This page was developed as a follow-up to collaboration on a related project with Drs. Melanie Walker, Kate T. Carroll, Michael R. Levitt, Eytan Raz, Erez Nossek, Nader Delavari, Osman Mir, and Peter Kim Nelson.
The publication version of this project is in JNIS — link here
The first case of MMA embo for cSDH was described by Mandai et al in 2000. A t r i c k l e of subsequent reports followed. Many in Japanese journals. As a field, we really missed the boat on this one — by something like 15 years. Why? The usual — preconceived notions and turf battles. Among the former is the devotion to bridging vein tear theory. Among the latter is the idea that this disease belongs in the OR, regardless of the level of excitement it generates. With that bland introduction, lets go ahead…
Normal and pathologic dural vasculature reference figure below
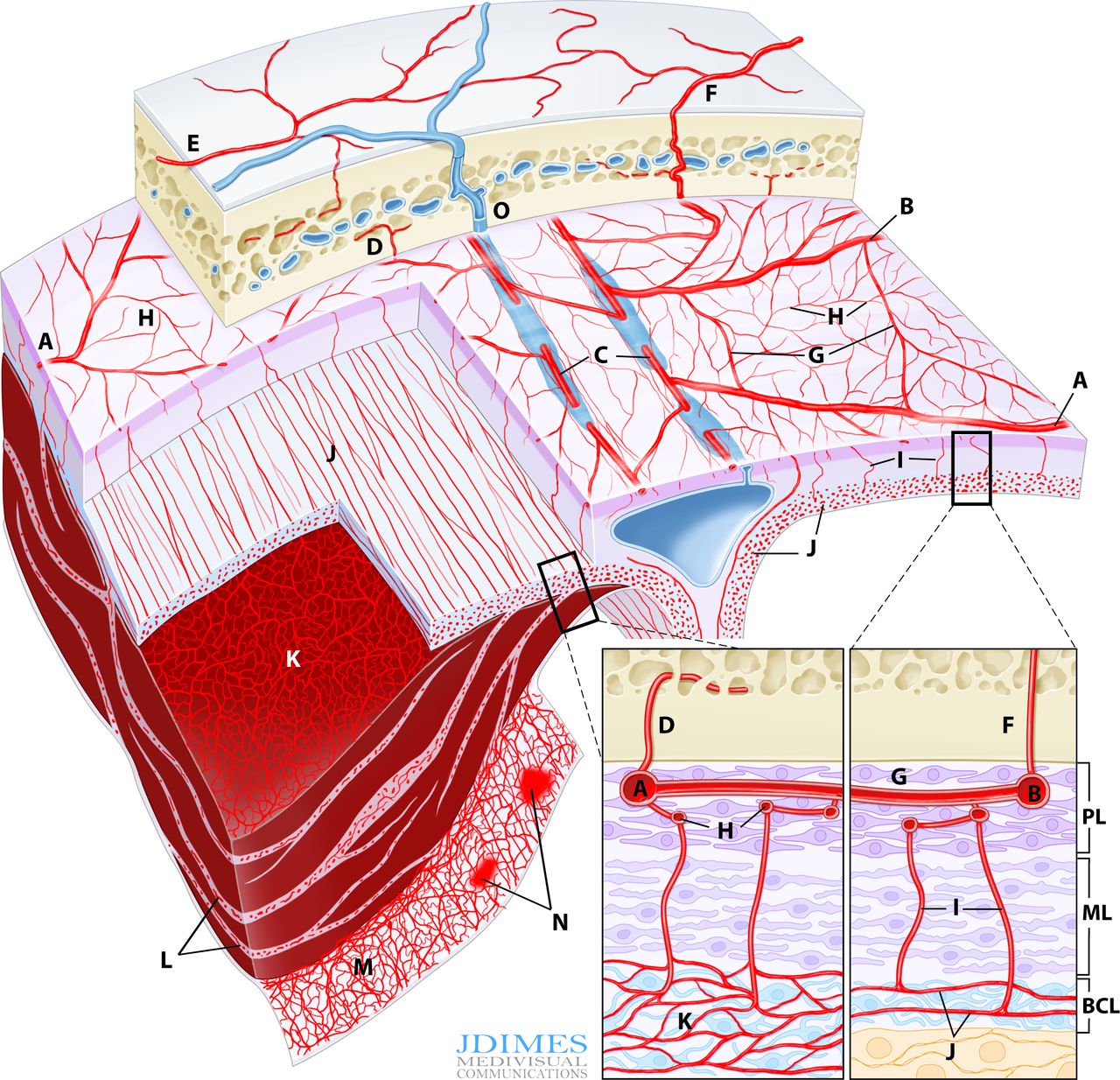
A, B – main MMA branches such as frontal (~400-800 um); C – arterial network in walls of sinuses (variable size); D – meningeal branch supplying skull; E – cutaneous branch supplying skull; F – cutaneous branch supplying skull and meninges; G – primary anastomotic network of the outer layer, connecting larger dural branches (100-300 um); H – secondary anastomotic network of the outer layer (50-90 um); I – penetrating vessels in the relatively avascular meningeal layer; J – normal inner capillary network in the border cell zone layer; K – proliferating border zone / inner network vessels in a subdural hematoma – the vessels are located within membranes (L) and on the inner surface of the hematoma (M) which is frequently covered by a membrane as well; N– areas of leakage / extravasation; O –dural venous drainage into diploic vein

Brief Background
Understanding why cSDH embo works requires knowledge of normal and pathologic dural anatomy. Briefly, the dura consists of three layers, each associated with their corresponding vascular network (see figure above). The inner vascular network is located in the “border zone cell layer” — a layer that is closely adherent to the arachnoid — in fact tighter than to the adjacent meningeal dura. The capillary network in this layer is responsible for the growth of cSDH in a cycle of hyperproliferation, fragility, bleeding, further hyperproliferation, etc. The “membranes” of cSDH are made up of border zone cells and their vessels. This also applies to the membrane on inner hematoma surface. In reality, the “subdural” hematoma is really intradural, or at least inside the border zone layer. The whole concept of subdural space — whatever is below the dura but superficial to the arachnoid — is very useful surgically. From a histologic perspective this is really not a great idea. There is excellent literature by l e g i t people about this — worth looking into — not the first time long-standing notions were proven to have issues.
What starts off the process of cSDH formation? The idea of minor trauma is still prevalent, with tear of bridging veins. Why should veins tear with frequent regularity in a tiny potential space between the arachnoid and the border cell layer? Too often, minor trauma is like a black cat in a black room. Whatever the case, the idea of cSDH connection with dural vascular hyperproliferation / instability naturally leads to the notion that MMA embolization might help.
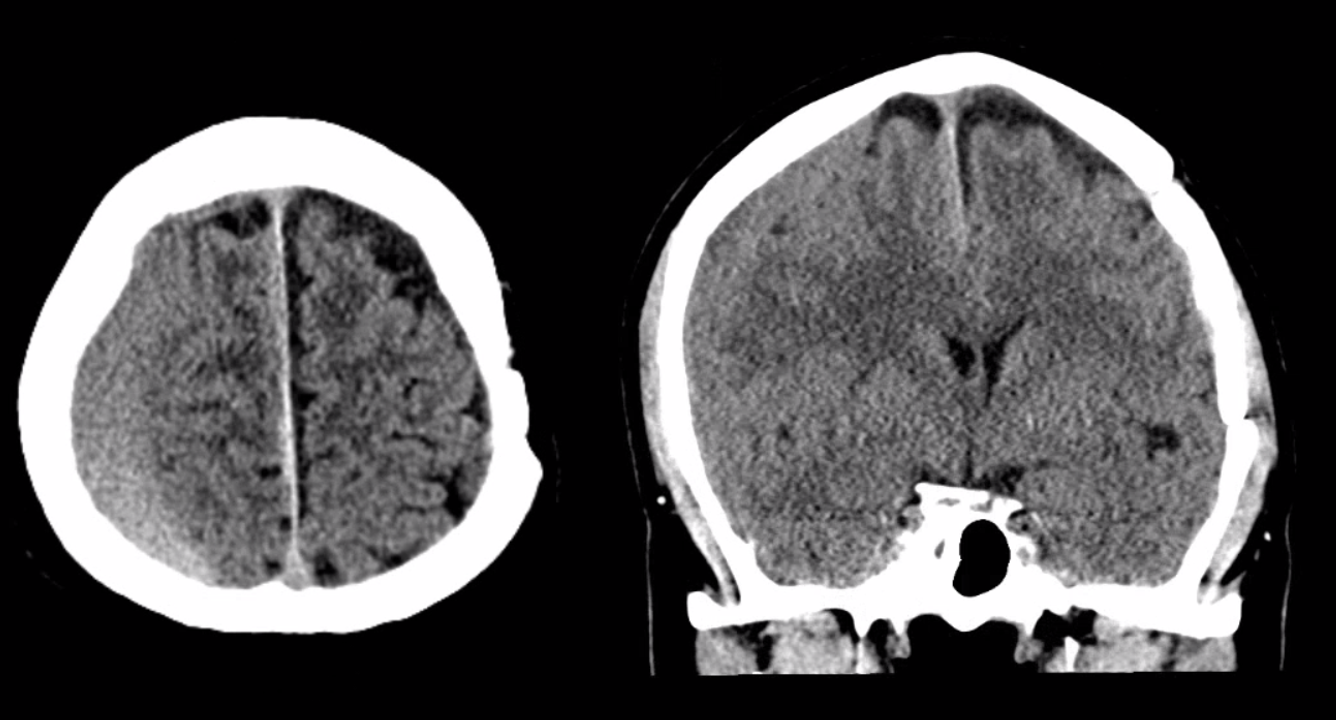
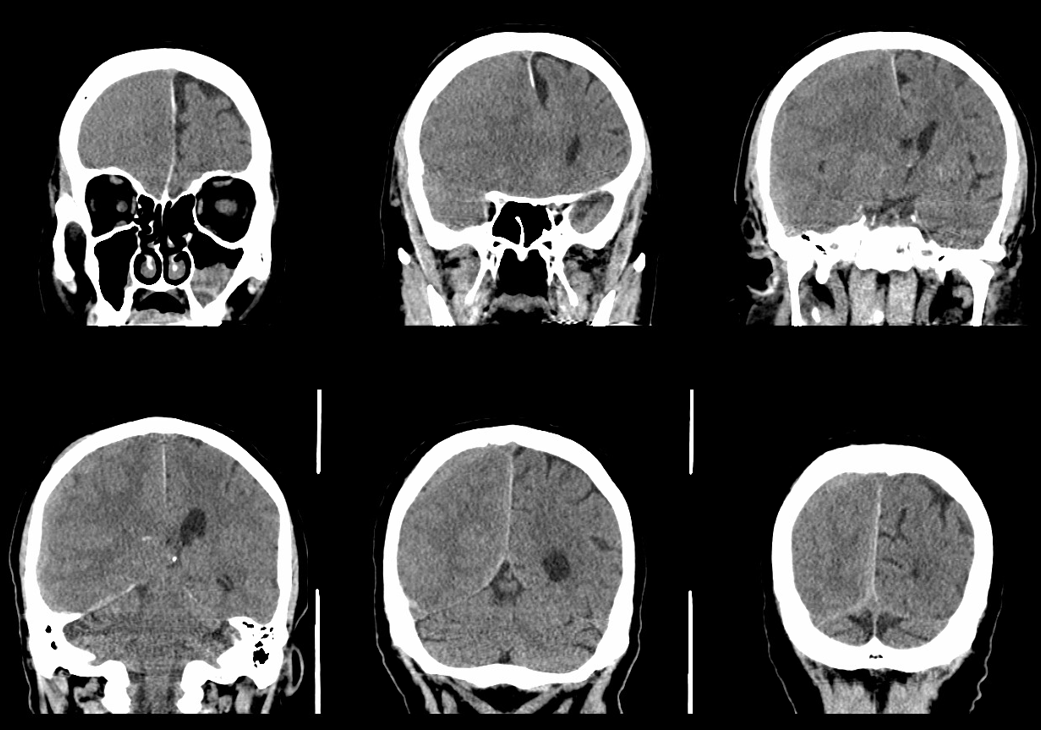
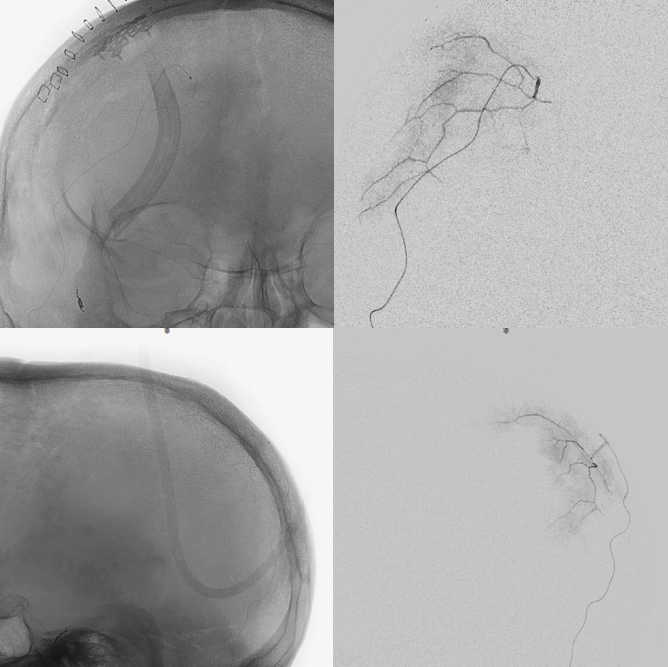
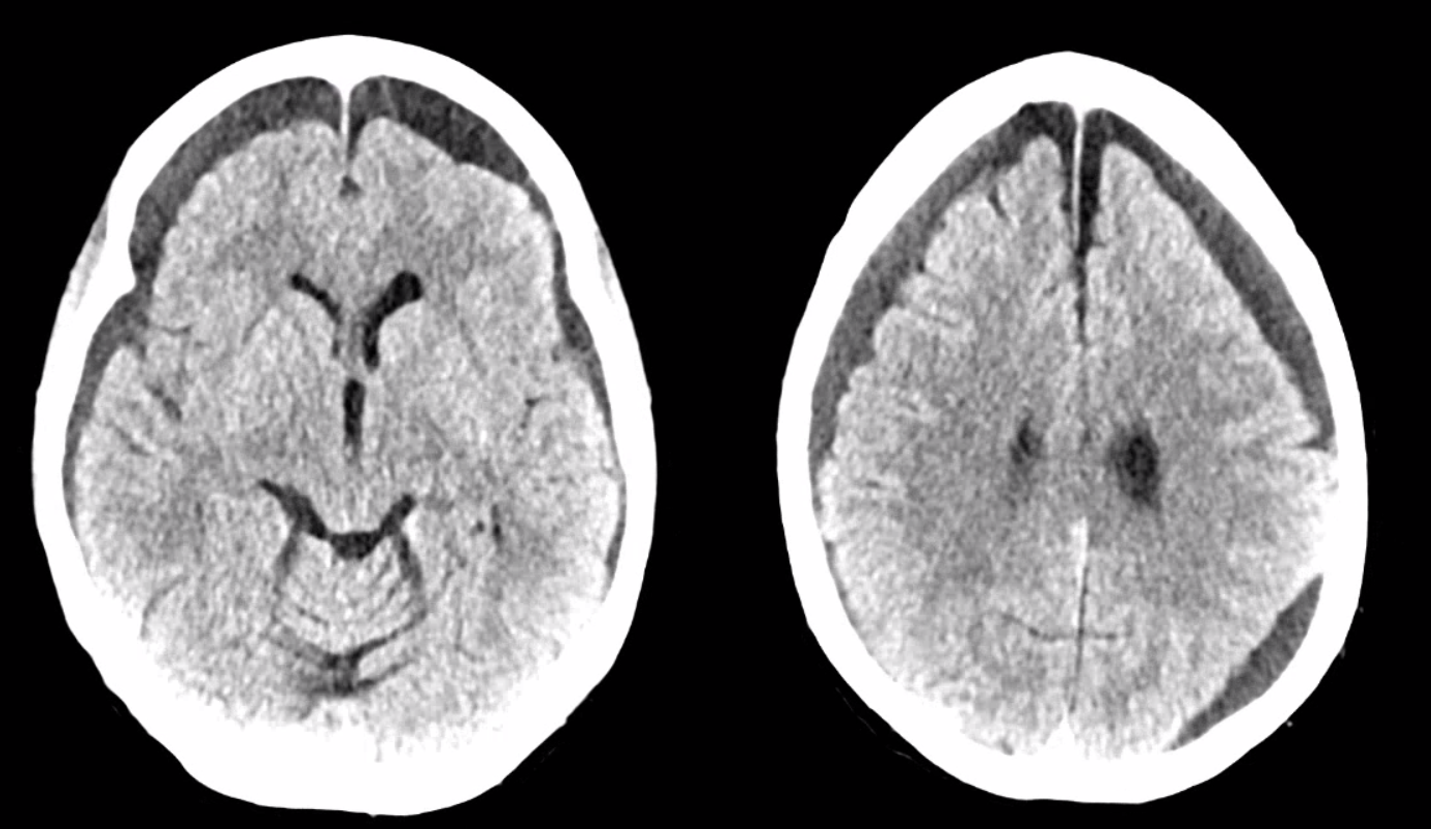
Below is a typical image of a chronic mixed density subdural hematoma — some areas look newer, some older.
