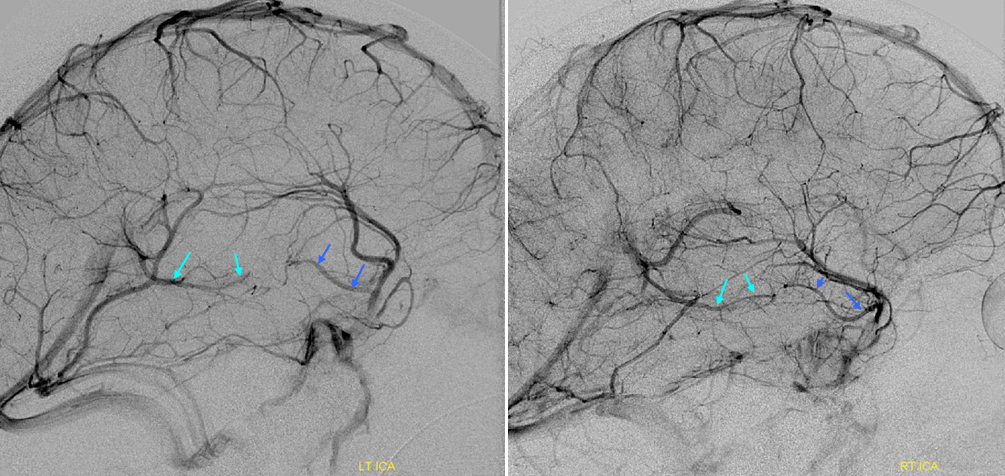
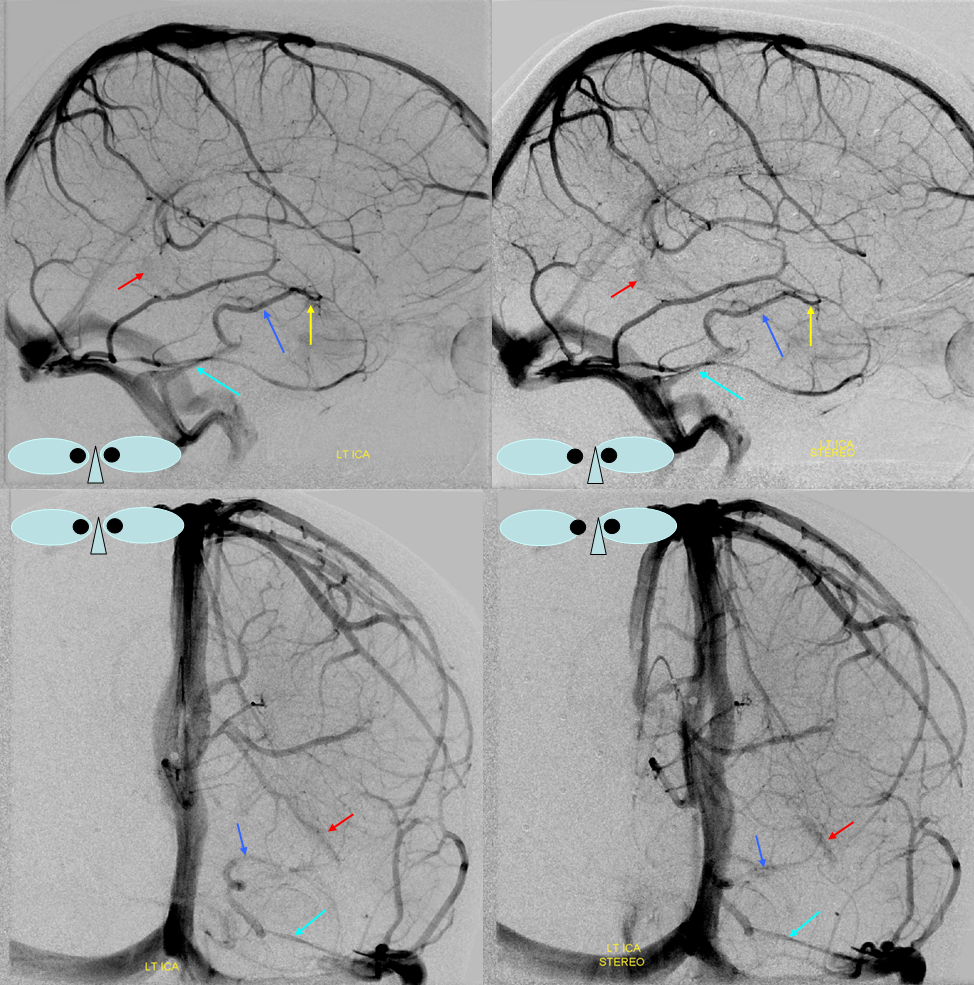
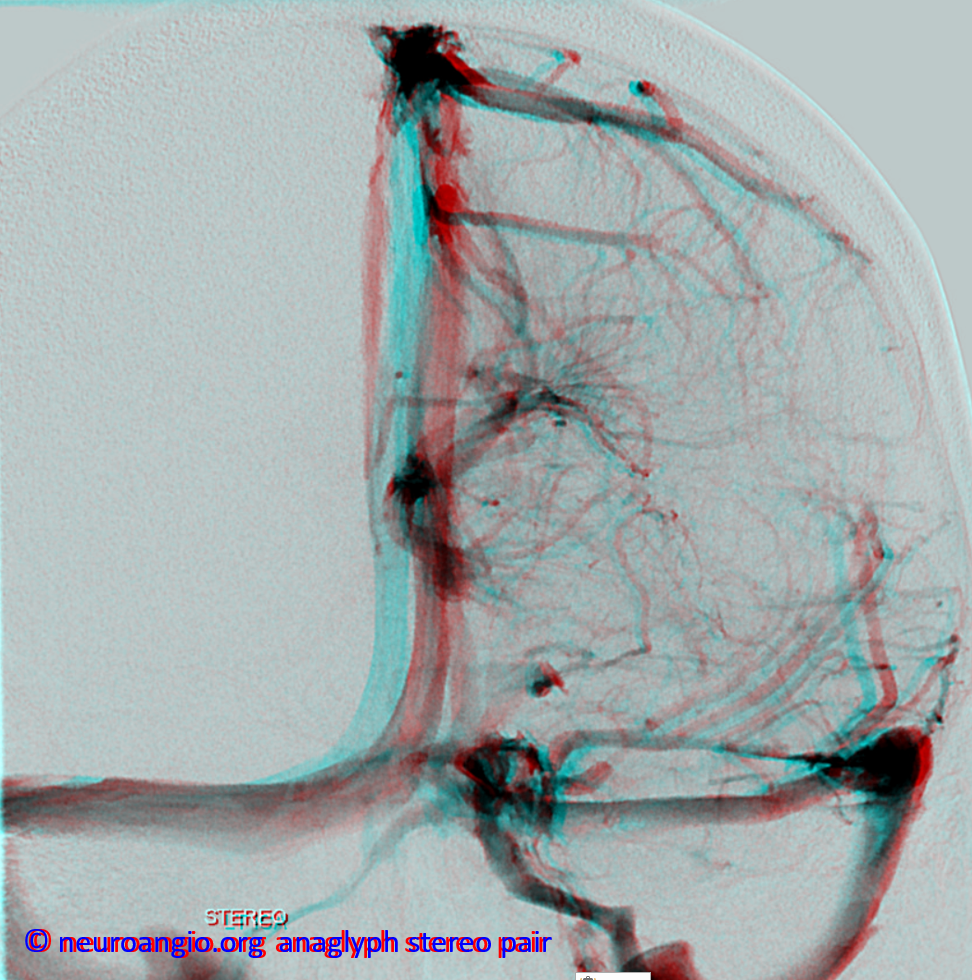
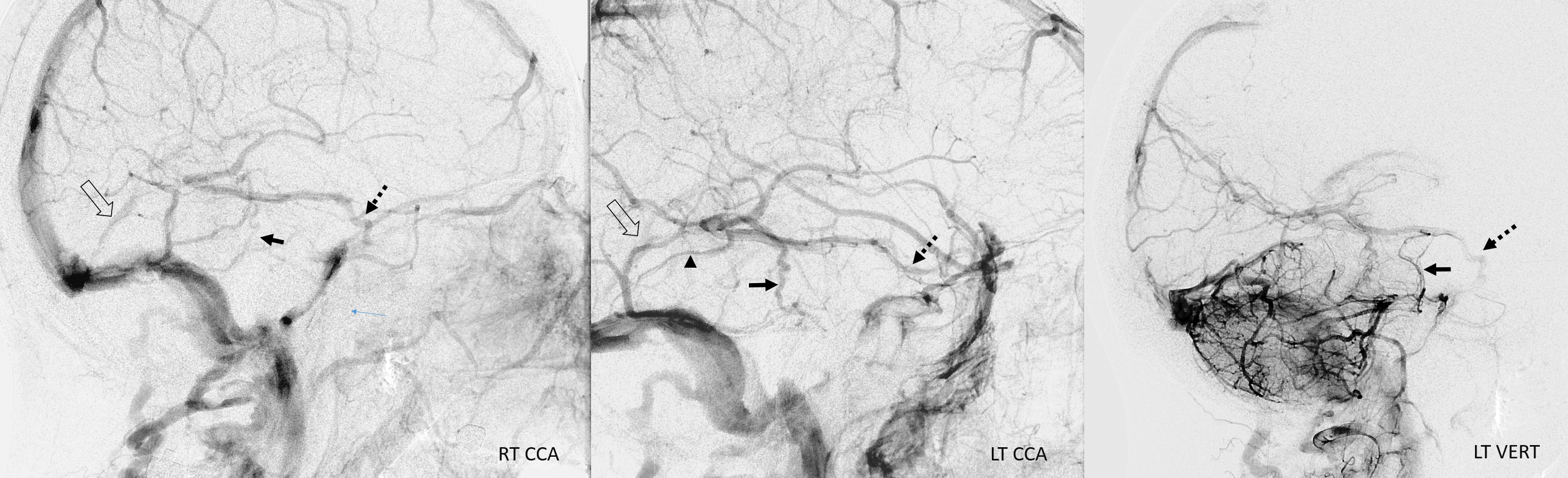
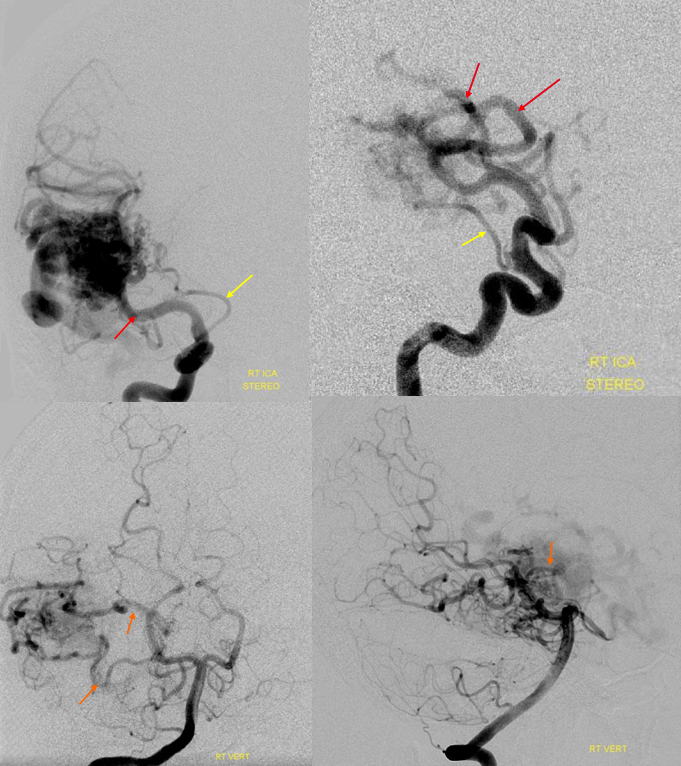
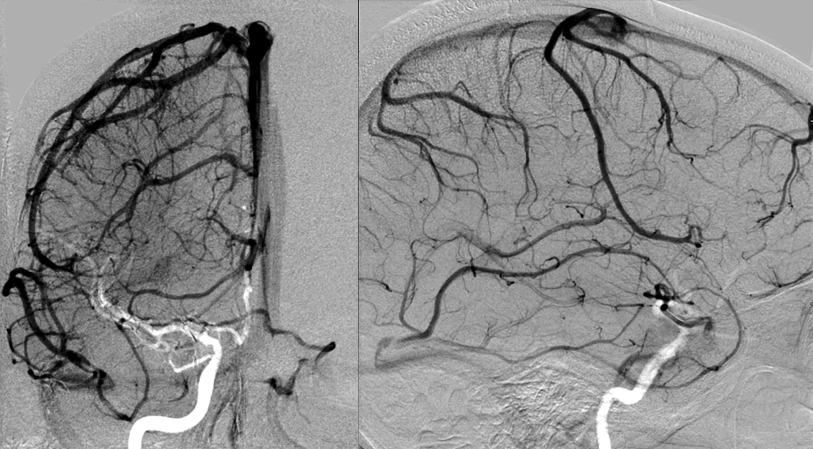