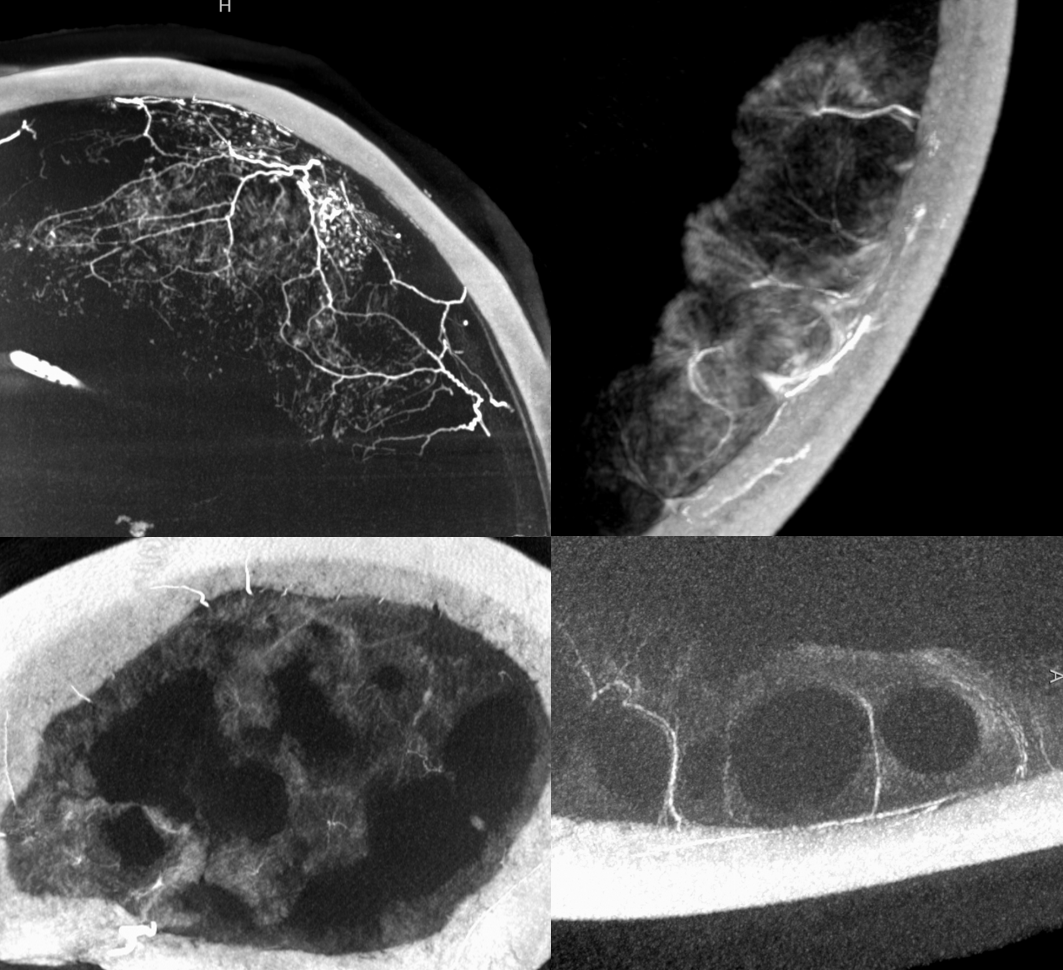
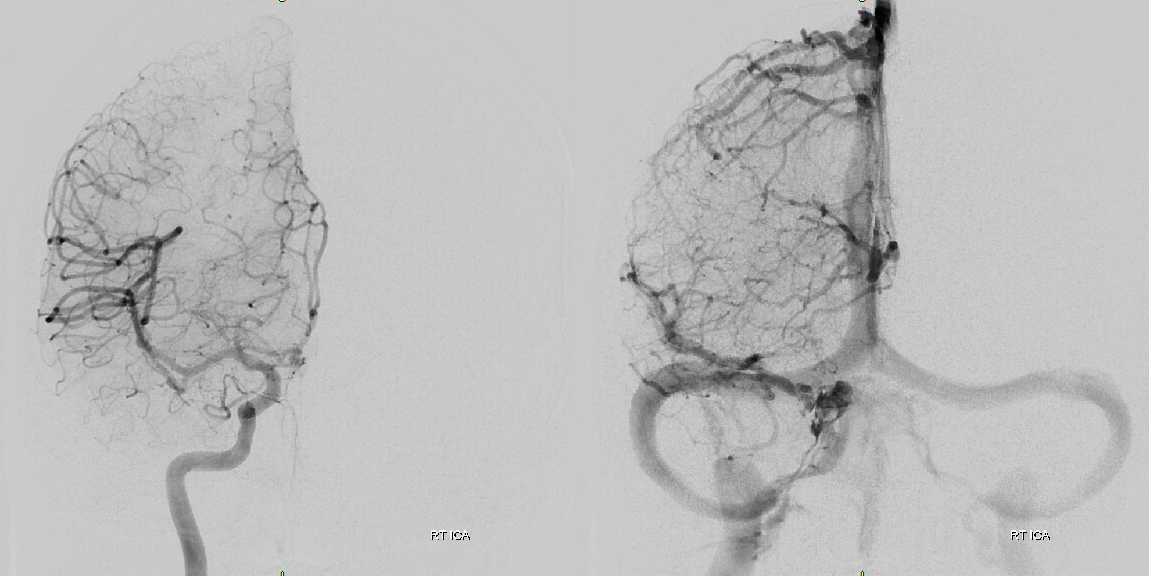
In a kind of “active” phase of microhemorrhage from the inner layer network, contrast leakage can be seen on angio, as in this extreme example
There is a lot variability to this. Below is a more homogeneous collection mostly isodense to brain — the classic “subacute” look
The angio is pretty bland though, with neither spot signs nor the “enhancing membranes” look
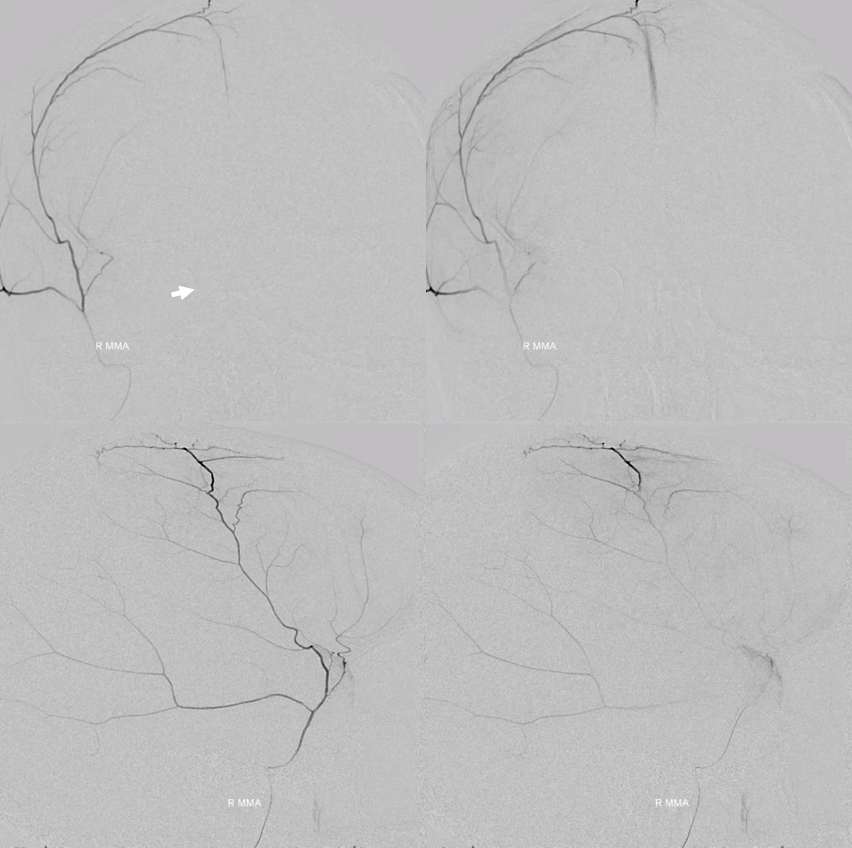
Nevertheless, several months post embo the hematoma is gone
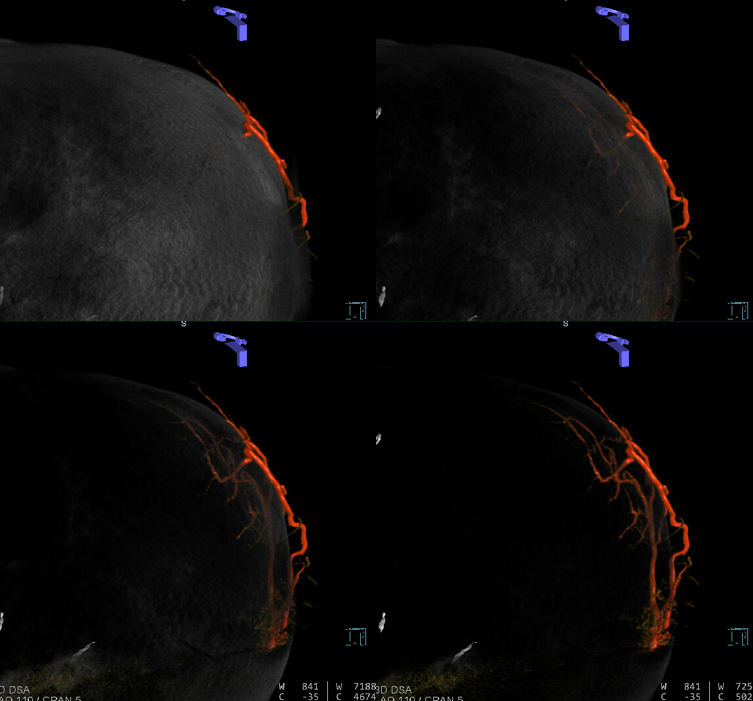
Sometimes, membranes can be extremely vascular, with stunning Cone Beam CT images

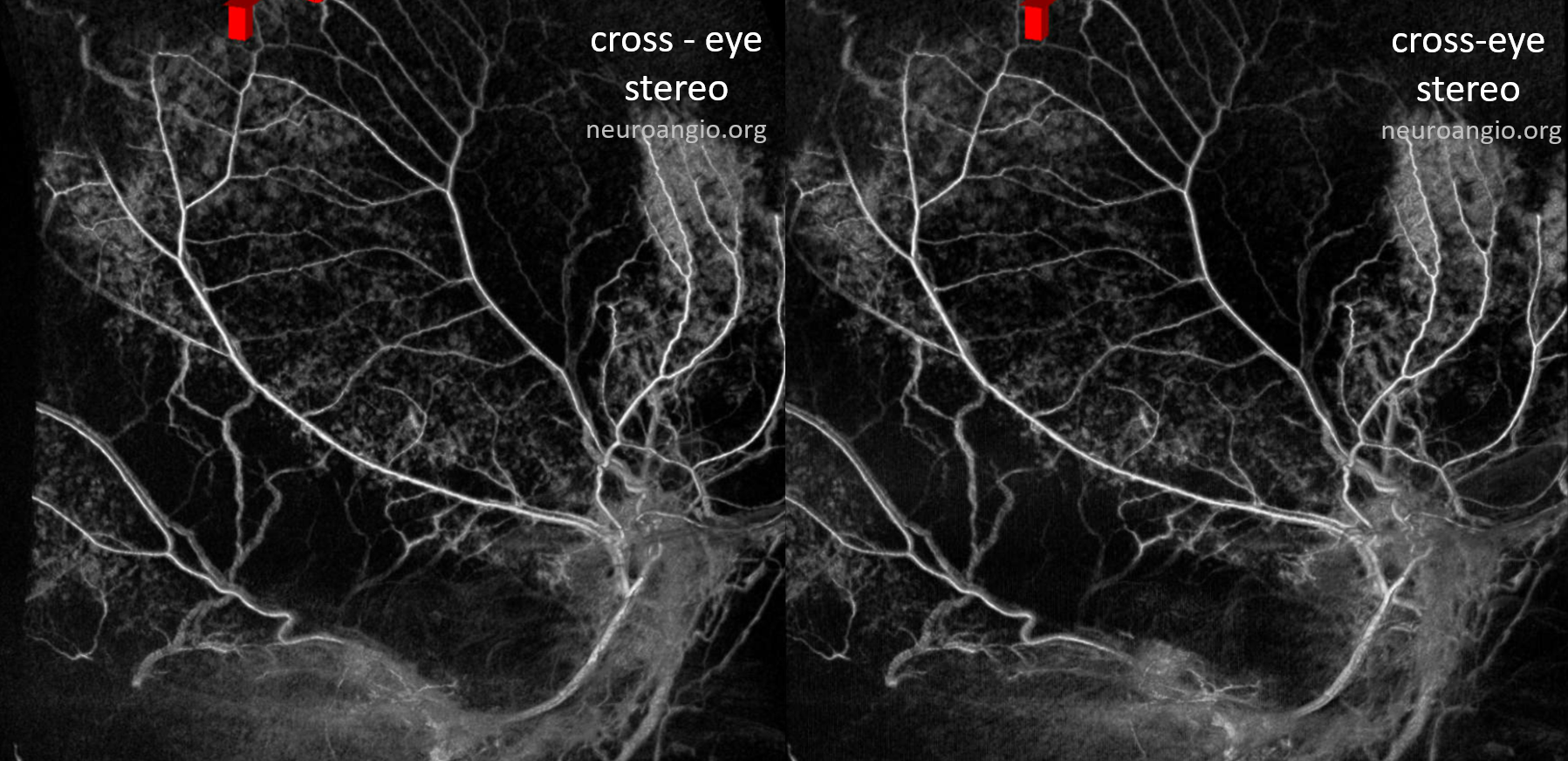
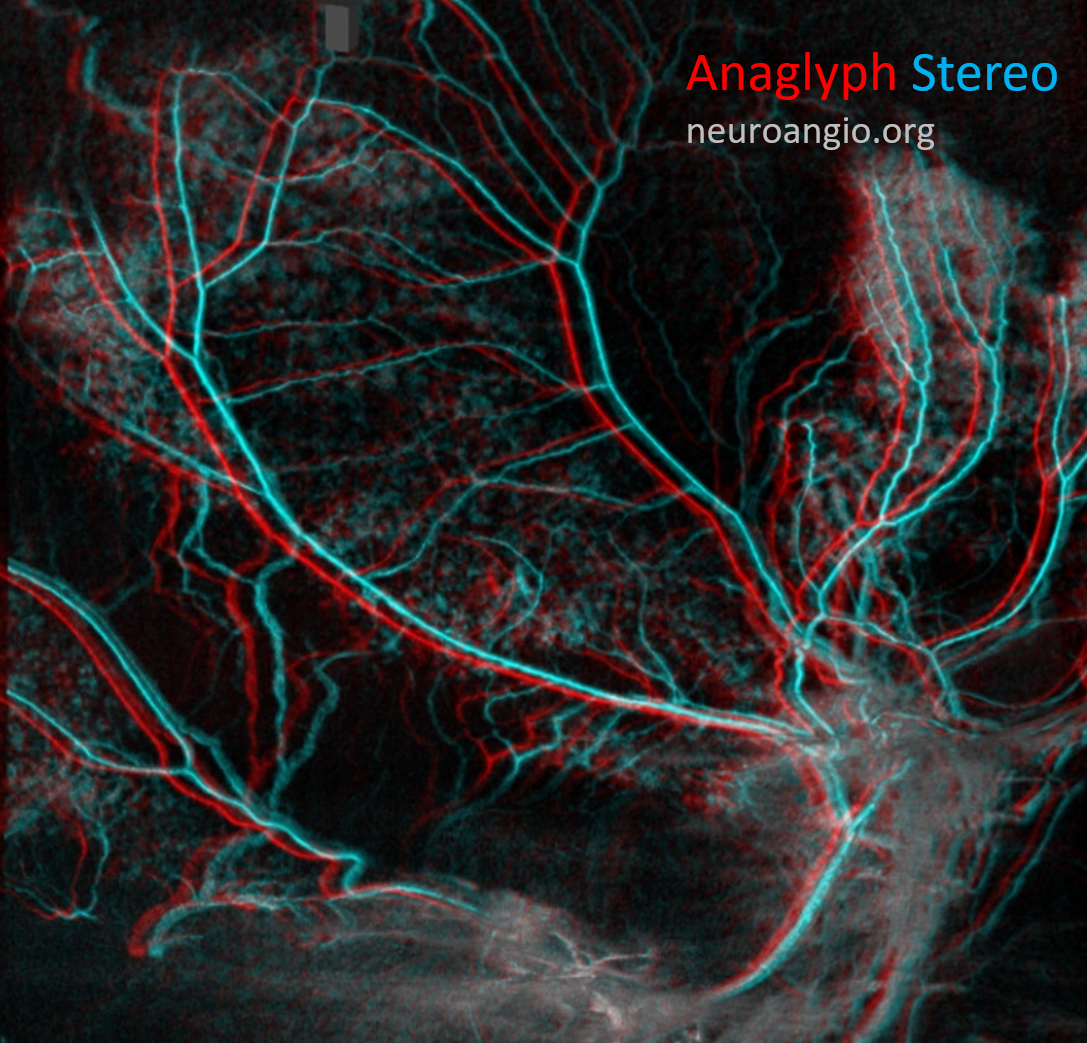
Anaglyph stereo
In the image below, there has been particularly efficient nBCA:LIPIDOL 1:4 permeation of the falx, allowing one to see its pathologic hypervascularity in upper right hand image (be careful of the Davidoff and Schechter anastomosis!) . The rest of the images are contrast injections of MMA showing exuberant convexity membranes. Note vessel extending from the inner table to supply the membranes.

Volume Rendered Movie of the same case
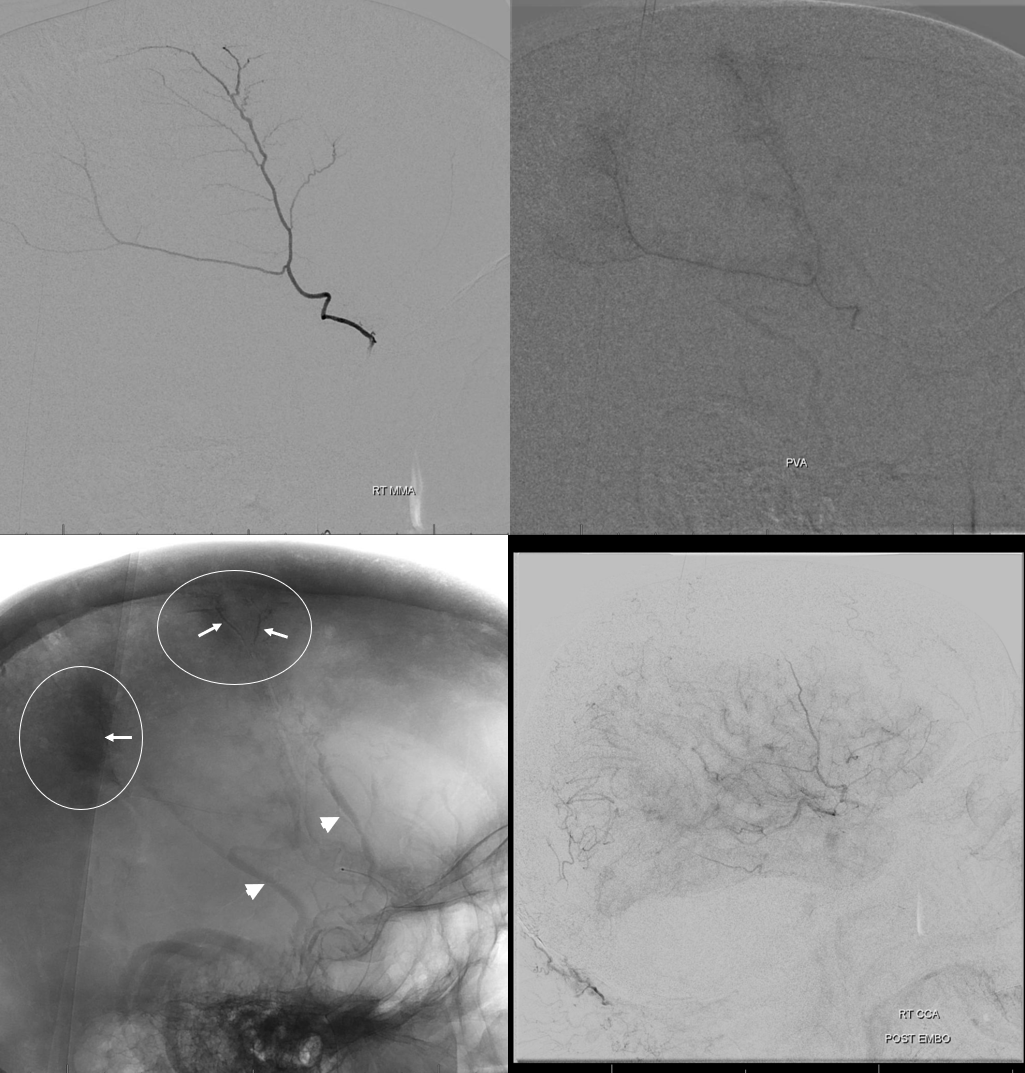
You have to be careful when describing “enhancing membranes” in cSDH embo reports. Both Dural Arterial and Dural Venous vasculature participate in supply and drainage of skull as well as dura. A lot of skull in fact is supplied by meningeal vessels. Differentiating skull and dural enhancement can be difficult. Tangential DSA images that show enhancement superficial to dura are certainly in the skull (white ovals in images below). But, for rest of the image, it could be either one — in fact image below is from a subject without subdural hematoma — the whole blotchy enhancement is very likely skull
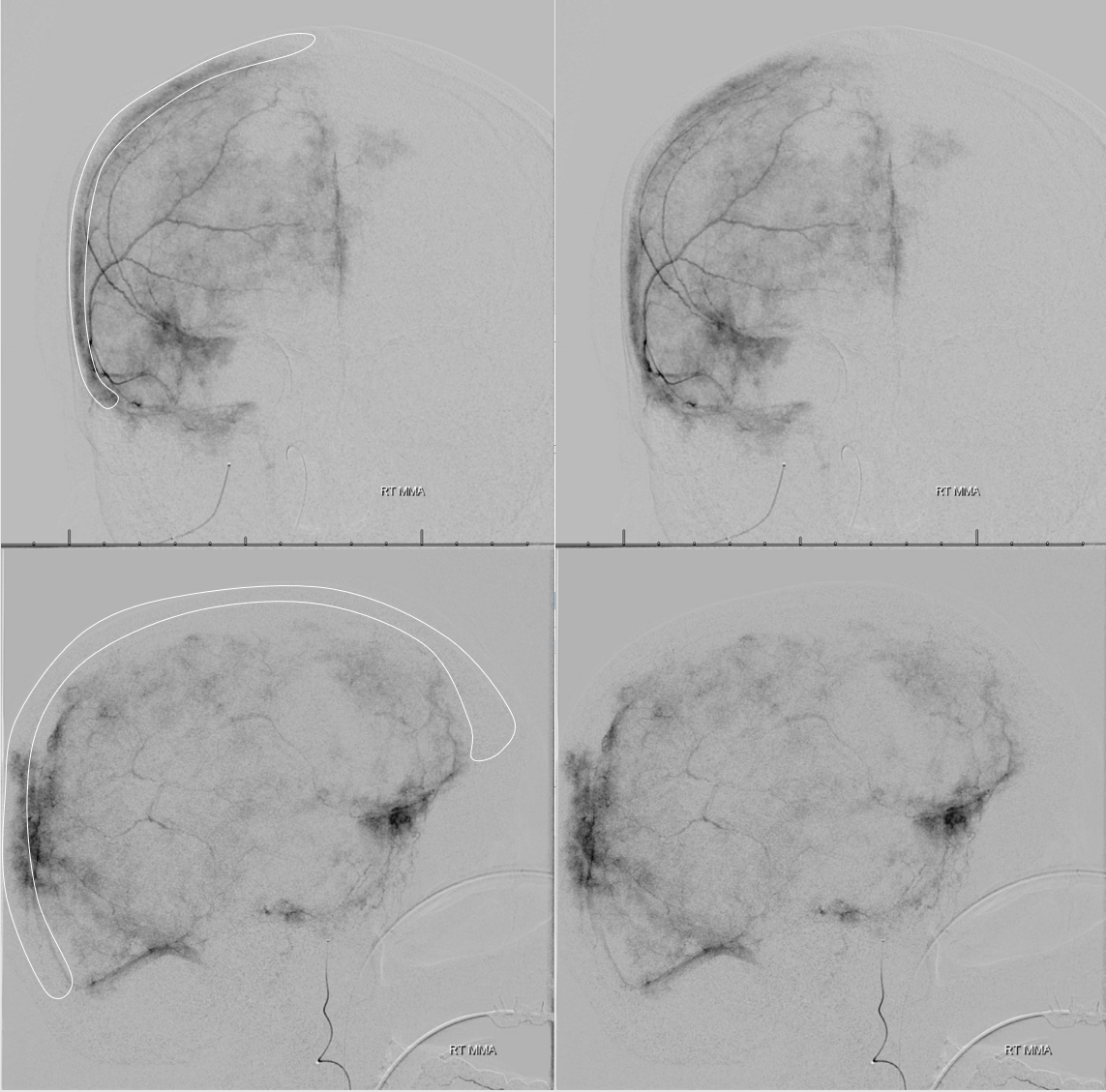
On the other hand, image below is from a subject with a cSDH. The enhancement is likely all dura, and does not extend to the paramedian skull
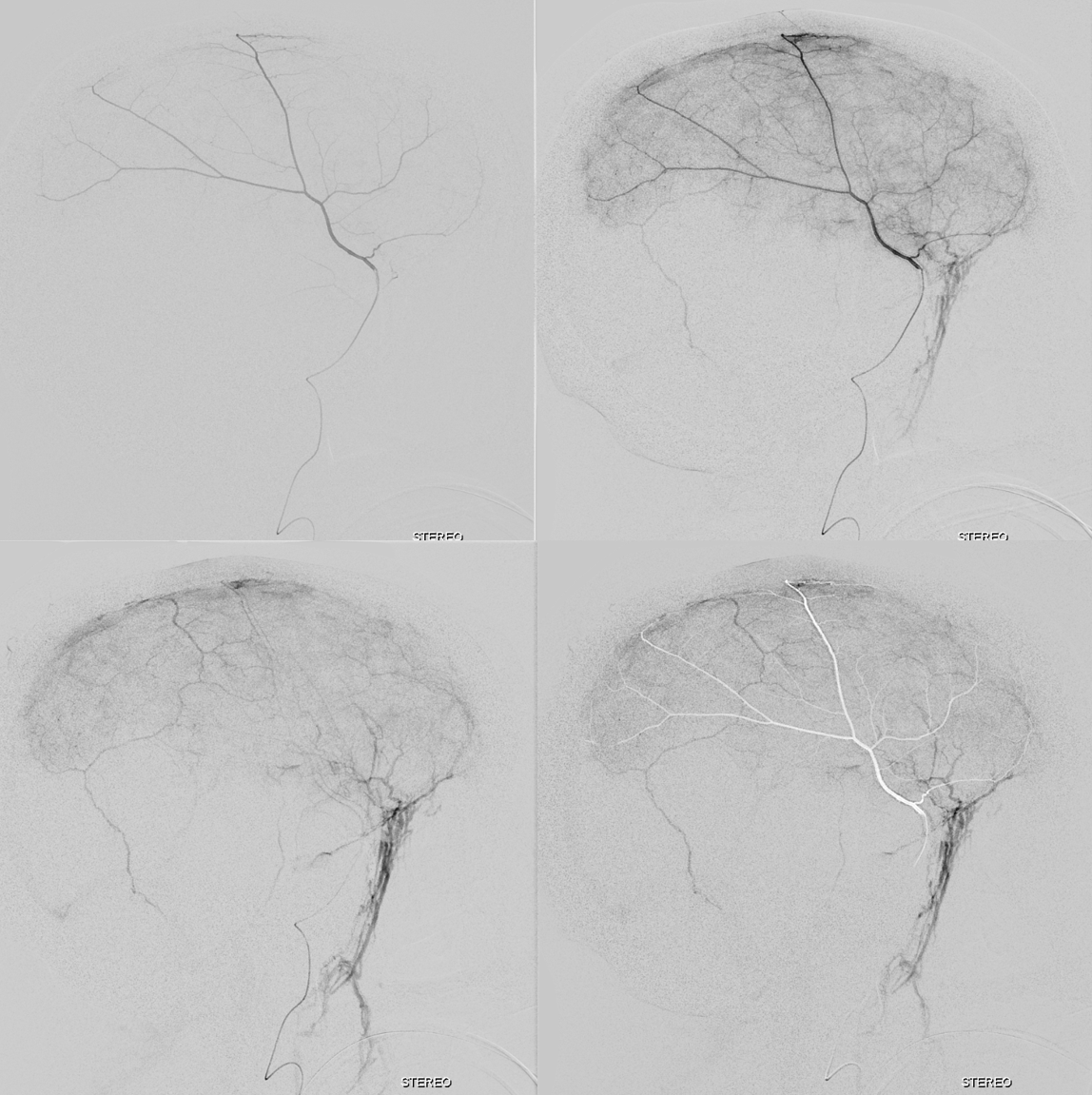
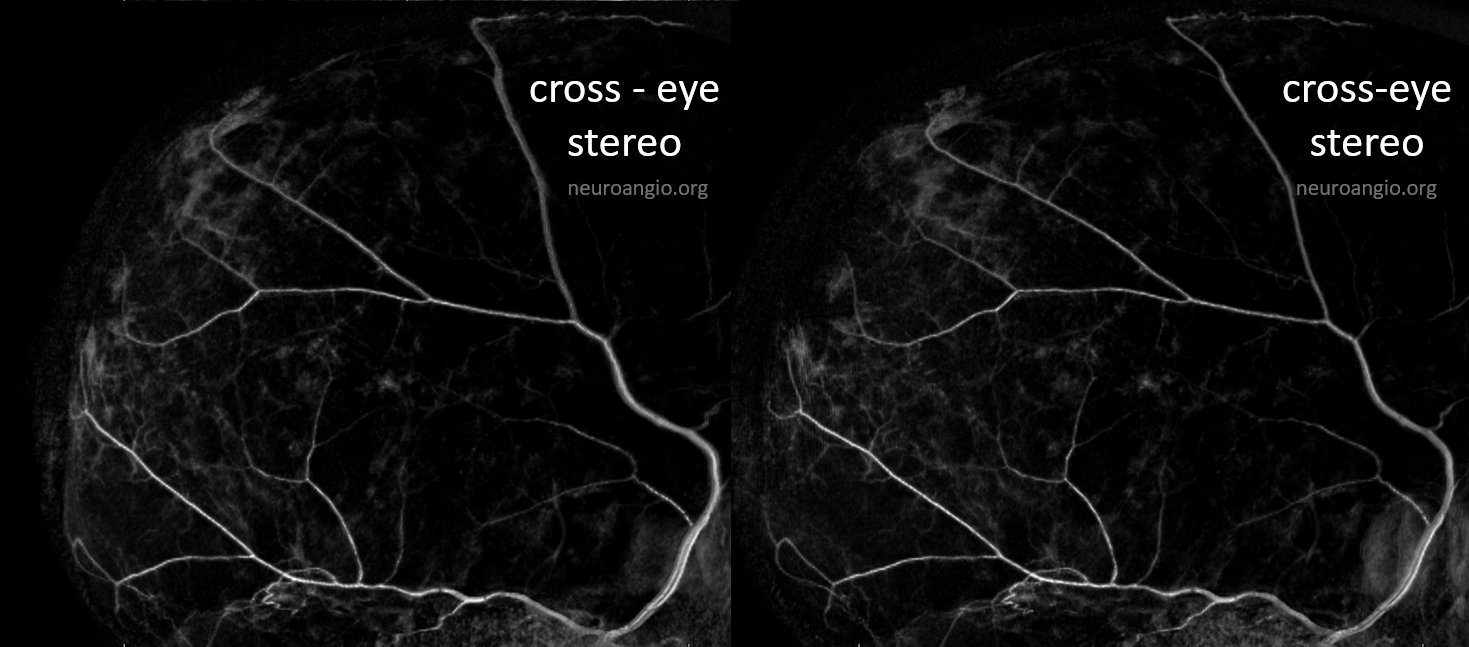
The stereo subtracted DYNA images below can help. It is not necessary to obtain these of course for the embo, but is important if any kind of investigation into the nature of dural enhancement is done. Stereo images below show enhancement deep to major MMA arteries in a man with cSDH, and therefore dural.
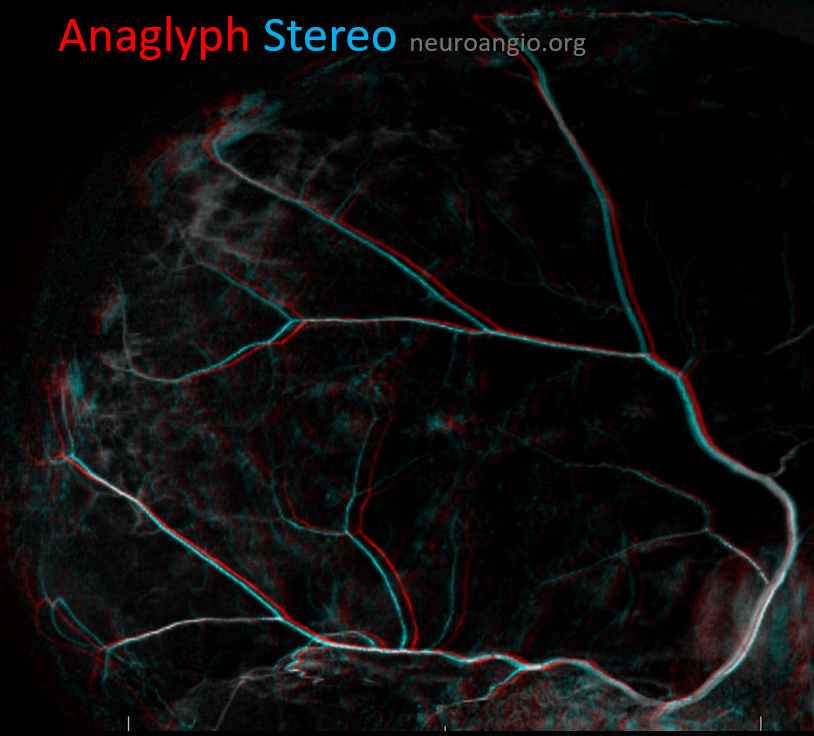
Anaglyph stereo of the same patient
On the other hand, the images below show enhancement superficial to the major MMA network, and therefore belonging to the skull. This patient has no dural pathology.
Here is an anaglyph stereo of the same
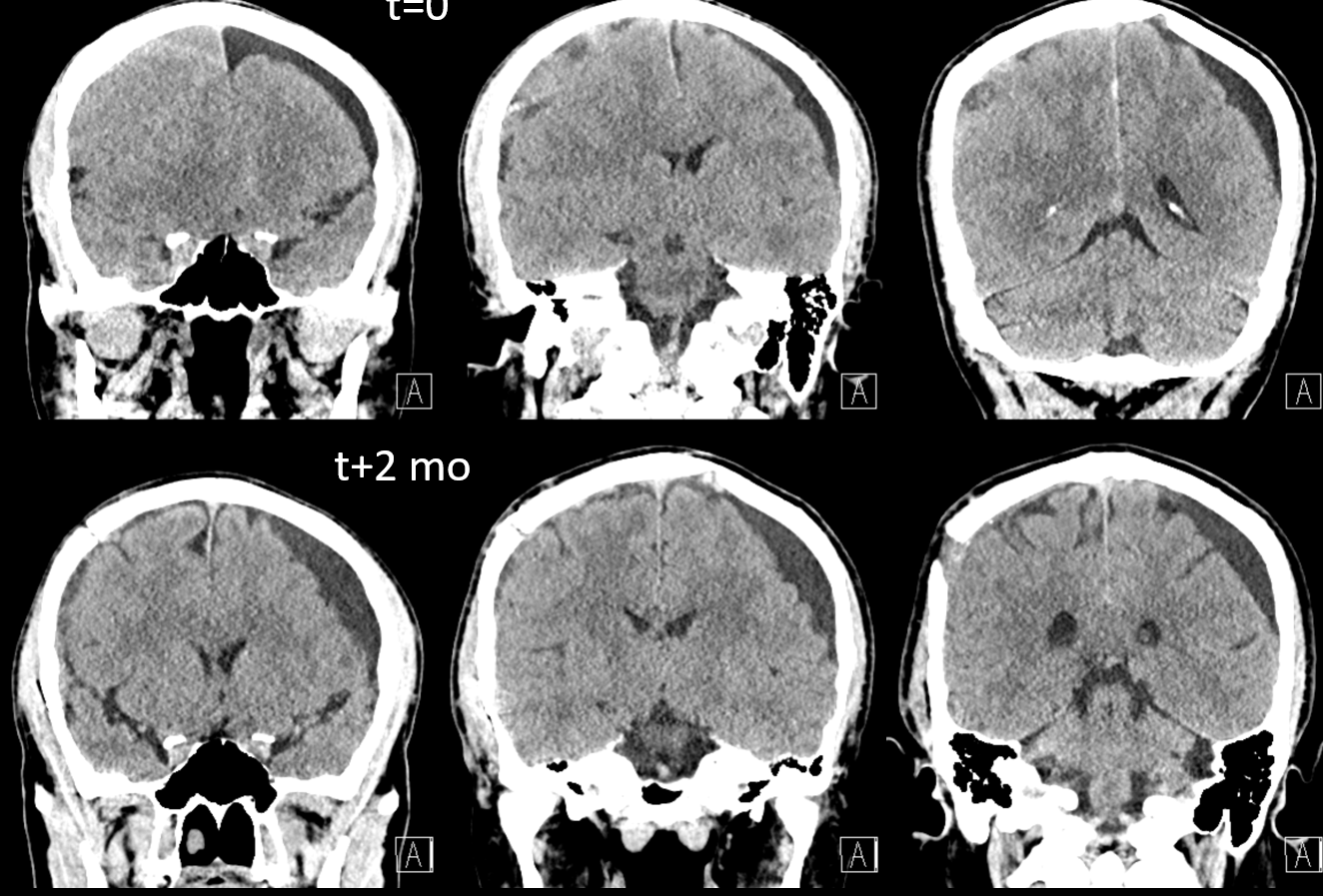
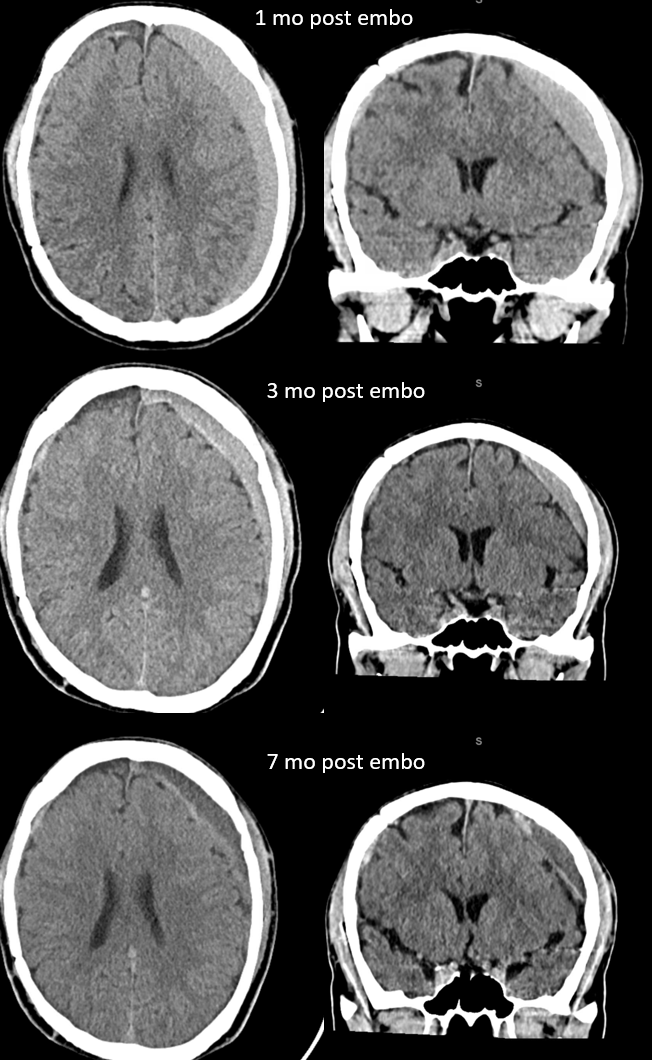
Here is a big subacute one on the right, with an older one on the left — the left one grows by a bit after 2 months. Right one was evacuated.
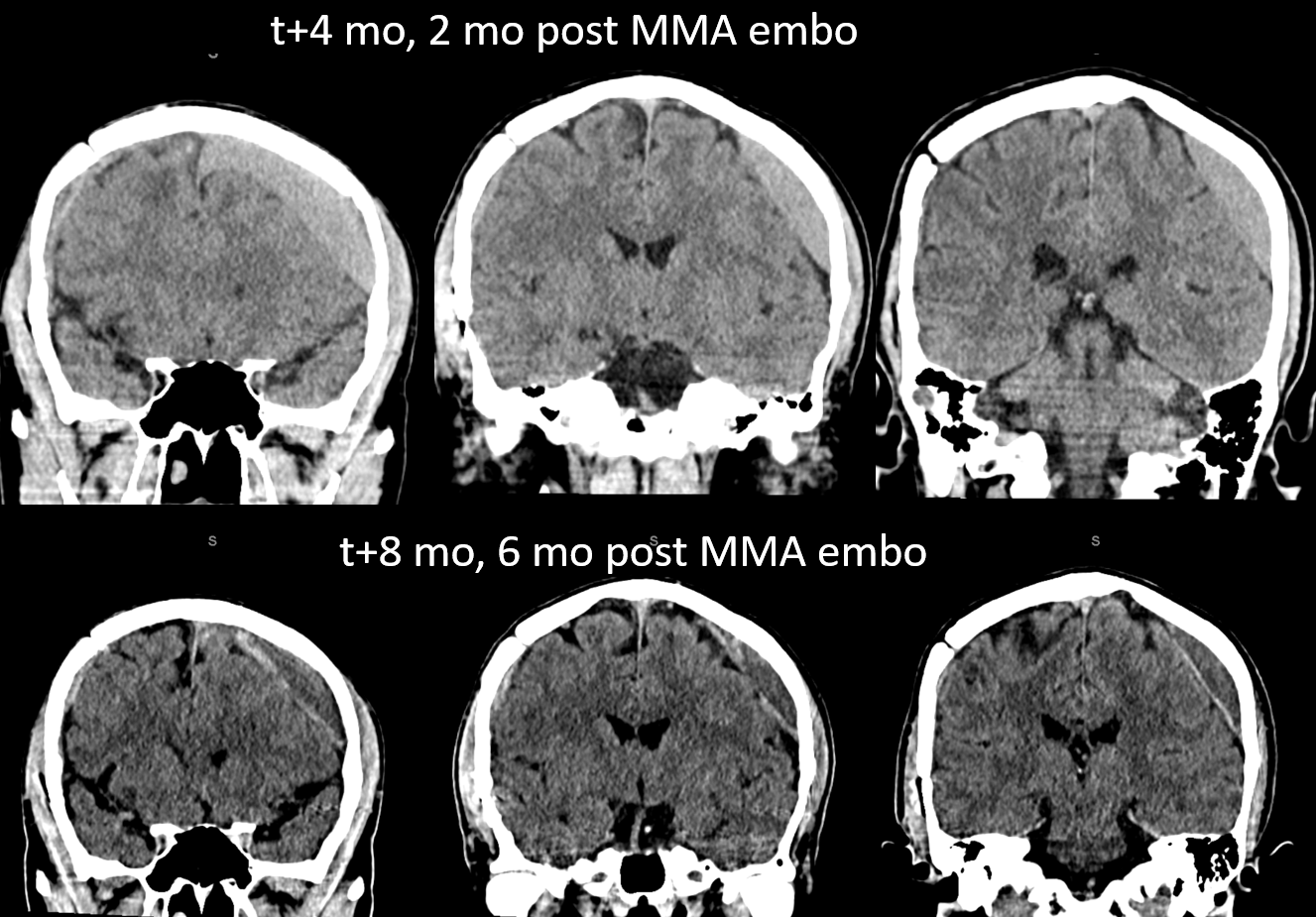
A left MMA embo is performed. Nevertheless, two months post embo, the subdural grows slightly larger, with new hyperdensity, finally to shrink some 8 months after (bottom row). Notice a hyperdense inner border of the collection — could this be small amount of bleeding, or developing calcification? Clinically, this is a success — no further treatment was required. There is a lot that needs to be established in terms of which patients benefit from embo, at what stage, with what material, etc.
However, technique is important, we believe here, as the following cases will illustrate.
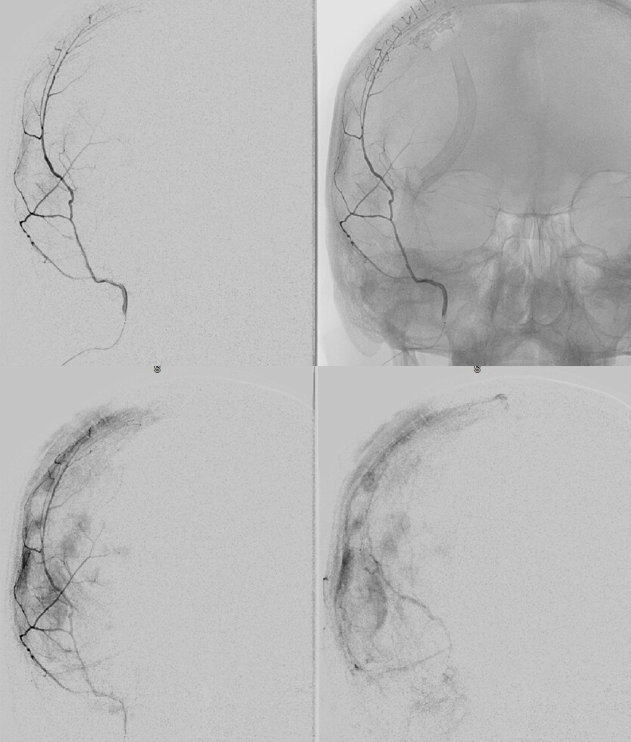
Typical Subdural Embo — with a small twist at the end 🙂
We don’t show boring stuff here… Besides, there is something to learn in every case, really
A classic “isodense” subdural
Frontal views after burr hole evacuation — note ophthalmic artery origin from ICA. Always the most important thing to look for!