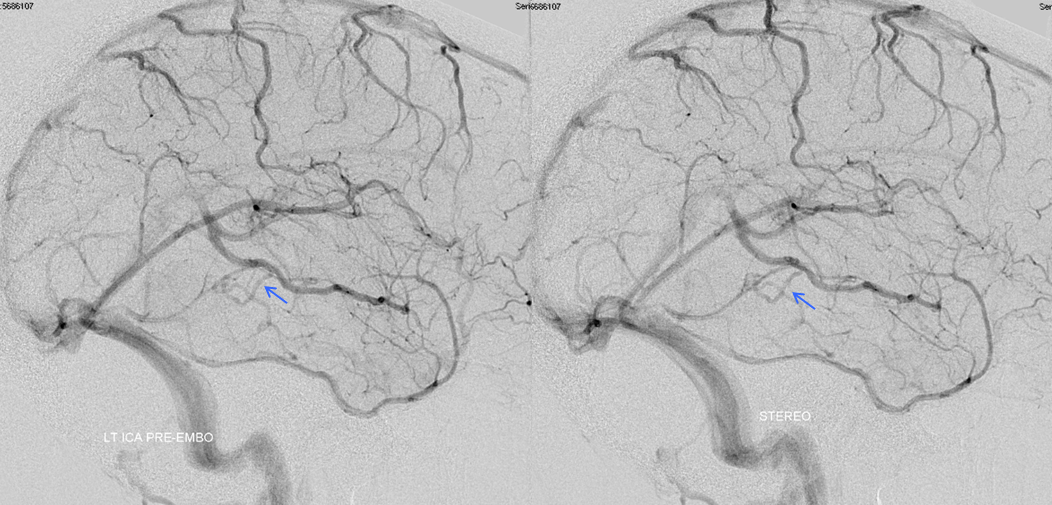
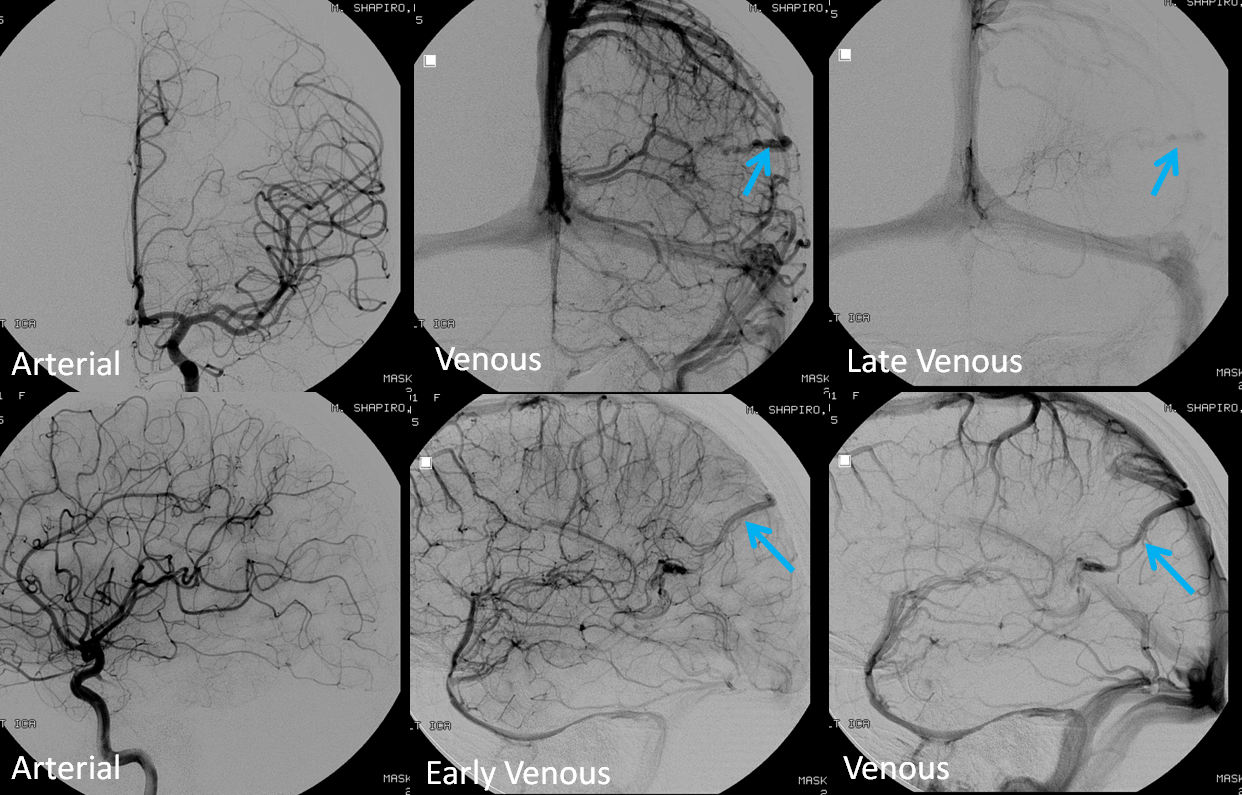
Stereoscopic views (top) and labeled image (bottom) of quasi-normal right ICA injection; convexity surface veins are present. A tremendous, tortuous basal vein (blue arrows) is working overtime. Some of its lenticulostirate tributaries, pathologically enlarged (why?) are labeled yellow. The convexity drainage is dominated by superficial sylvian veins (purple). What vein is missing?
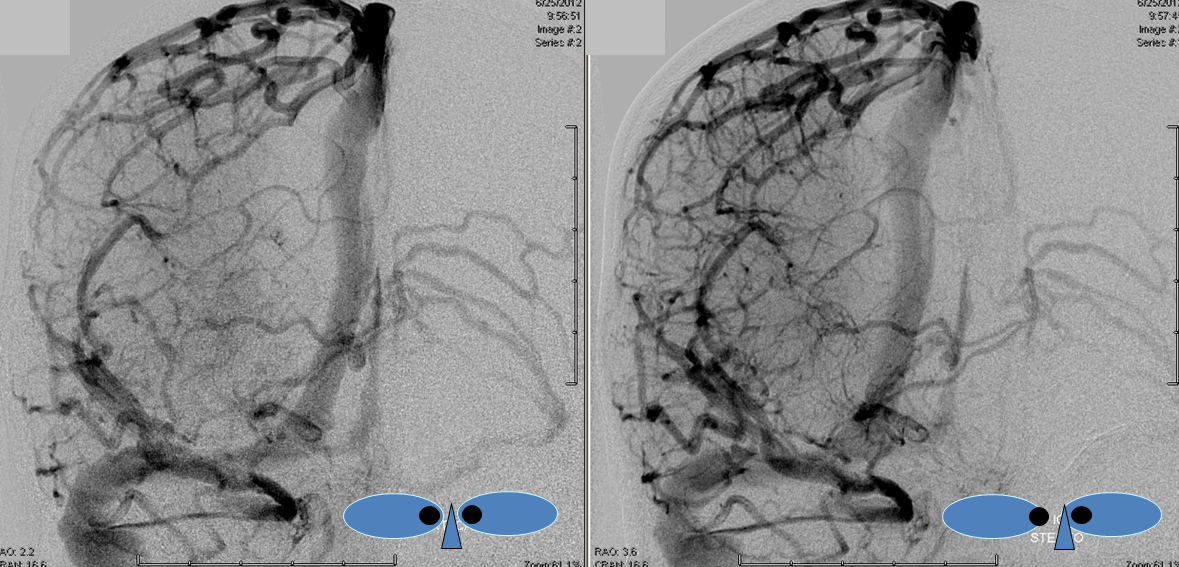
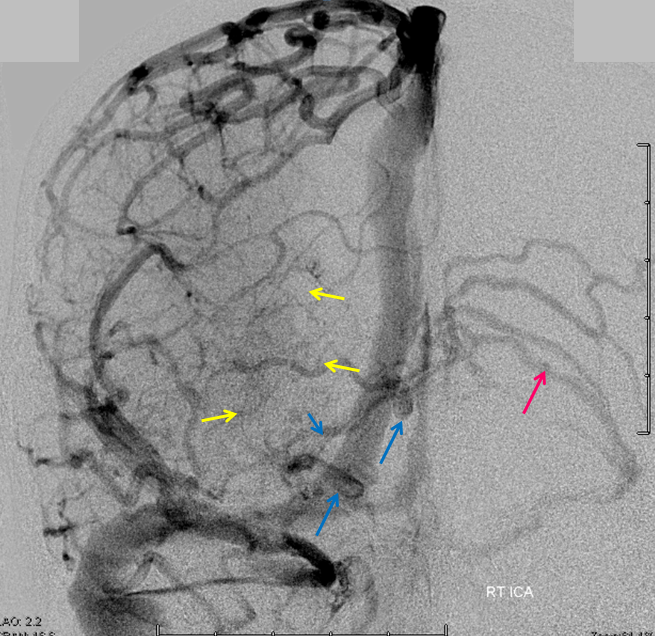
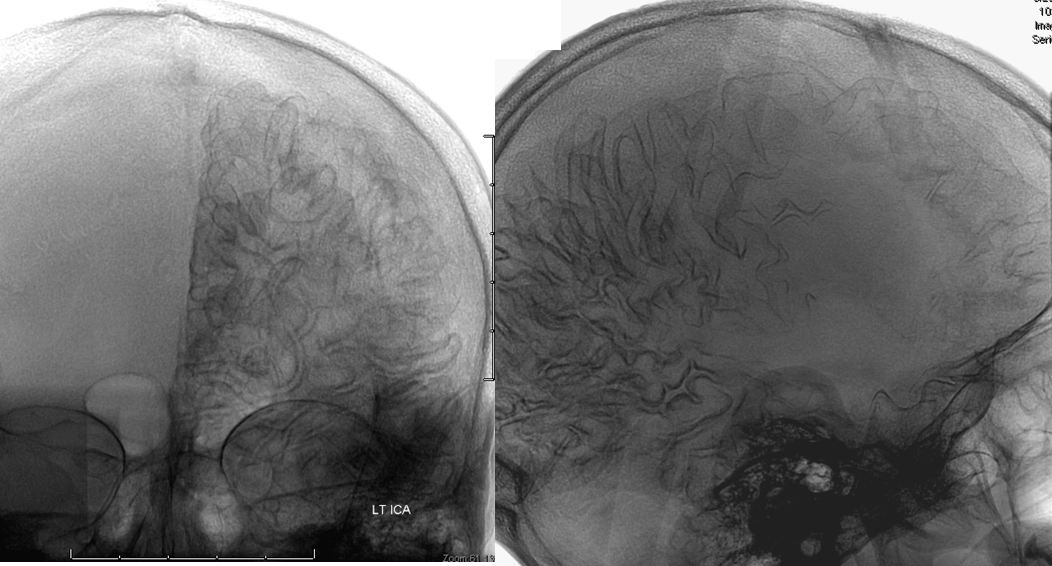
This is a lateral view of the abnormal hemishere, left ICA injection, in late venous phase. The image is characterized by near-complete absence of surface veins and tremendous venous congestion. Appreciate how gyral calcifications, seen on the x-ray image above, are greatest over the areas with least