Global external
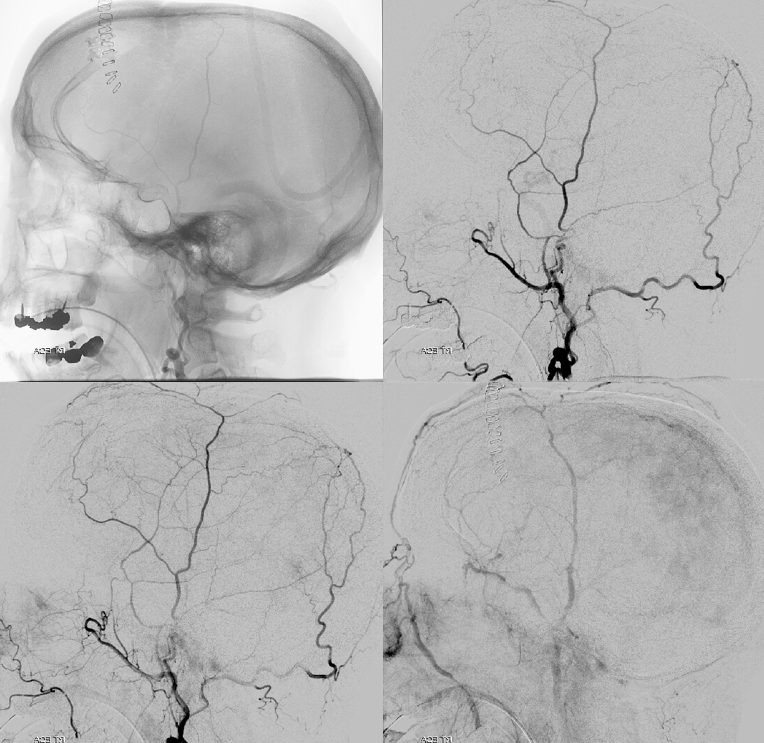
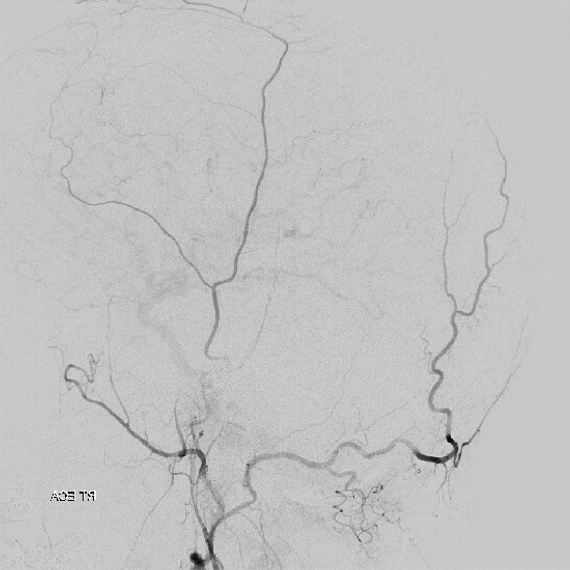
MMA — typical blotchy, likely dural enhancement
Frontal MMA views — in fact some of the enhancement is skull. Notice that there are no vessels projecting over the orbit — those frontal views are very useful that way. Anything projecting over orbit on MMA injection is likely in the orbit as it does not usually reach so far medial posteriorly — that’s posterior meningeal territory.
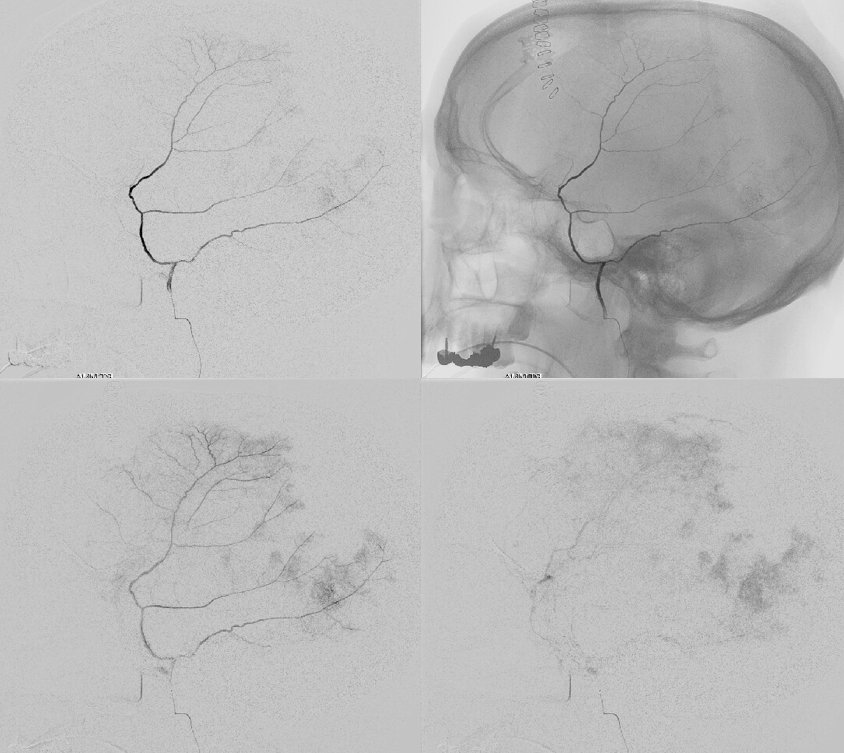
Our usual choice is 45-150 um Contour particles (read on for more on that). This is typical sequential imaging of a good particle embolization — a nice strong blush initially, followed by gradual “trimming” — like a tree loosing its leaves — until bare trunk only is left in image 4 below. It is very important to monitor vigilantly for any hint of orbital connections — even if none were demonstrated initially — again more on that below. As embo progresses the resistance in dural target territory rises, setting up possibility for penetration into territories via connections with initially higher resistance
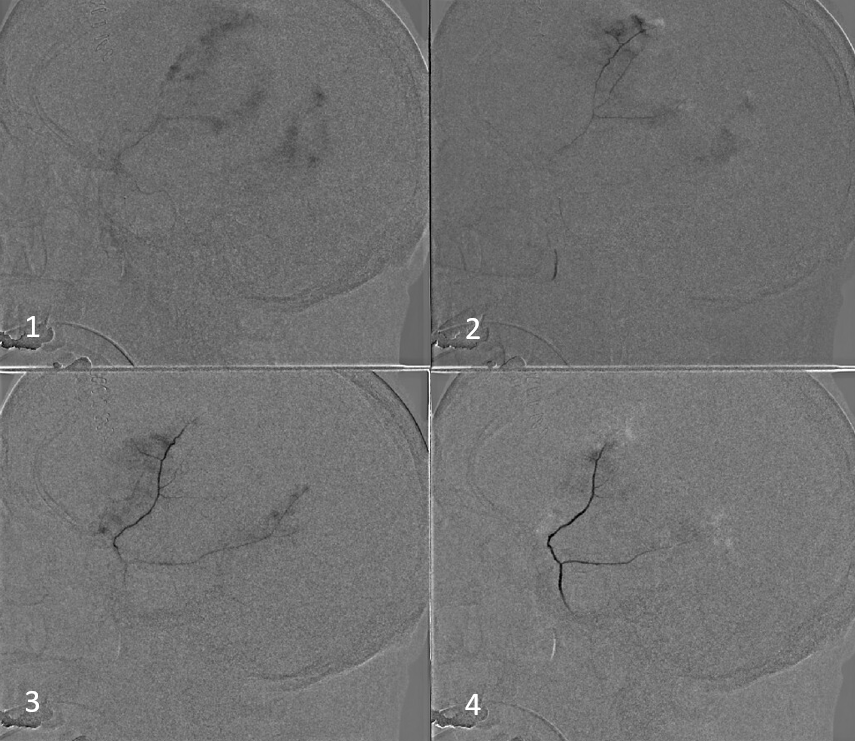
We put a coil at the end to minimize recanalization — this is a detachable coil. We generally prefer pushable fibercoils, which require an 021 microcatheter, but are about 10 times cheaper than detachable ones.
Here is the little twist — look at some of the parietal dura still enhancing (skull is not enhancing on those tangential views, so likely its all dura. Plus — look above — there was a piece of parietal dura not seen on lateral MMA injections)
Sometimes, parietal dura is supplied by a transosseous branch of either the STA or occipital (letter “F” in our top diagram). Its probably totally fine to leave it alone. But, why not try? We don’t feel it adds morbidity enough to preclude this kind of nice catheterization with a duo and a hybrid 0.07 j-wire. Got to get all the way to the ostium to minimize skin issues
We get some nice images before particles. 45-150 is safe here also, but if you are skin-concerned, 150-250 will do fine too. Contours are aspherical and produce less necrosis than spherical types, so better for both skin and nerves, in our opinion
Post-embo
Common post — always do that to make sure there are no surprises
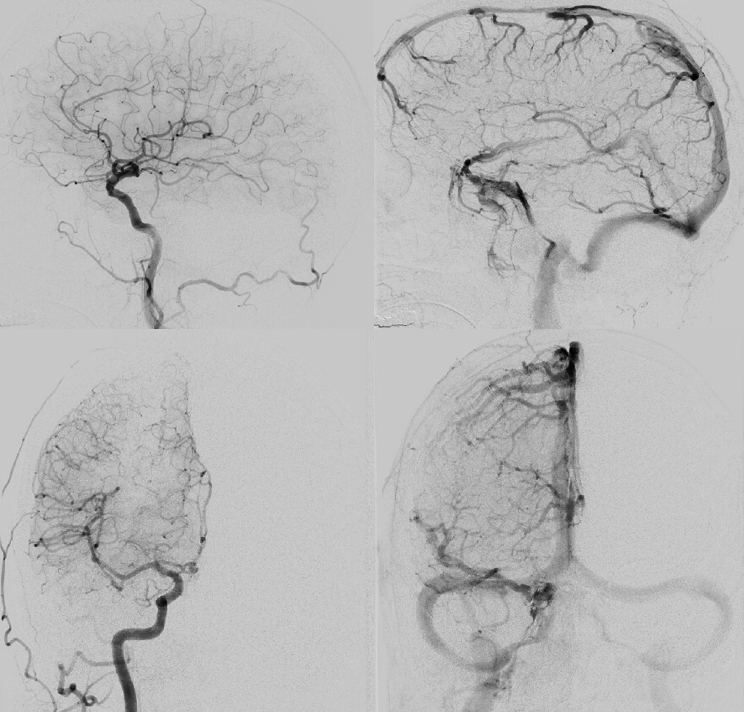
So, thats the basics.
Importance of Technique
Bilateral subdurals, one on right was evacuated. On left looks older
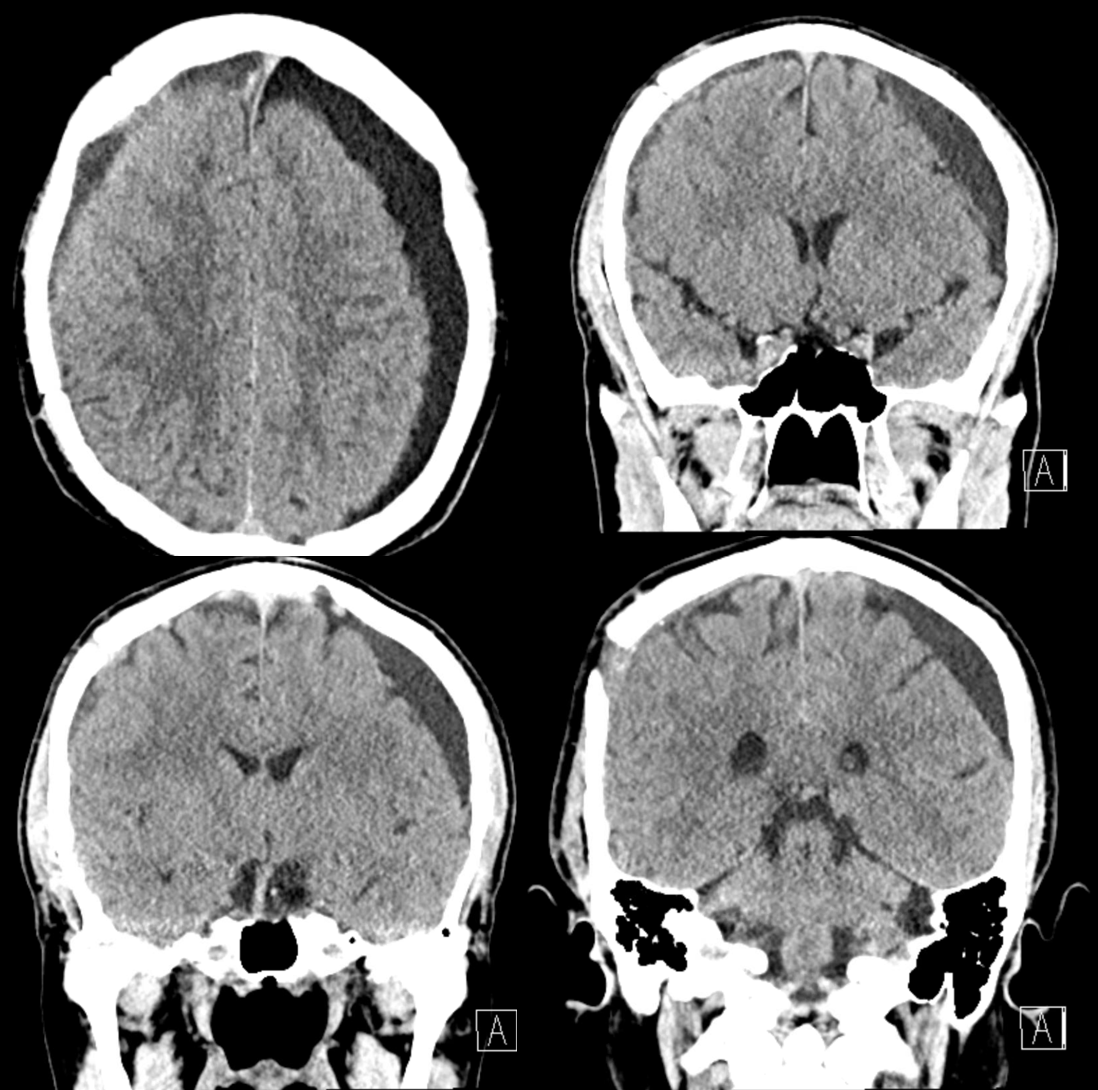

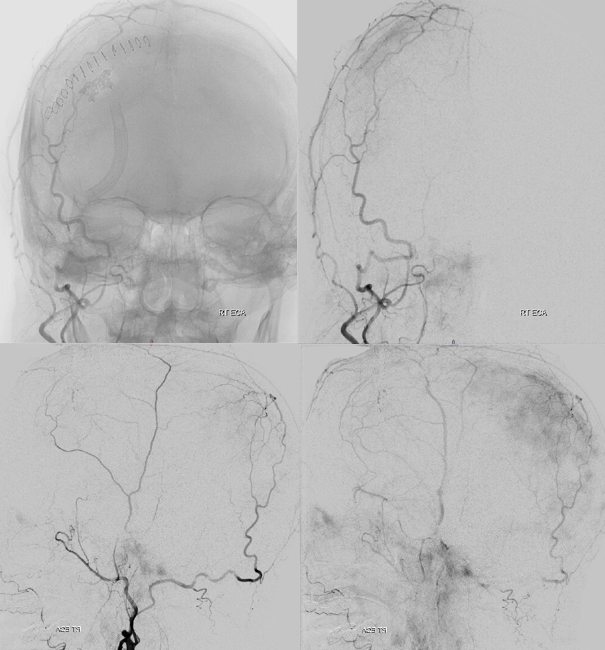
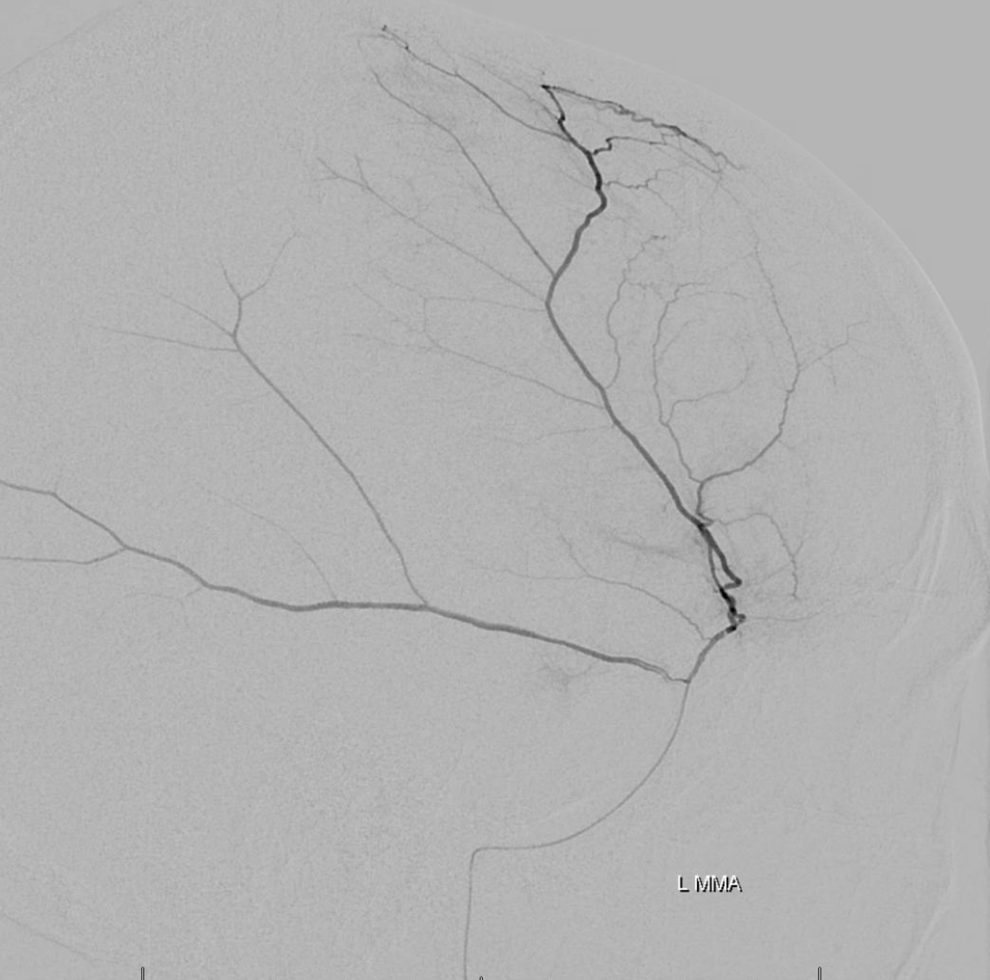
Pre left MMA embo
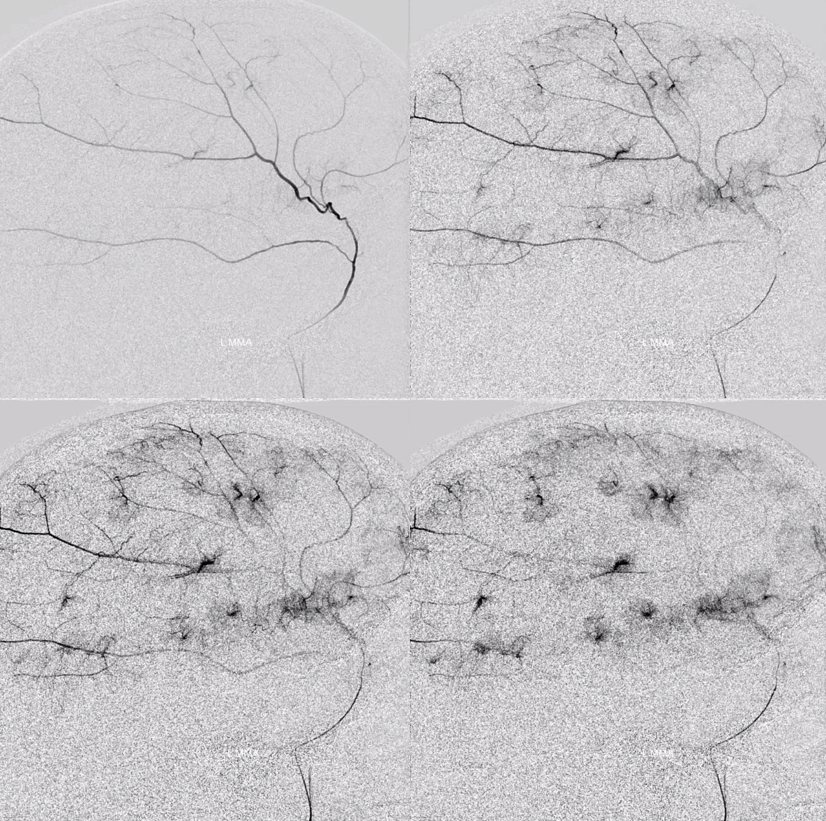
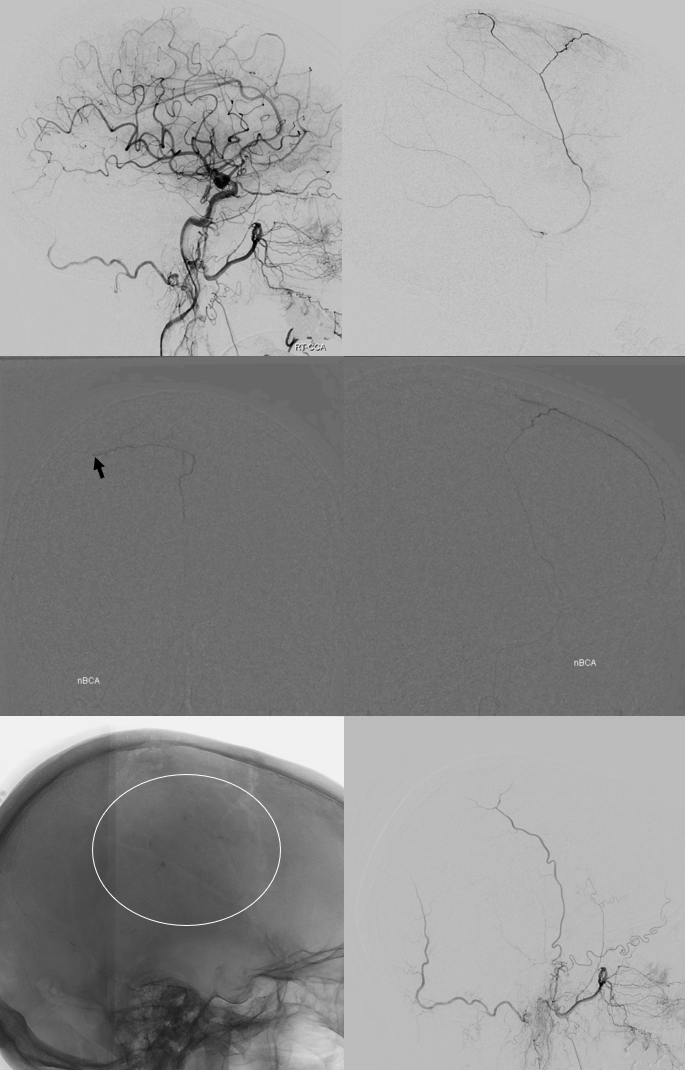
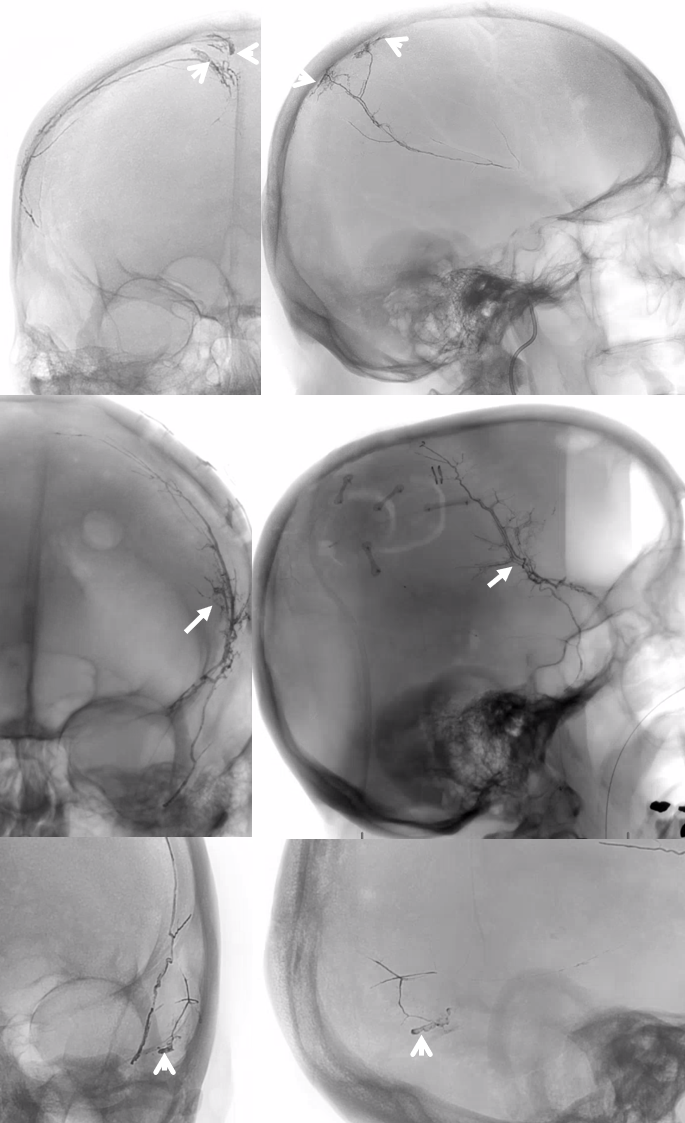
The frontal branch is embolized with particles. Next, the petrosquamosal/parietal branch is injected (below). Notice however that numerous previously not visible primary outer network anastomoses with the just embolized frontal branch are present, not only opacifying its convexity dural territory but also showing presence of several “spot signs” — contrast extrav/ leakage into the hematoma (arrows). This suggests insufficient particle penetration into the distal frontal branch territory. Why? Perhaps the catheter was in a “wedge” position in the frontal branch or maybe particles clumped. Its not size, as 45-150 um Contours were used — plenty small to get into distal territory. Whatever the case, you can see how an embo of frontal branch alone like might not succeed.