superficial venous drainage. Over the frontal lobe, where several surface veins (pink) have developed, the calcifications are less pronounced. The basal vein is labeled in blue. Why is the basal vein seen?
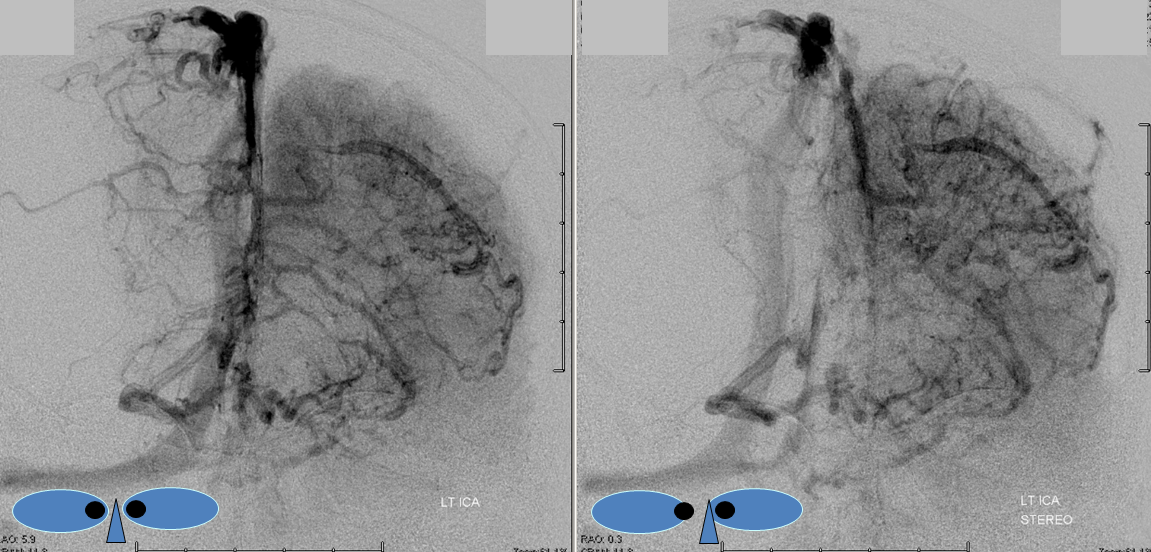
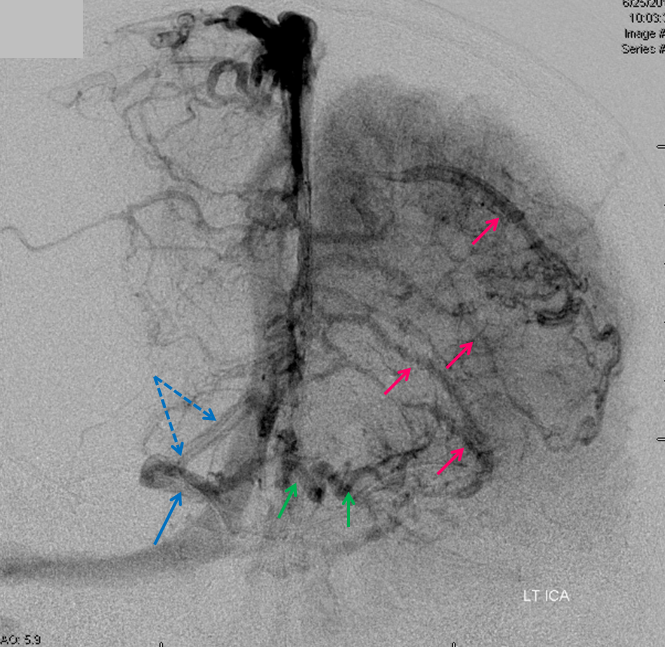
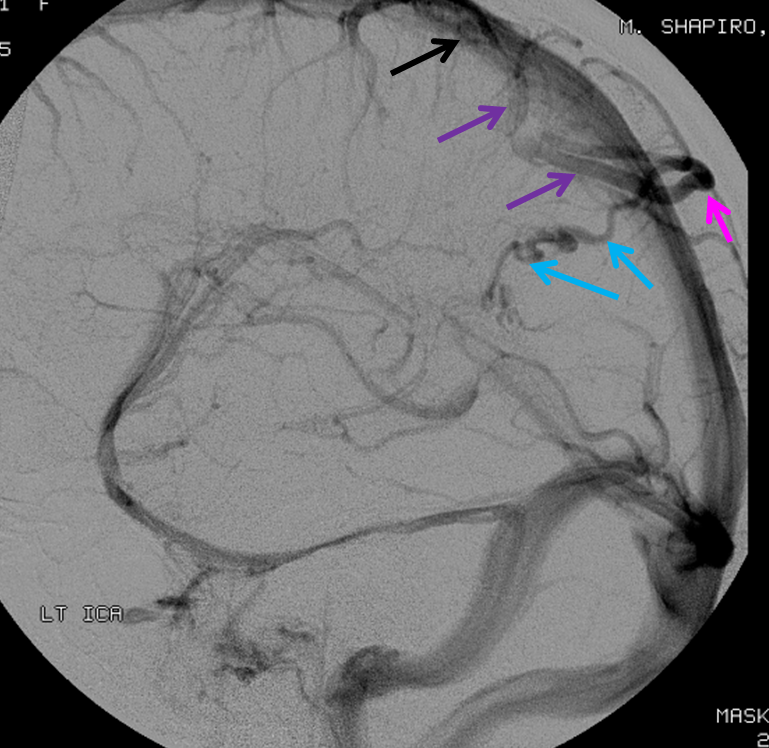
AP views of left ICA injection. The basal vein seen on the lateral study belongs on the right side. It