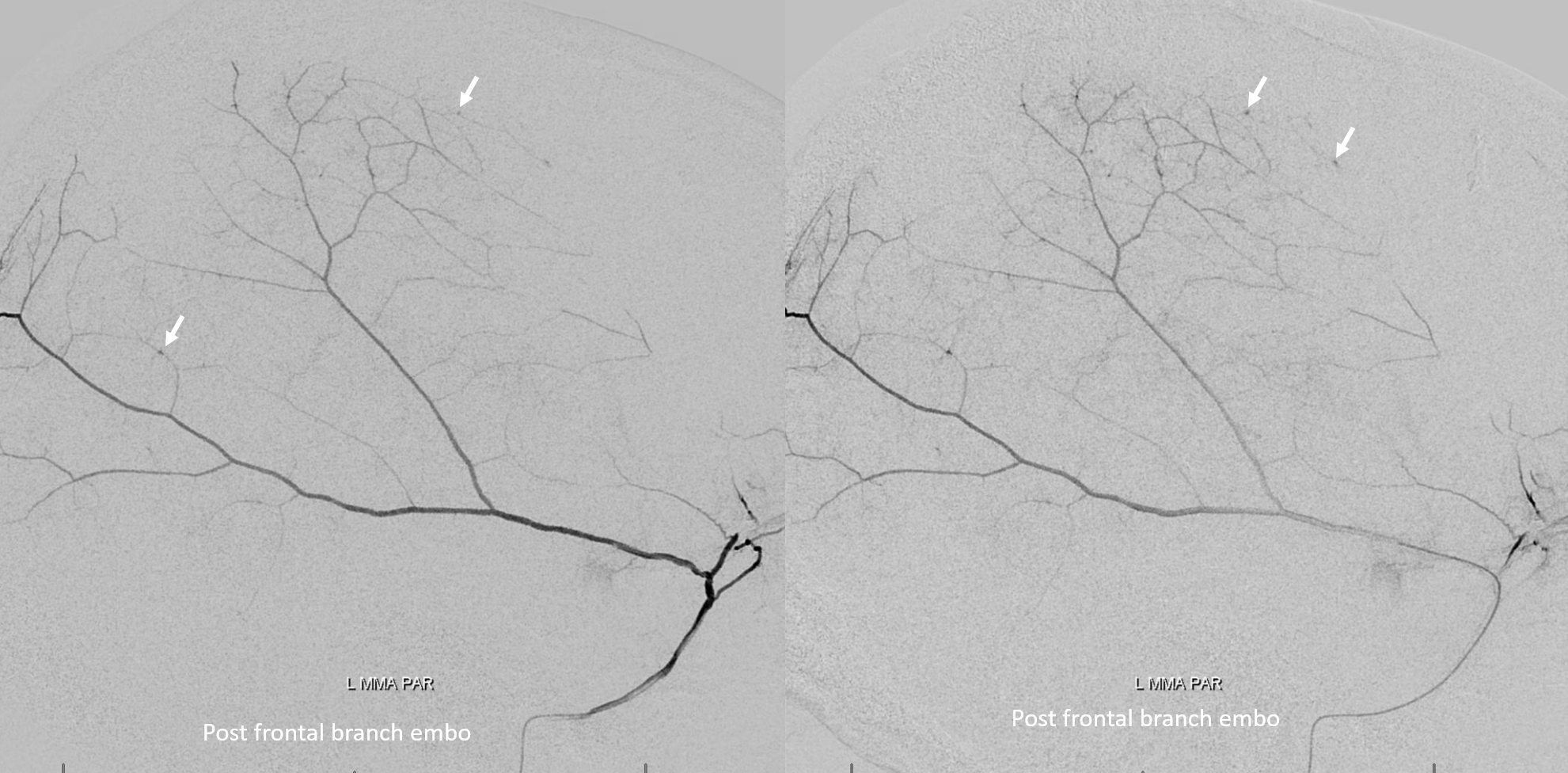
The above shows both extent of primary outer layer anastomoses, and potential mechanism for failure of subdural embolization. Below is a final ECA image after embo of the above branch. Coils (arrows) were placed into both frontal and parietosquamosal branches after particle embo
 Post embo DYNA nicely shows both inner hematoma membrane (arrow) and contrast layering in various compartments (open arrows)
Post embo DYNA nicely shows both inner hematoma membrane (arrow) and contrast layering in various compartments (open arrows)
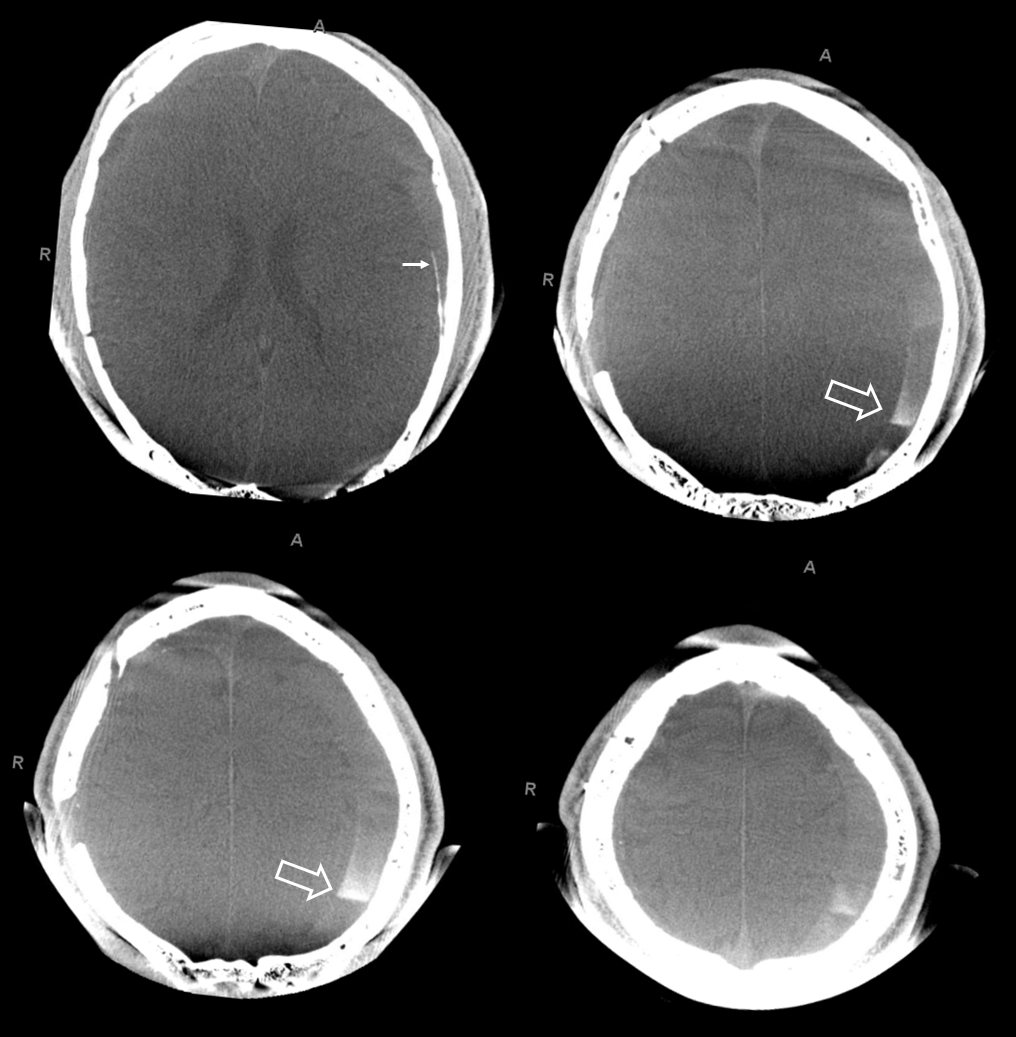

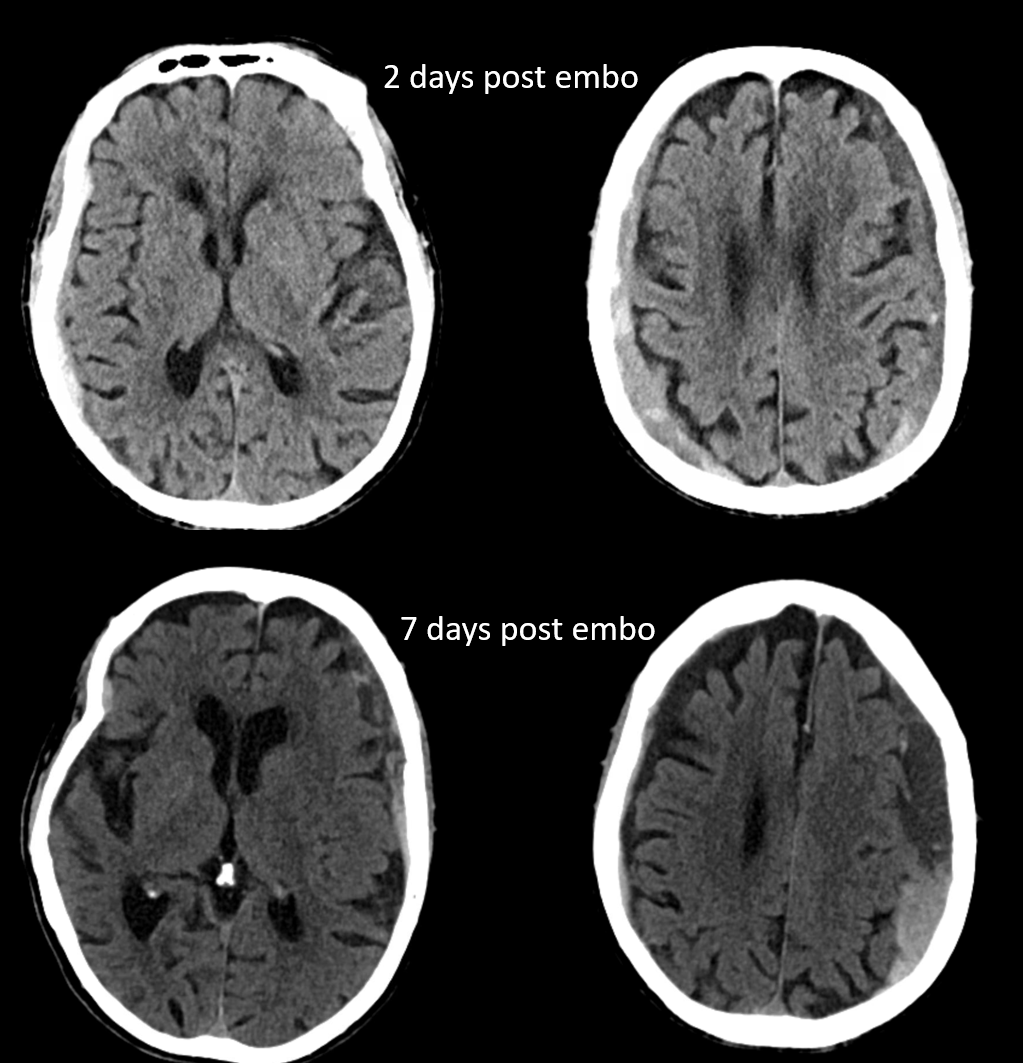
The hematoma is slow to resolve, but again is a clinical “success” — the question is would it work better with more embolic material penetration. We don’t know. We also don’t know what is the nature of fluid hyperdensity 1 and 3 mo. post. Of course it could be rebleeding. Could it also be residual contrast that cant go anywhere?
Here is another interesting case — pre-embo MRI below. Notice parenchymal hematoma also
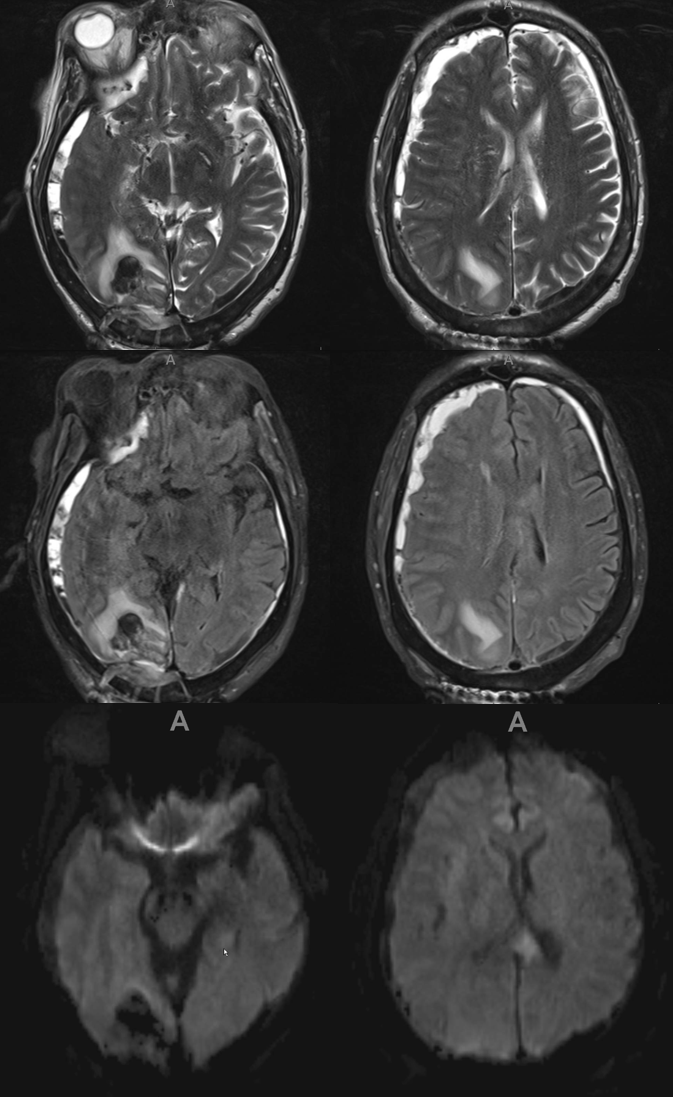
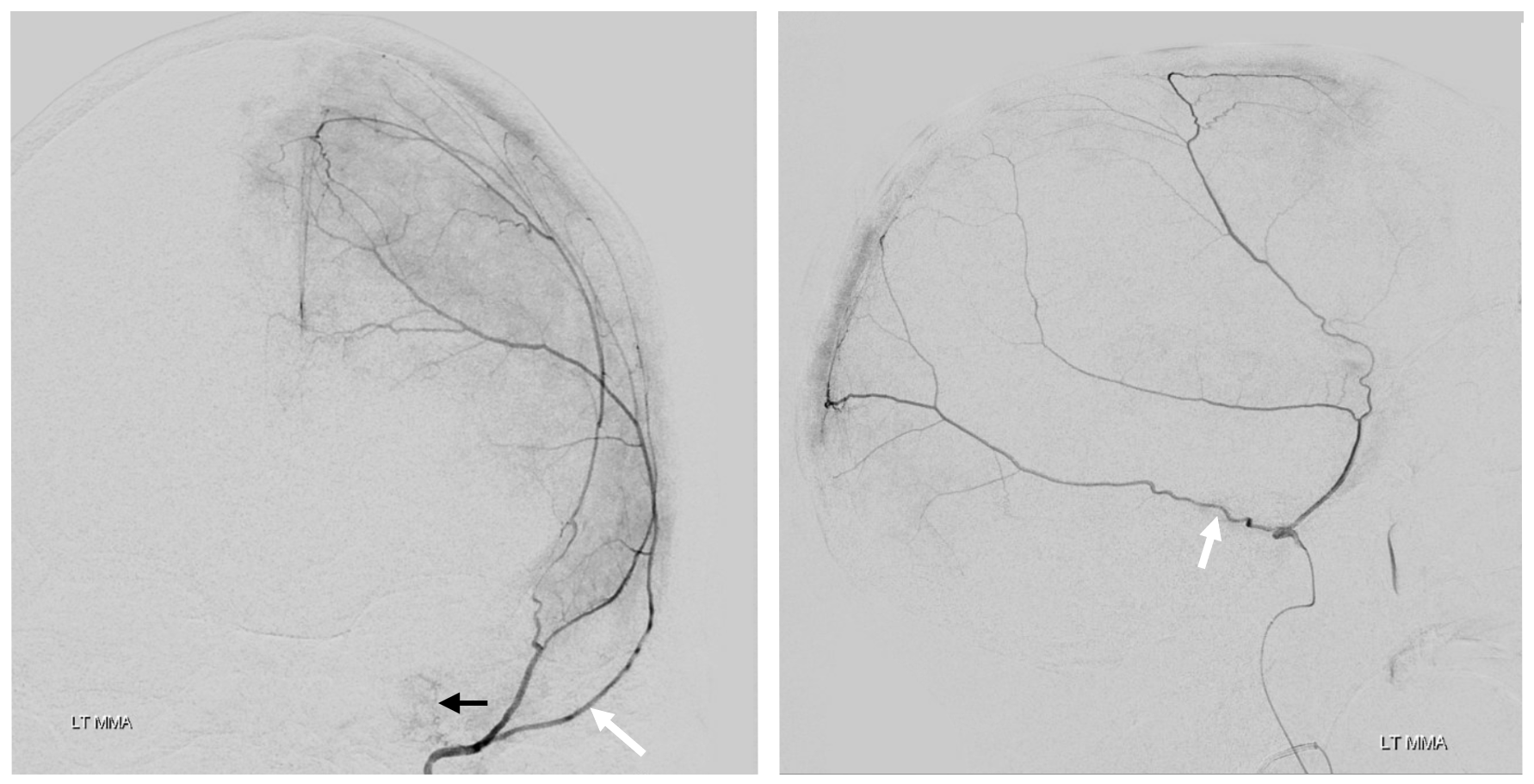
Right side embo only — this is what an adequate particle embo looks like — there is persistent staining of the dura and contrast stagnation in distal branches (arrows and ovals), persisting into venous phase of injection (arrowheads)
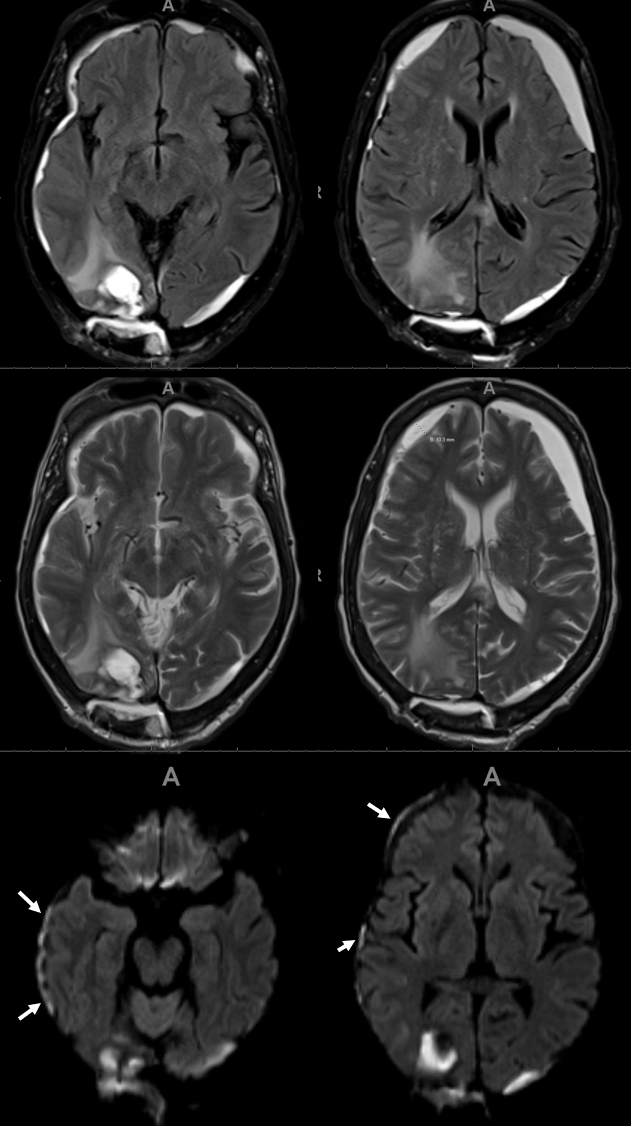
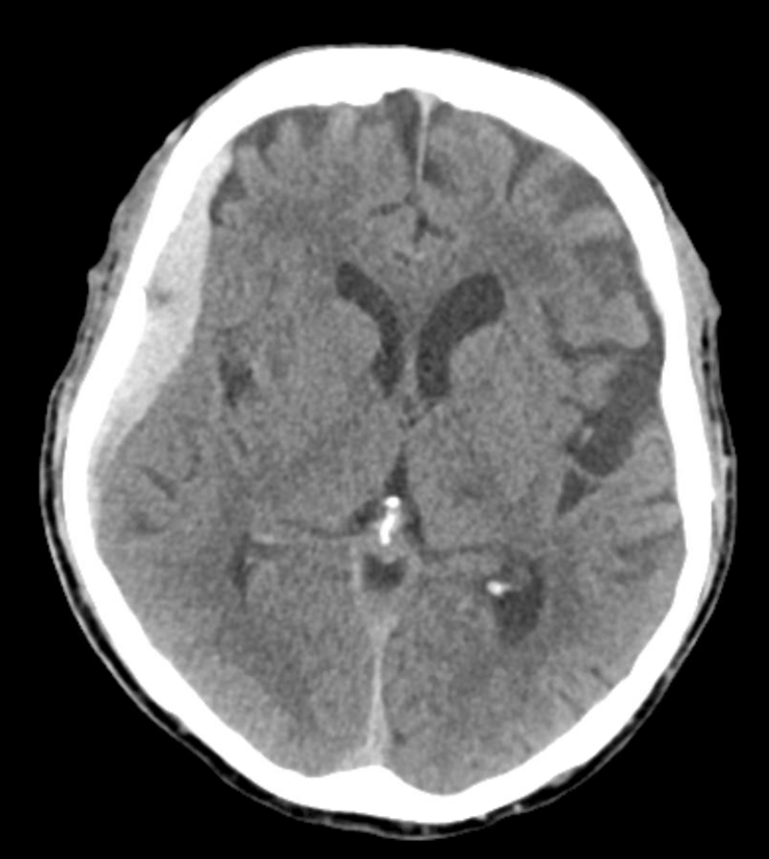
1 month later, we have an internal control — right hematoma is smaller, left one bigger! Also notice something u don’t see every day — diffusion restriction in the dura post embolization (arrows). Yes, we know, its a very heterogeneous blood-filled environment there — nevertheless, this is actual diffusion restriction we believe — a marker of good embo
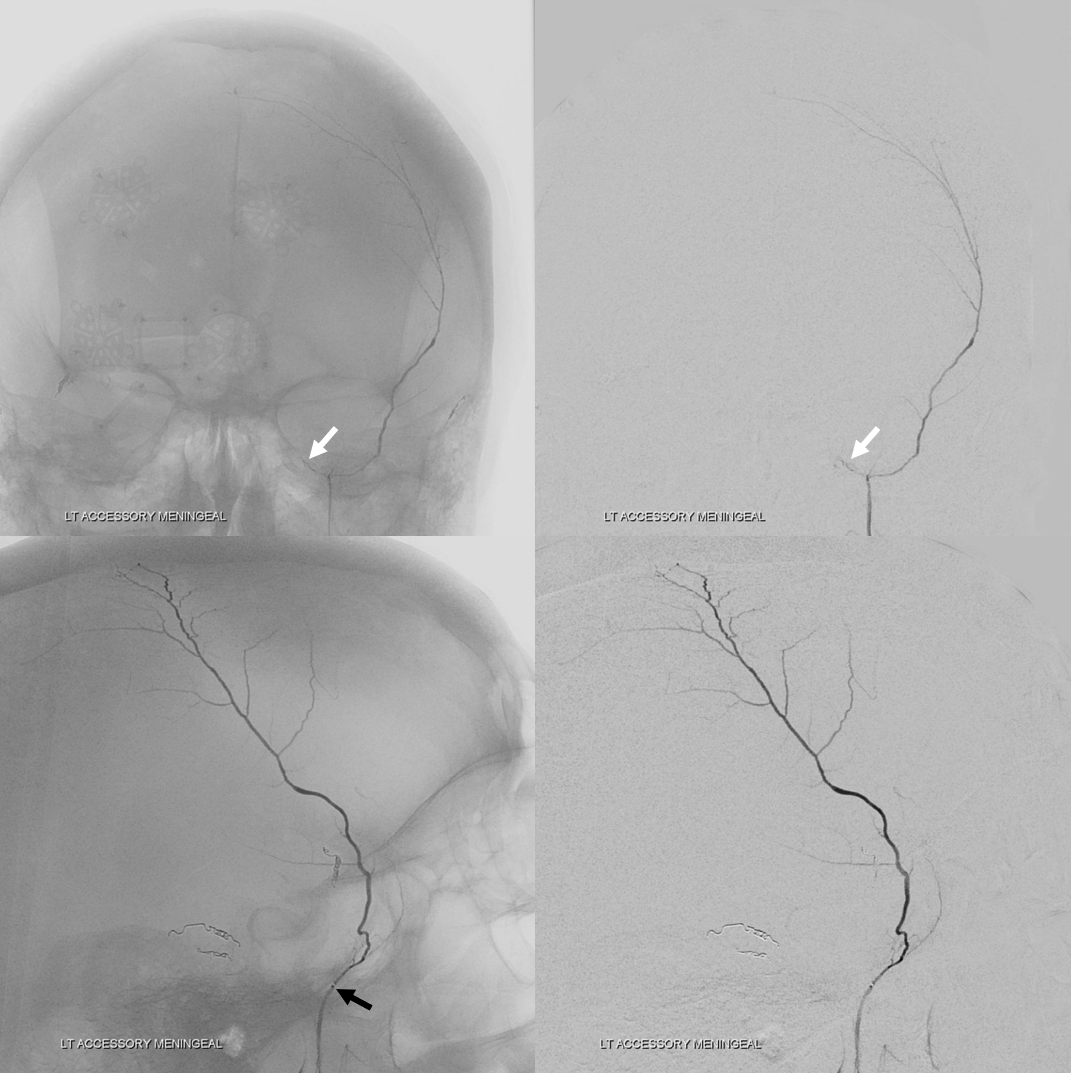
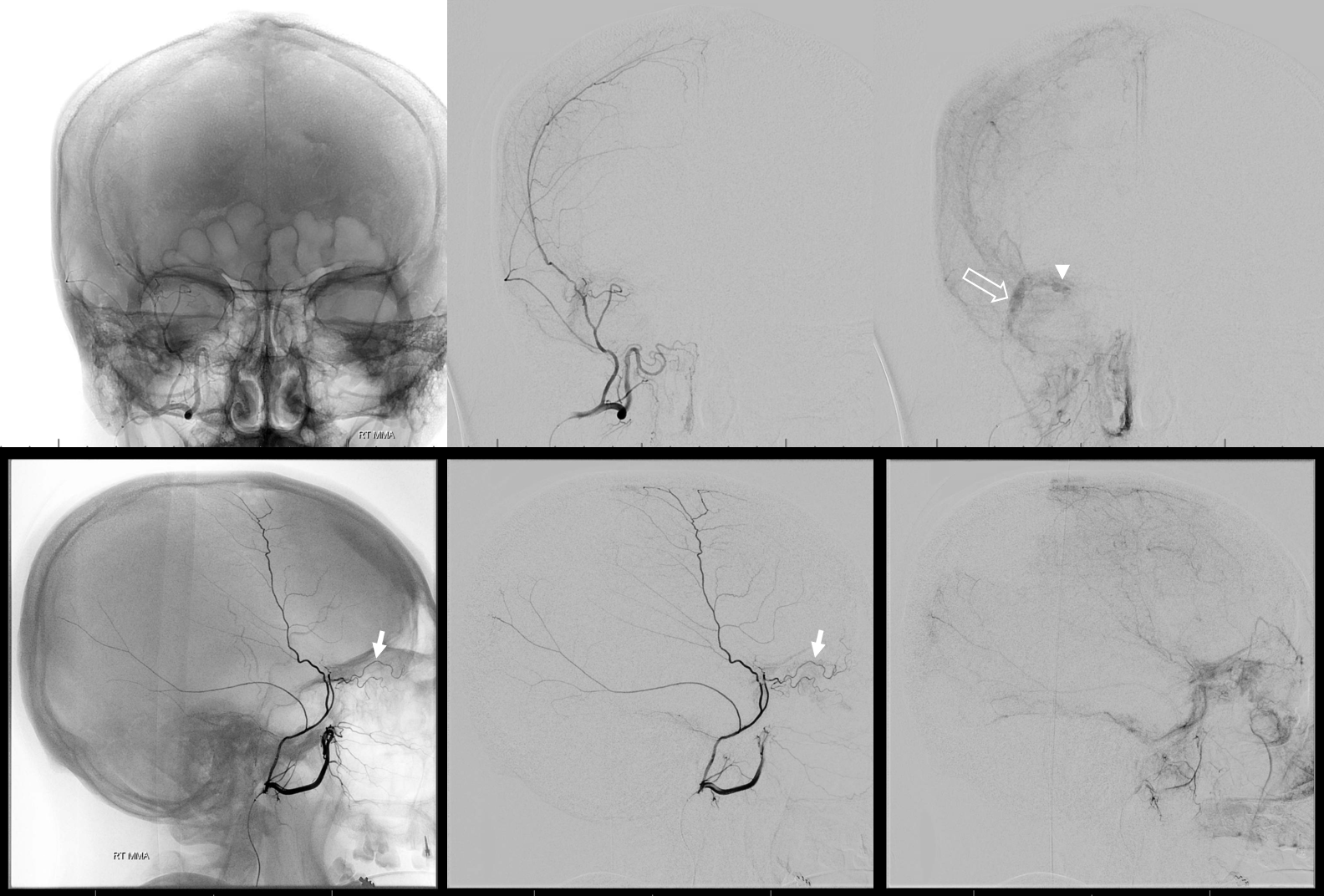
Below is a rare variant — the frontal branch is supplied by the accessory meningeal artery — see separate origins of frontal and parietosquamosal branches?
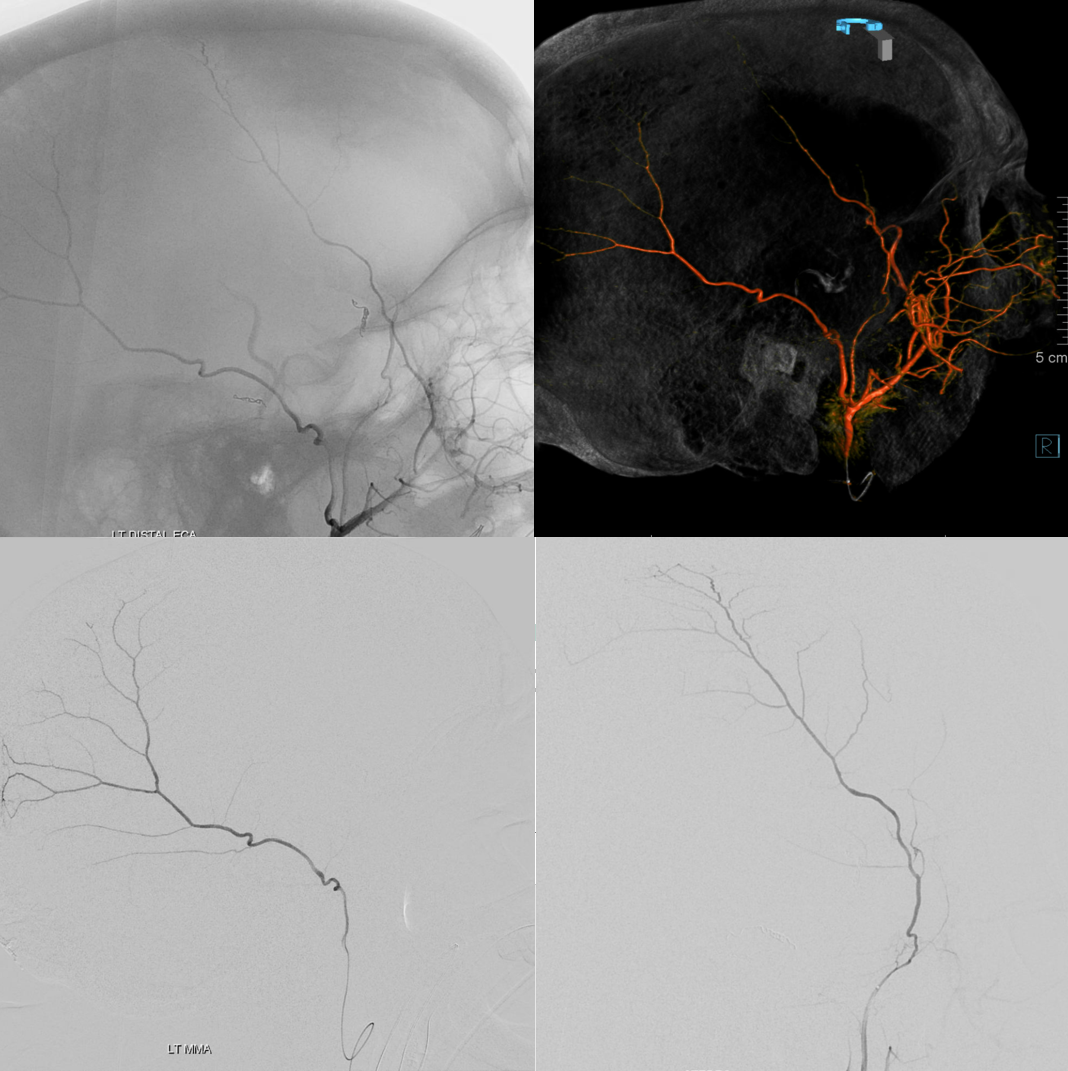
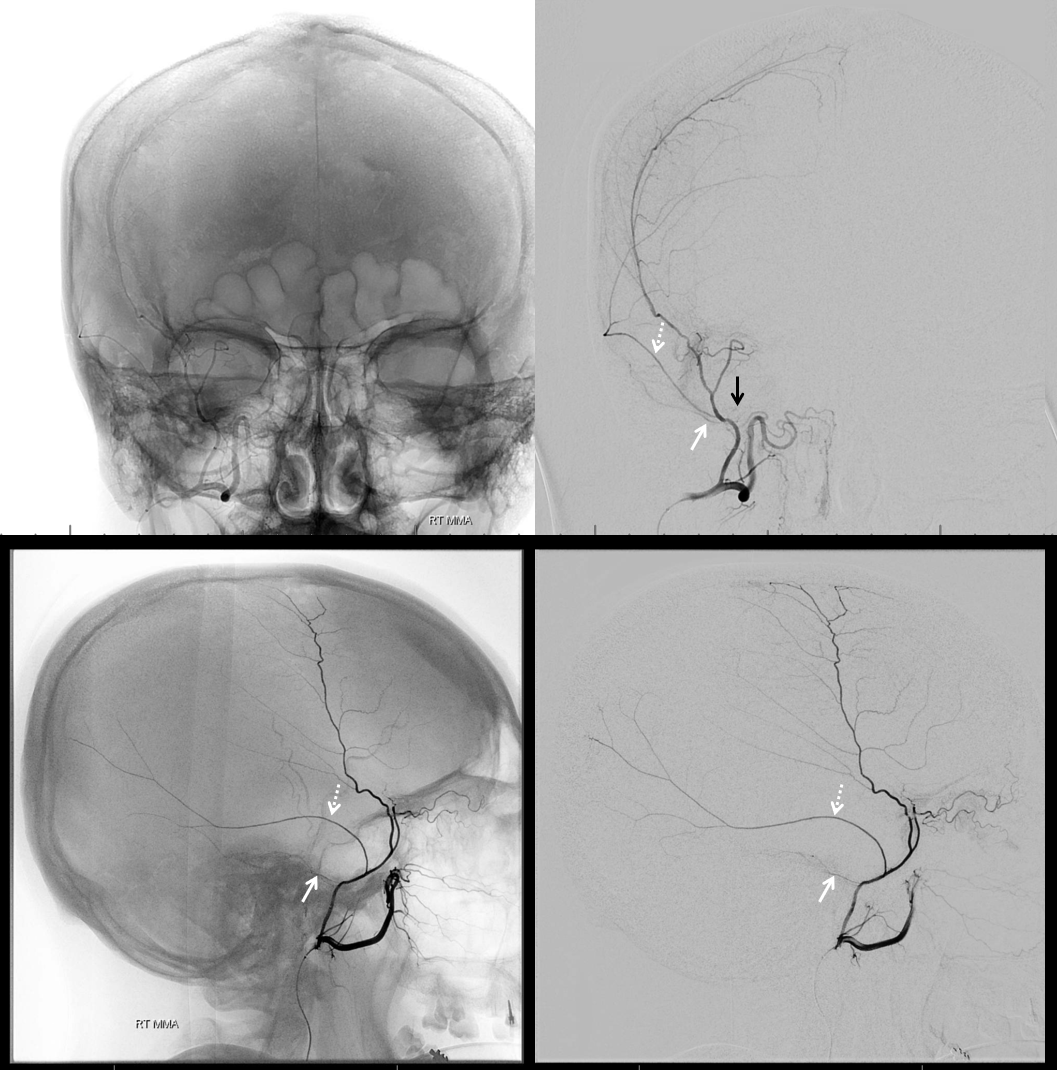
More good stuff follows — check out the true petrous branch (white arrows). It characteristically projects superiorly on frontal views, and is obscured by dense petrous bone on lateral views. Black arrow shows catheter position in foramen Ovale. Embolization from this position, in addition to facial and greater petrosal nerves, would also risk V3 (Ovale)
So we go a bit distally. But wait, there is more! Look at images below — “suddenly” we see the ophthalmic artery (white arrows) — projecting characteristically over the orbit in frontal views. Why? Because we have a “wedge position” — there is no flow around the microcatheter. Contrast injection pressure then opacifies a sphenoid ridge artery anastomosis with the ophthalmic system (not visible from the Ovale position injection). So many points here…
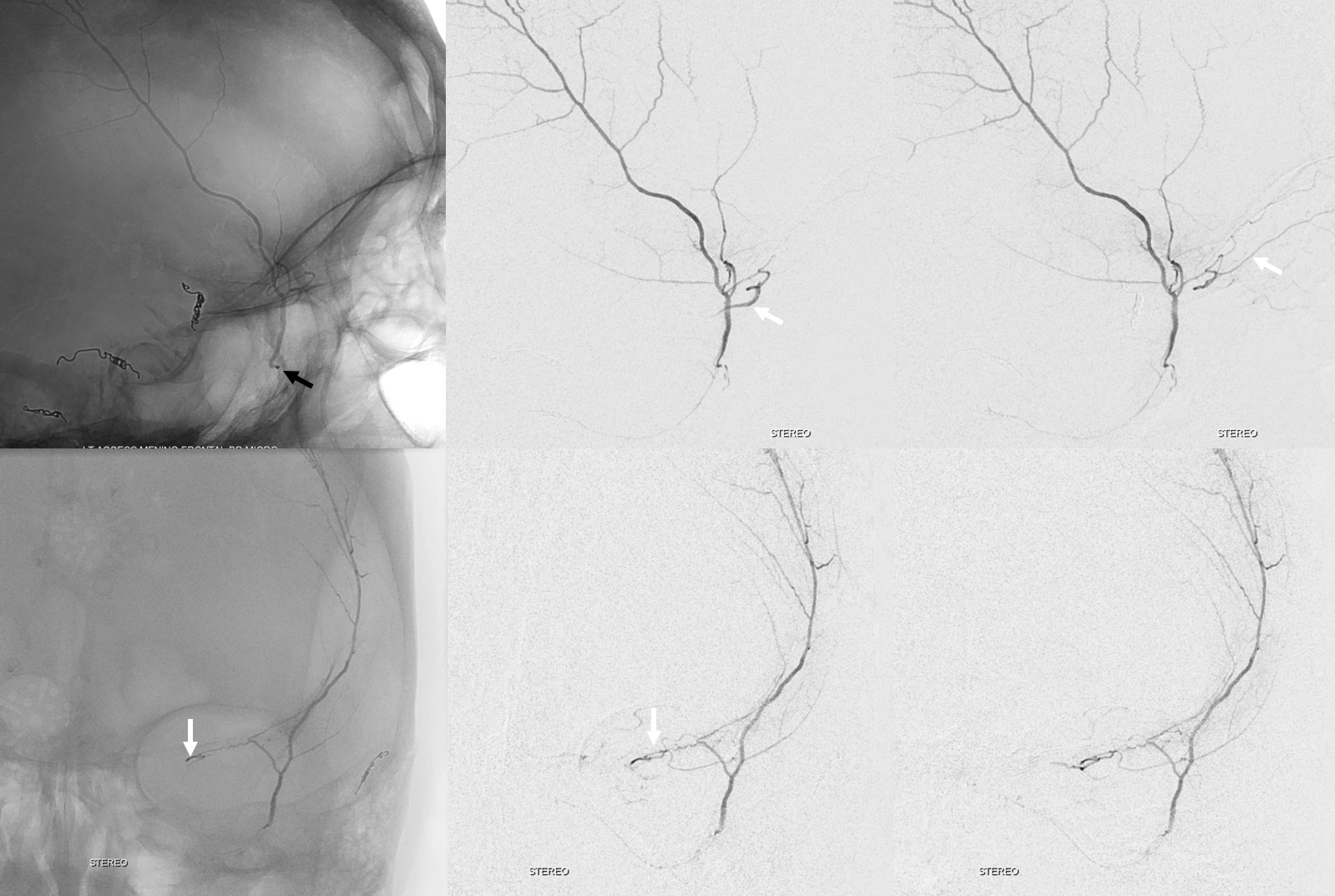
What to do? We can use particles and inject gently — safe enough. Or try to go beyond the origin of sphenoid branch — probably the best option. if we dissect the vessel trying, that’s no worse than what we ended up doing instead, which is just coiling it at the microcatheter position above (black arrow). Chickened out this time — not the optimal way to go.