is draining both hemispheres, hense its large size. Left-sided tributaries (green arrows) reach the right side accross the posterior communcating vein (interpeduncular vein) which connects the two basal veins in front of the brainstem.
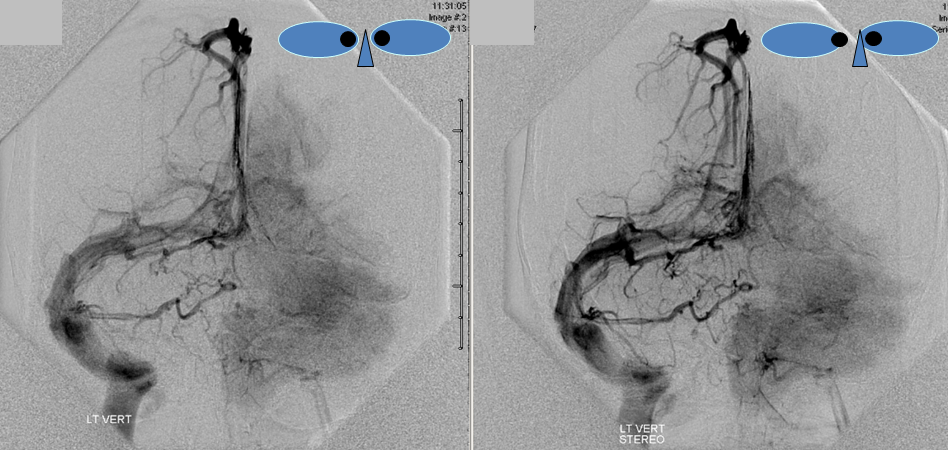
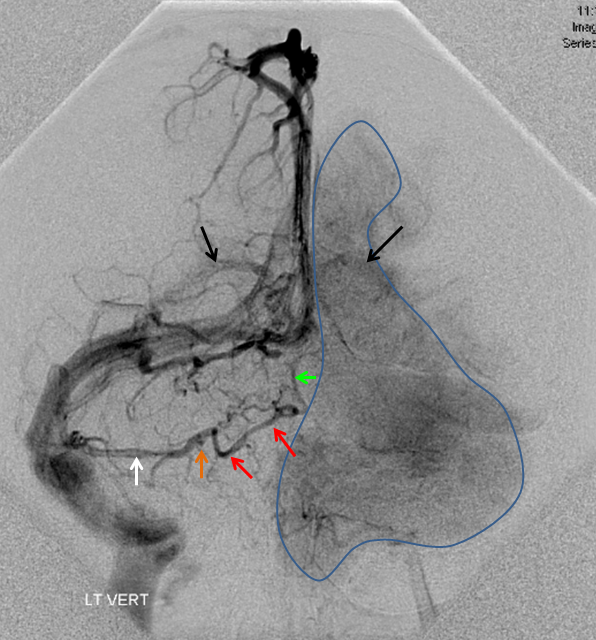
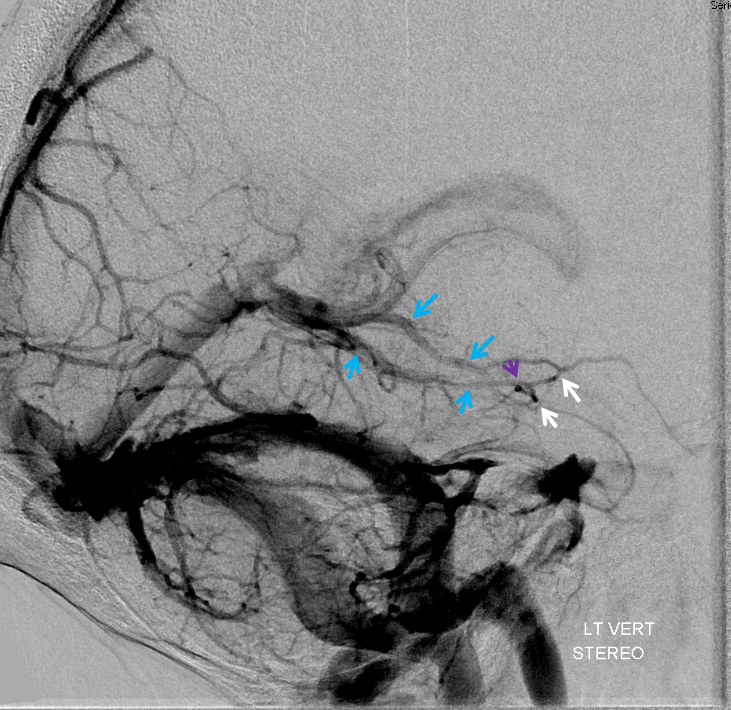
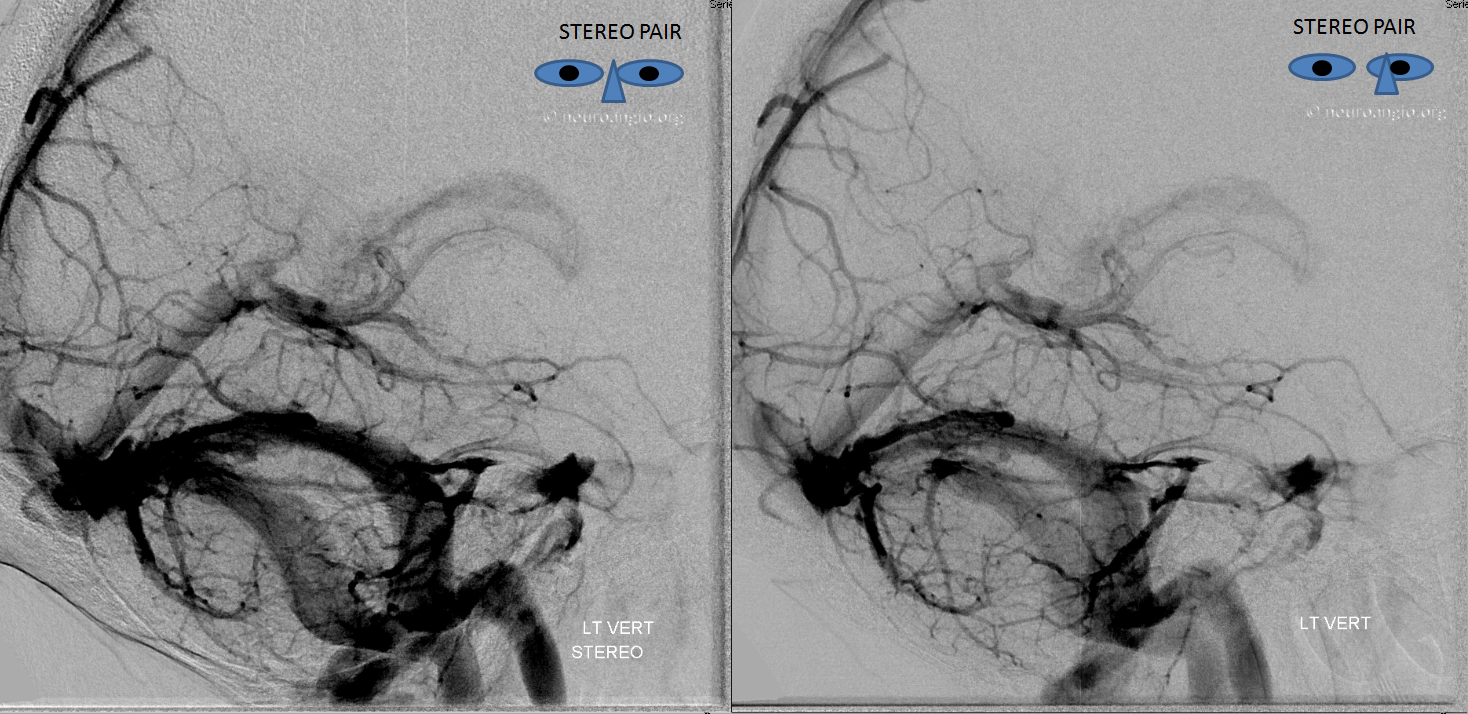
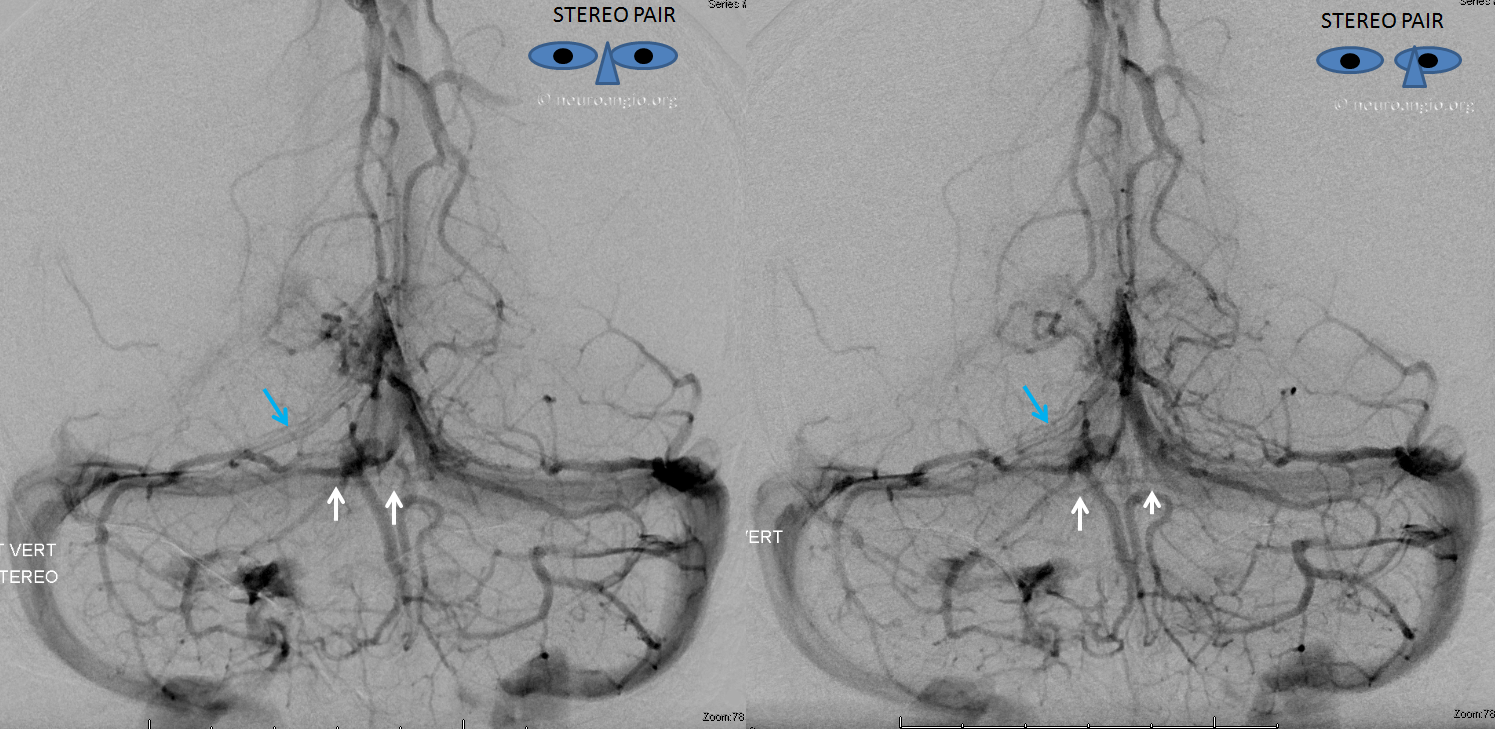