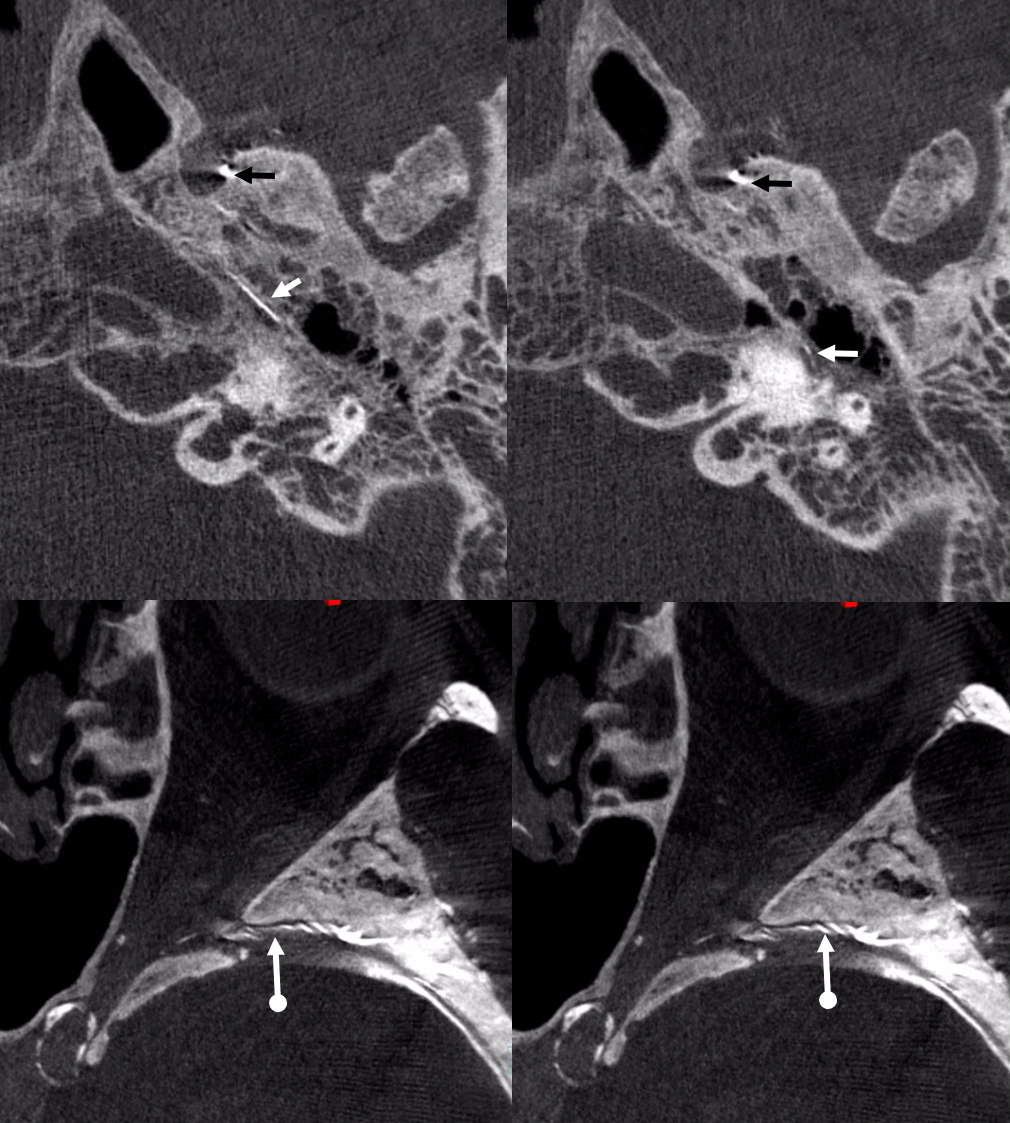
Below are DYNA CTs. The upper two images were done with catheter tip in Ovale (black arrows) — the petrosal branch is labeled with white arrow. The lower two DYNAs were done from the higher position where we saw the ophthalmic — with sphenoid ridge branch shown by ball arrows
Final post below (white arrow on frontal branch coil)
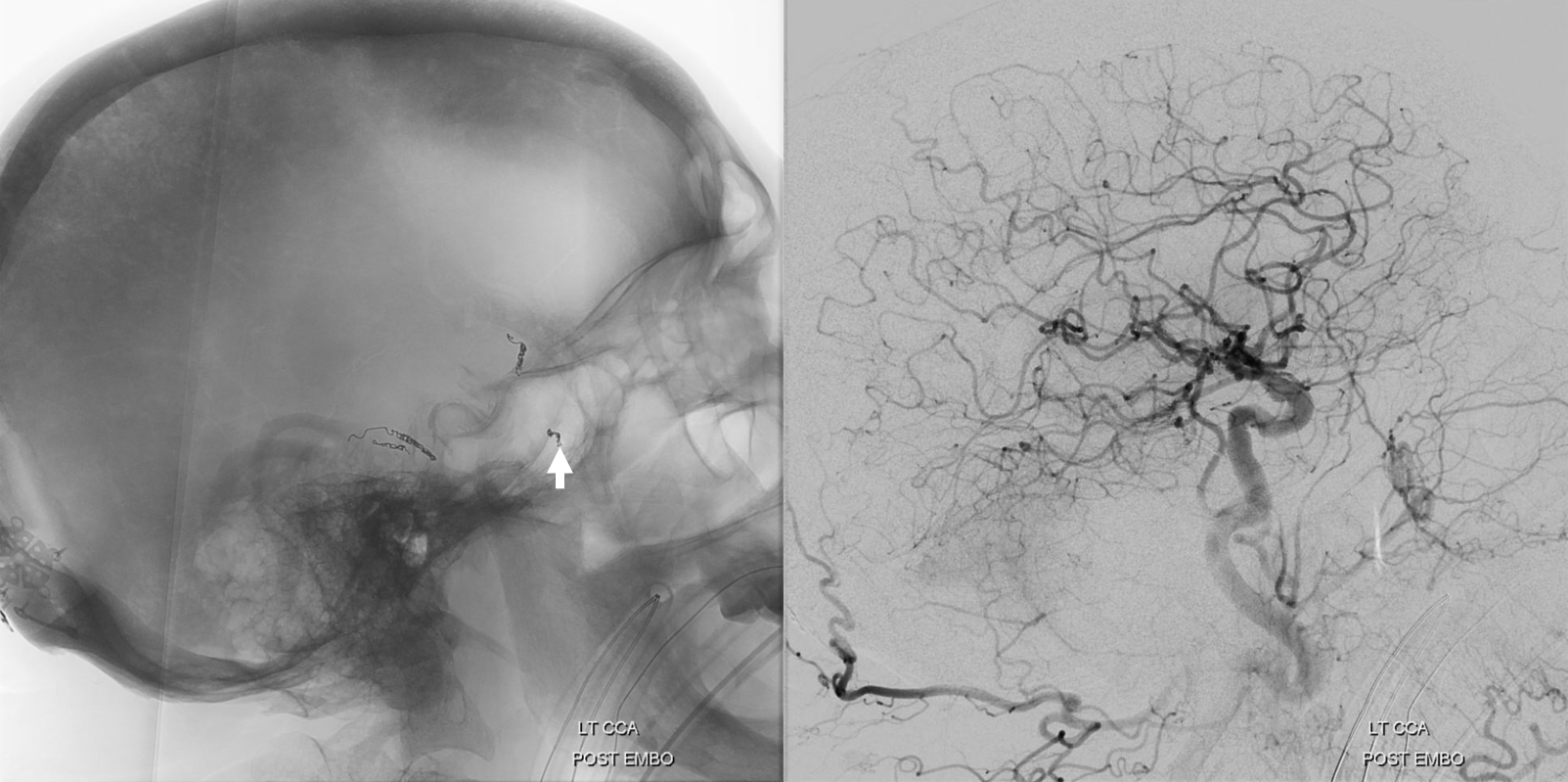
A few weeks later, the parietal convexity hematoma is gone. The frontal one is a bit smaller, but again — would it have been better if we used particles or liquid embolic in frontal branch?
Meningeal-ophthalmic Anastomoses
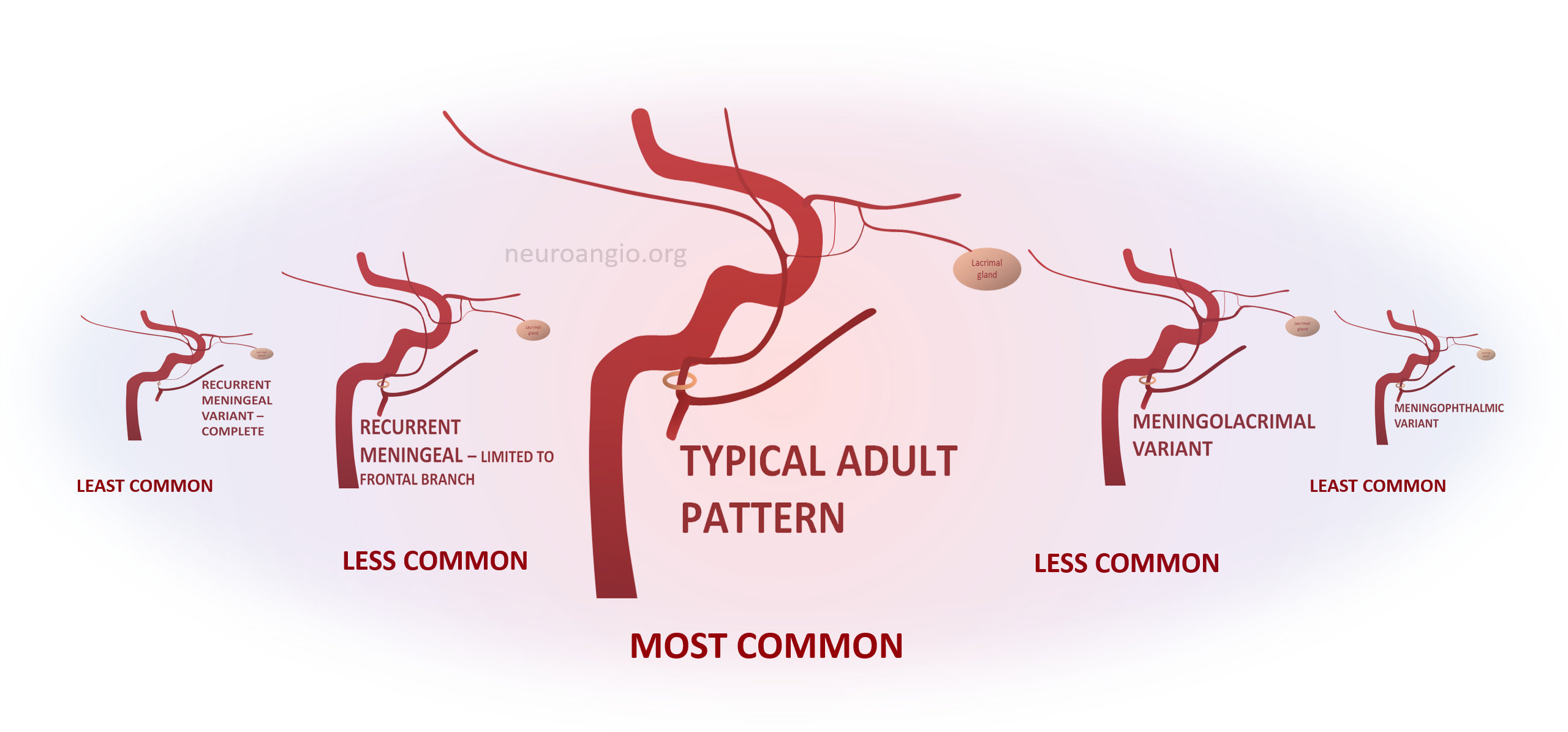
These have been covered in endless articles, as well as here — see ophthalmic, meningeal system, and middle meningeal artery pages. What is frequently missing is the big picture of how we look at variants in general. The idea is a “spectrum” view of variation. Any two interconnected vascular territories show variable balanced, partial, or complete dominance of one territory over another, with extremes being progressively rarer. The AICA–PICA and PCOM–P1 relationships are common examples. Middle meningeal-ophthalmic relationships follow the same principle.
The two systems are interconnected. The most common disposition is “separate” MMA and ophthalmic territories. In “variant” disposition, either artery can supply the territory of the other, to variable extent. Partial takeovers are more common than complete ones. Makes perfect sense now.