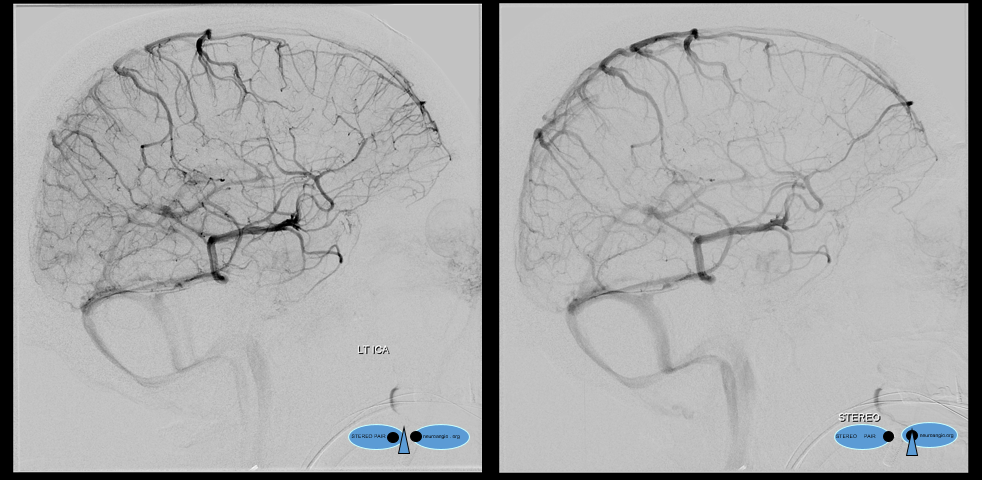
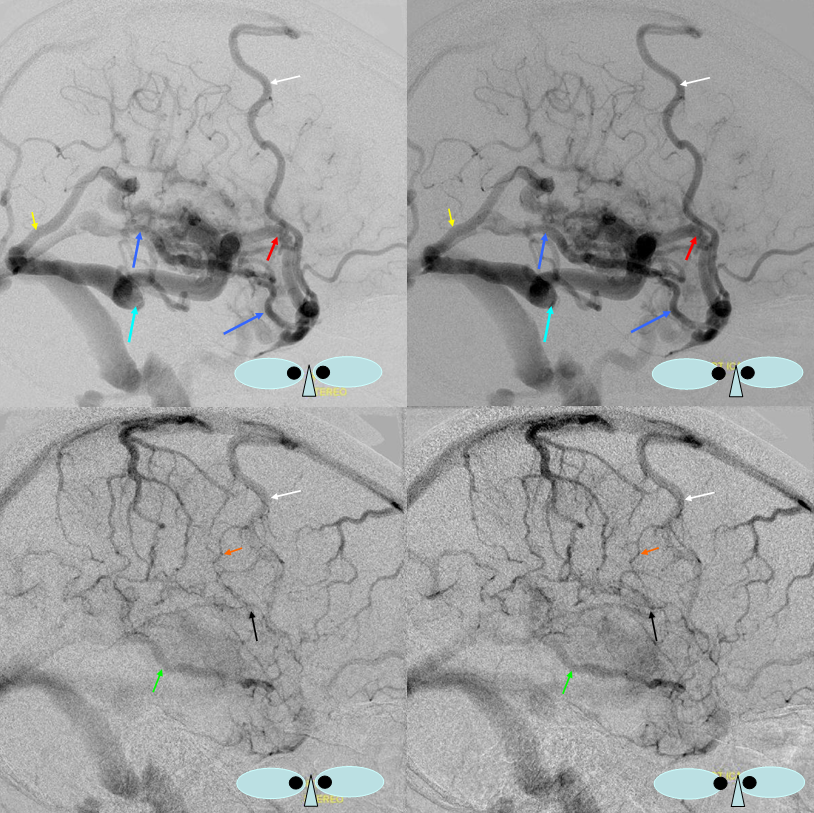
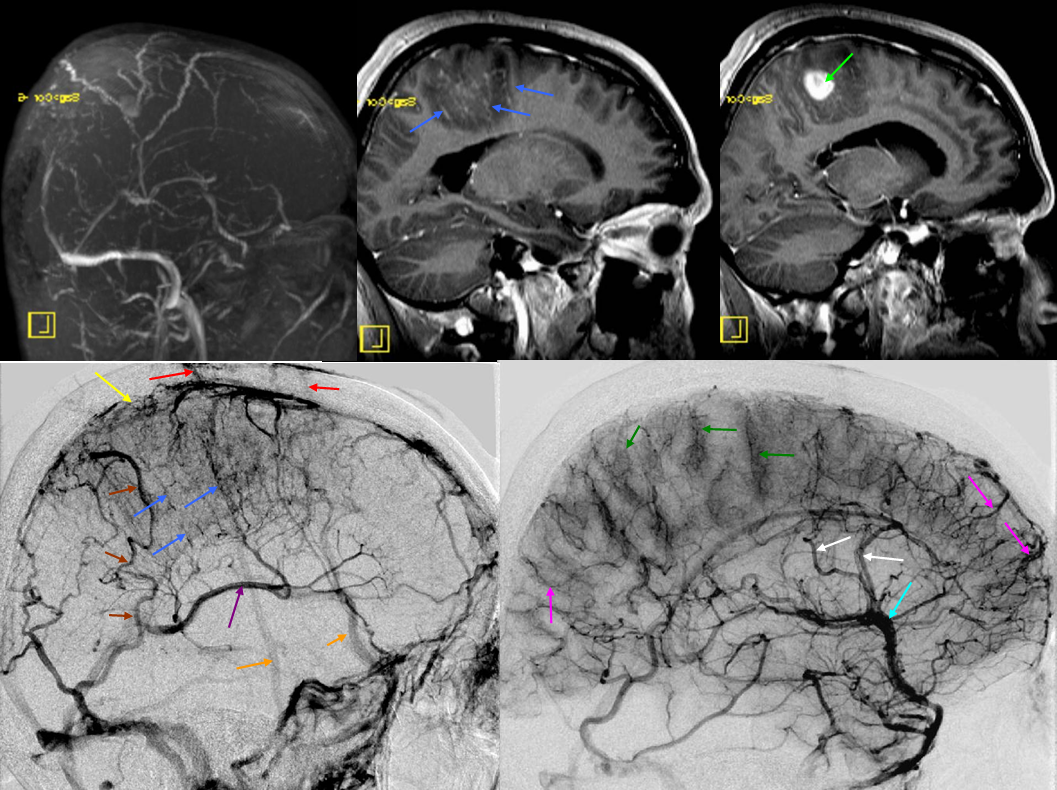
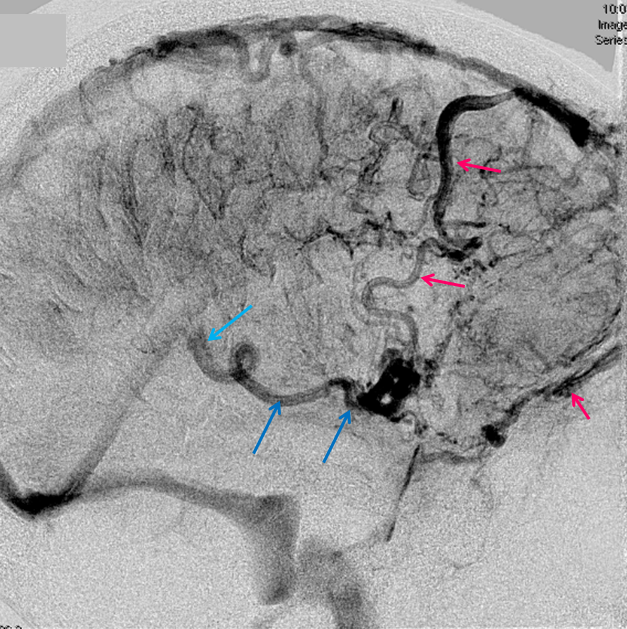
The disease is not limited to anterior circulation. Even more tremendous venous congestion (blue outline), wtih no effective surface veins, afflicts the left cerebellum, brainstem, and posterior cerebral artery territory, in stark contrast with normal right side. The brainstem drains almost exclusively via a large lateral mesencephalic vein (red), into the petrosal vein (beige), and ultimately into the superior petrosal sinus (white). An anterior mesencephalic vein (green) points straight up from medial aspect of lateral mesencephalic vein.
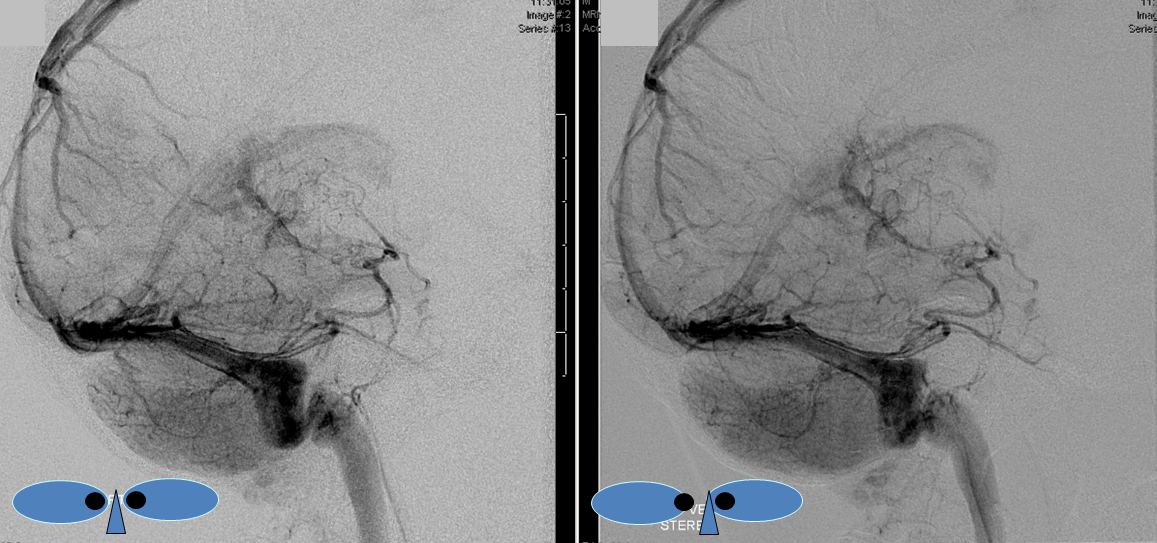
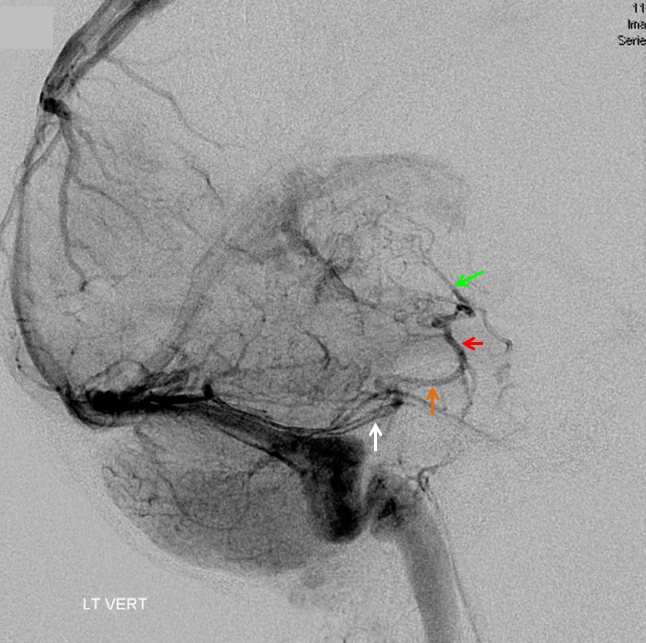
Lateral view of the same left vert injection.