Meningo-Ophthalmic Spectrum
When MMA supplies part of whole orbit. The more common one is “meningo-lacrimal” — when supply is “limited” to lateral orbital territory. At least 5% incidence in our experience. Typical appearance is of a vessel projecting over lateral orbit on frontal view. Lateral also projects over orbit but both views are very helpful (ovals)
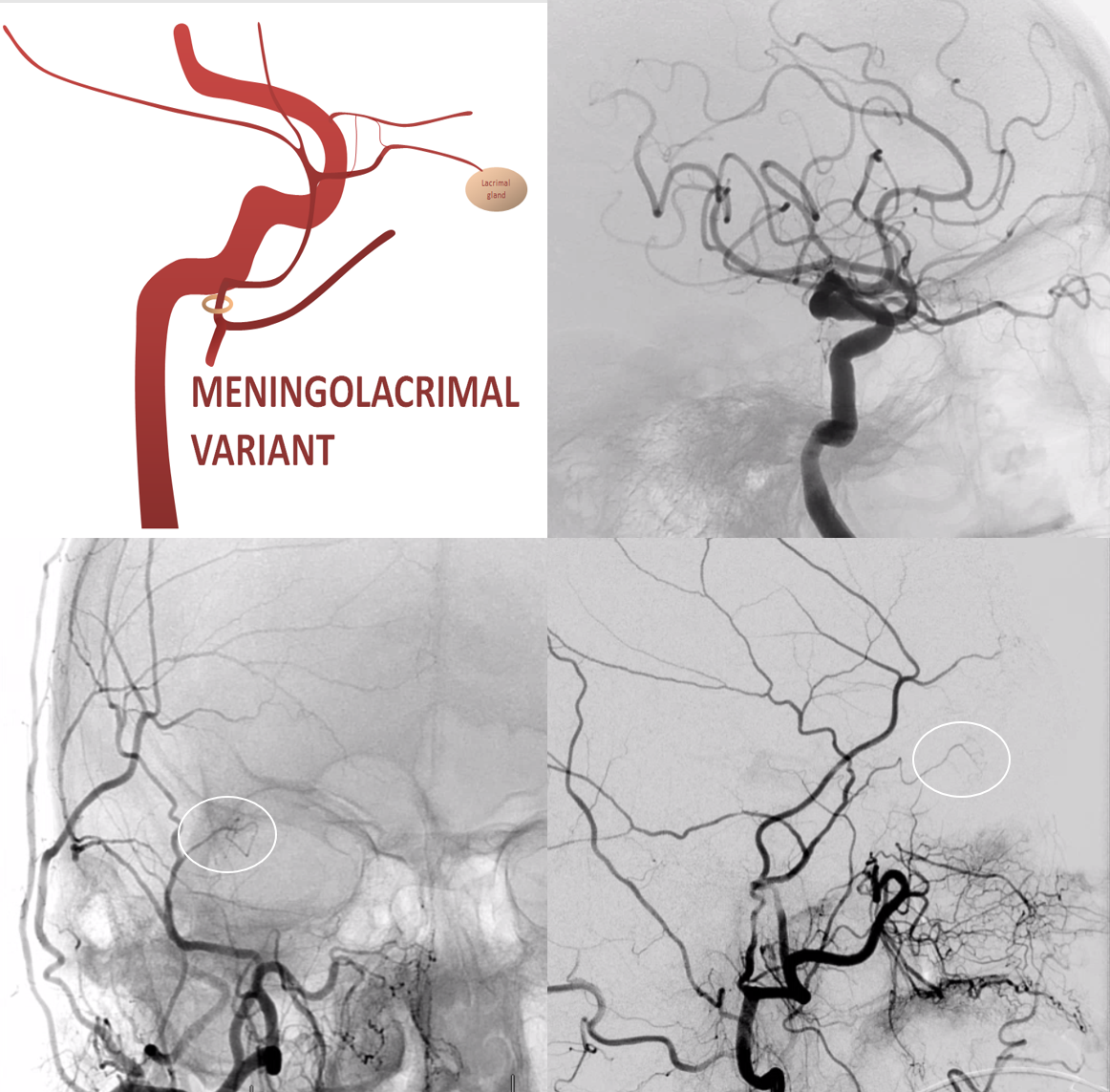
There is a lot more of this on MMA and ophthalmic pages
Meningo-ophthalmic Variant
“Complete” takeover of ophthalmic artery by the MMA — and thus less common than meningo-lacrimal. Again, meningo-lacrimal and meningo-ophthalmic are not discrete states, but a spectrum — larger and smaller chunks of lateral orbit can be supplied by the “meningolacrimal” for example — will show below. In the classic view below, there is no identifiable ophthalmic artery from ICA, with choroid blush etc. supplied by MMA. On frontal view, the artery extends to the medial orbital rim (arrow)
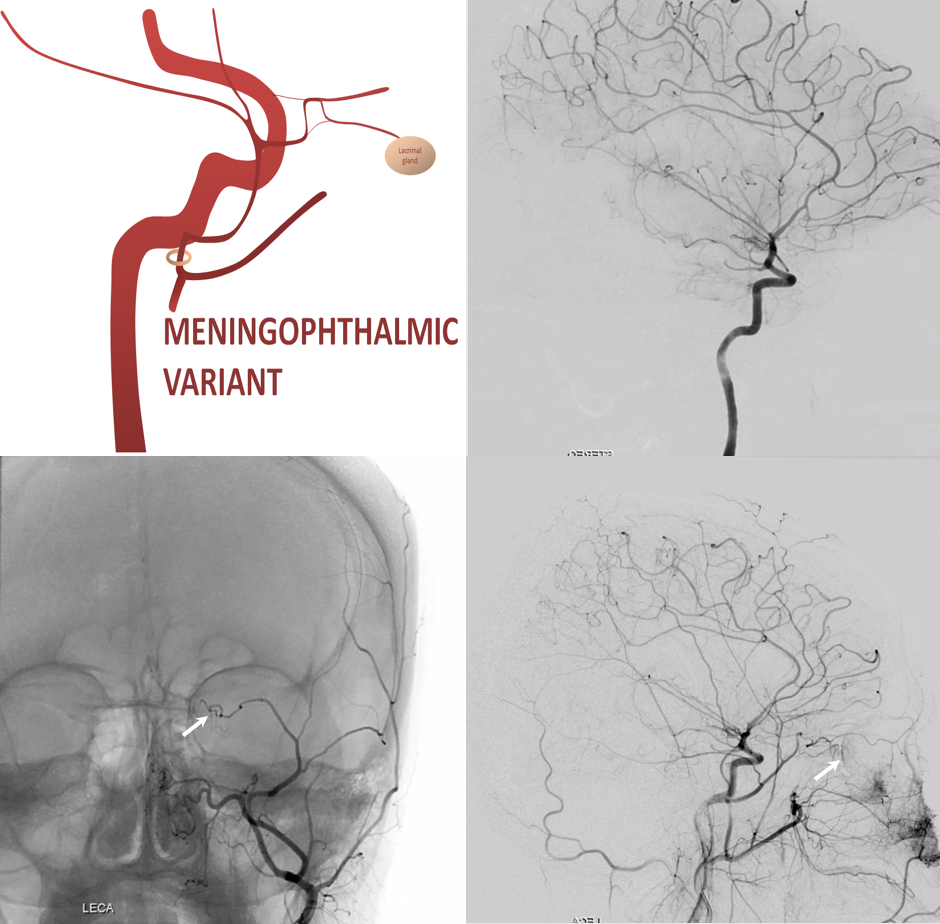
Recurrent Meningeal Spectrum
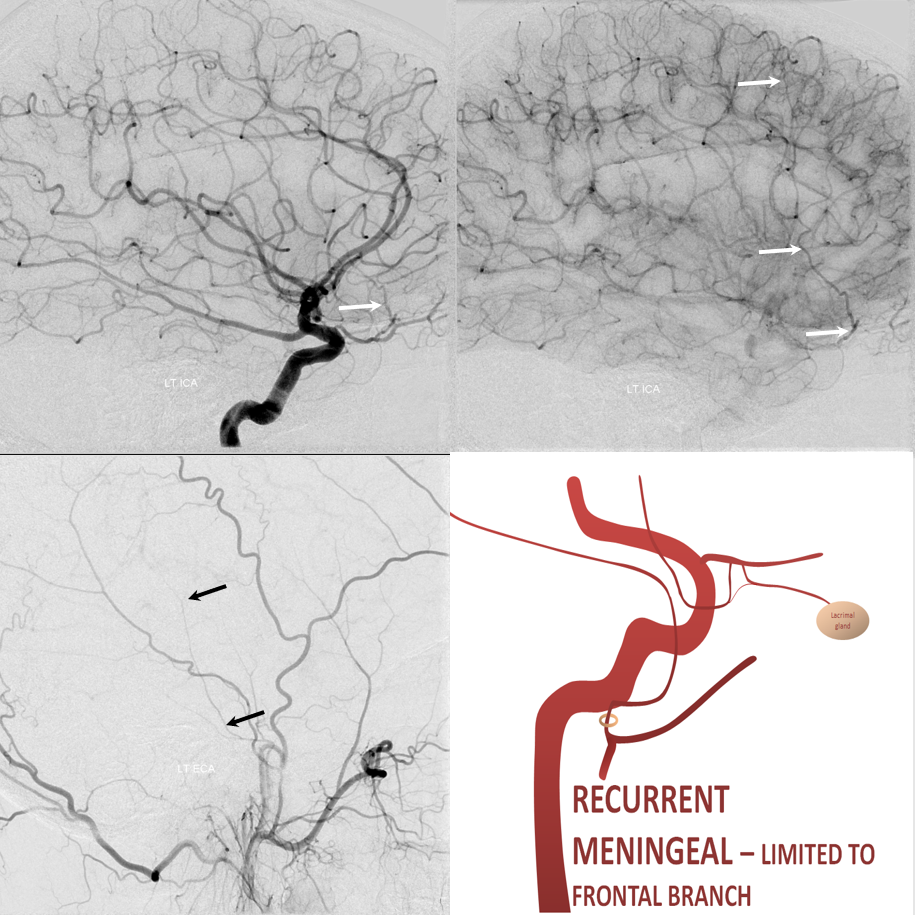
The other side of spectrum is ophthalmic “takeover” of MMA territory. Here the spectrum manifests also in extent of takeover. More commonly, only the frontal MMA branch (white arrows) is supplied by the ophthalmic system, as below. The parietal branch (black arrows) is as usual. Incidence is ~2% in our experience
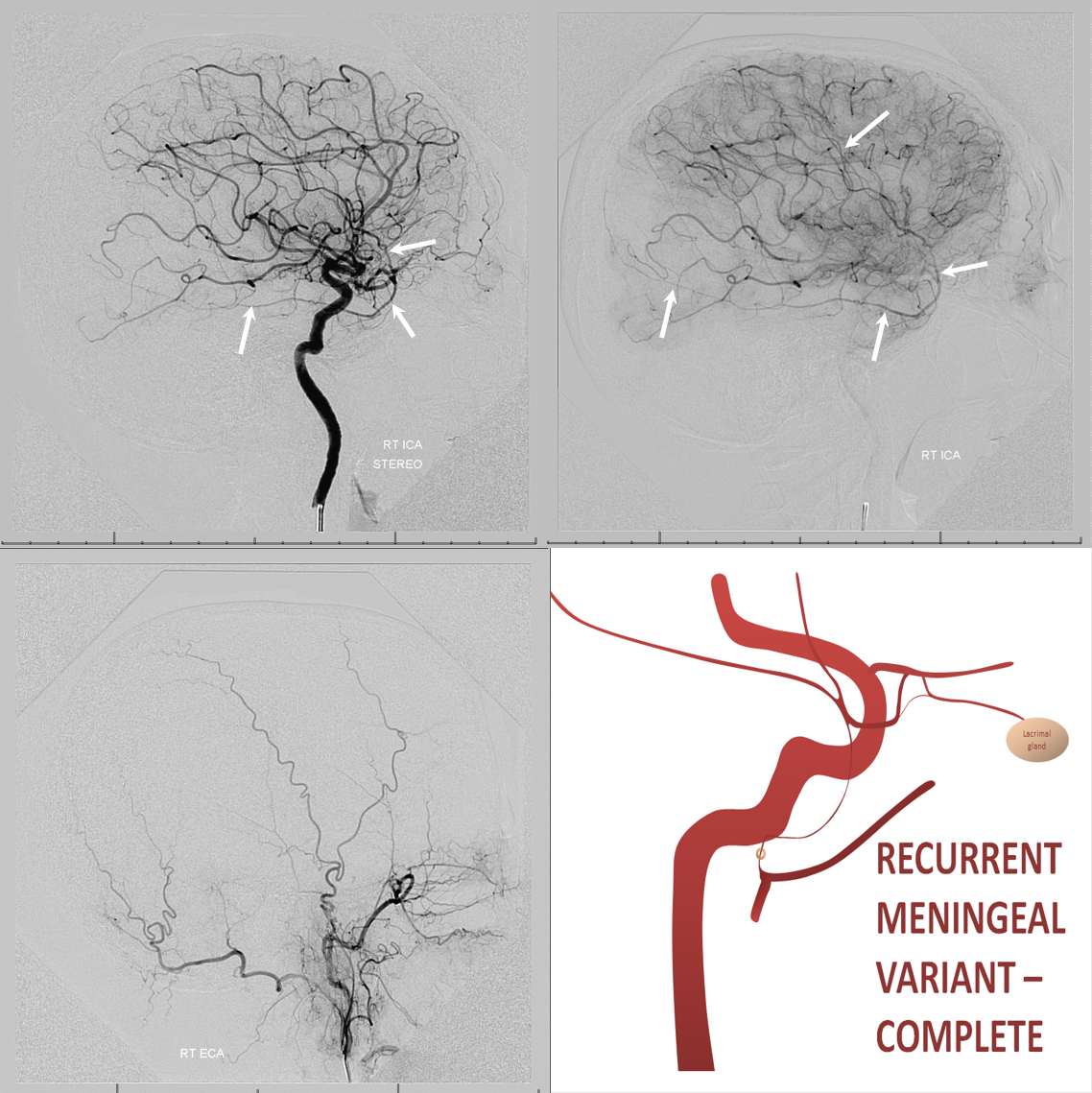
In the rarer ~0.5% disposition there is complete supply of MMA territory by the ophthalmic.
Implications for cSDH embolization — safety, efficacy, technique
Recurrent Meningeal Spectrum
Of course, these variants impact cSDH safety and efficacy. Recurrent meningeal spectrum mostly impacts efficacy, limiting territory accessible for embolization without catheterizing the ophthalmic artery. In most cases, petrosquamosal and parietal territories can still be embolized. Furthermore, it is sometimes possible, using several techniques, to deliver embolic material into at least some dural territory suppled by the ophthalmic through the primary outer network anastomoses. Remember, these measure about 100-300 microns. So, 45-150 micron particles can penetrate these to some extent. The other option is liquid embolics — however their penetration routes are often unpredictable — more on that below.
Here is an example of patient with bilateral subdurals
On the left, there are better anastomoses (arrowheads) between the “normal” origin parietal branch and the frontal branch supplied by the ophthalmic (white arrows). On the right, the anastomoses are not good (black arrow — frontal branch)
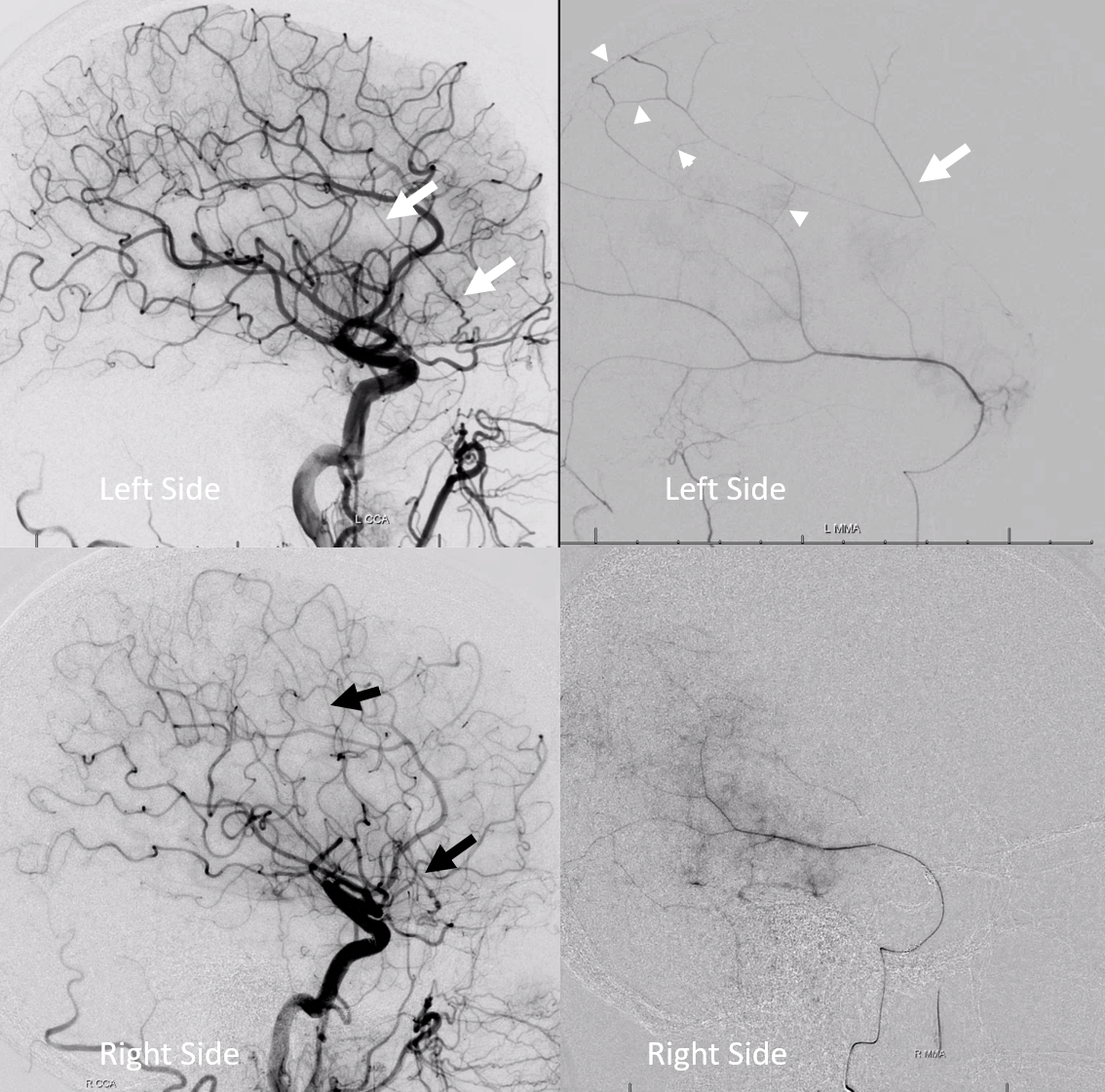
Following embo, the left subdural is gone. Right one is smaller over parietal region, but here is a small new hemorrhage in frontal dural territory (open arrow). Again, this is a clinical success — no further procedures required — but all important learning points.
As with any one of our procedures, technique is key to success. Given how variable both tumor and MMA embo techniques are across the board, we are likely to see a spectrum of MMA embo outcomes as well.
Another example below — bilateral also, worse on right it seems
On left side, there is a recurrent meningeal (arrow) supplying both frontal (arrowhead) and parietal (ball arrow) branches — spectrum again! We use nBCA in hope of permeating as much of recurrent meningeal territory as we can. In the end, there is penetration into parietal territory, but frontal branch is still well seen on post embo LT CCA injection (arrowhead, lower right image).
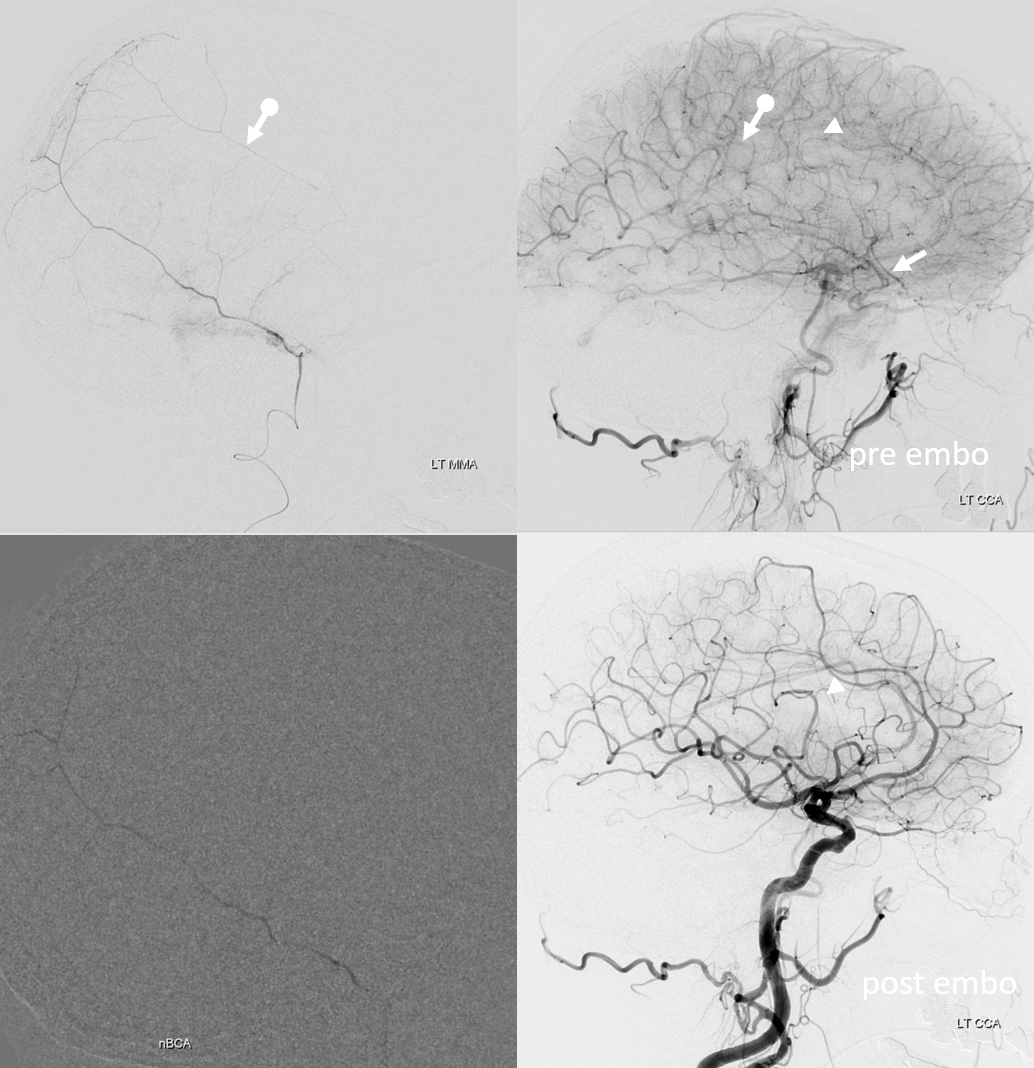
On right side, the disposition is “normal”. We go as distal as possible in frontal branch (black arrow – microcatheter position) and push more nBCA to see if we can permeate across midline — but the connections are either inadequate or glue does not fill that territory for another reason. We fill a lot of anterior meningeal artery and stop injection when we reach ethmoid region. Then we particle embo the rest of frontal territory (white oval — post embo dural contrast stasis) and finish the rest (lower right image)
The patient was restarted on anticoagulation for afib. Follow up shows that there is no magical thinking here. The right subdural quickly shrinks, while the left one grows — a prime example of failure due to unfavorable anatomic disposition. Anticoagulation was stopped. If stroke risk is high, catheterization of recurrent meningeal via the ophthalmic may be considered as a reasonable option.
Meningo-ophthalmic Spectrum
Here, the issue is mostly safety. However, we don’t want to sacrifice too much efficacy either — by not embolizing the frontal branch at all, for example. How to achieve these dual goals? In most cases, safe and efficacious embolization can be done by catheterizing the frontal branch distal to origin of its ophthalmic anastomosis — the sphenoid ridge branch. A position above orbital roof is likely safe. At this point, if there is still flow around the catheter, small particles can be used while monitoring for reflux, followed by coiling the branch to minimize recanalization. If catheter is in a wedge position, we prefer liquid embolics. To further minimize reflux risk, a dual lumen balloon microcatheter is ideal — however it will likely be “wedged” given its larger cross-section compared to an 017 microcatheter. At this point, we prefer Scepter and headway duo for these jobs.
Here is an example
The “meningo-lacrimal” branch contributes to supply of the superior rectus also (arrow), with blush of lacrimal gland (open arrow) and superior rectus (arrowhead) seen in parenchymal phase — “spectrum”
We put a Scepter C into the MMA past the orbital connection (via sphenoid branch). We are “wedged” even with Scepter not inflated — but we will inflate a bit for nBCA injection anyway — not too much, or you will make a fistula — more on that below. (If you do make a fistula, stay calm, they are benign — a bit of coil of liquid embolic will take care of it). Also look at those connections to the anterior meningeal arteries (dashed arrows) and supply to falx (arrowhead). Ball arrow points to a small dural branch we often see at orbital roof — its safe.
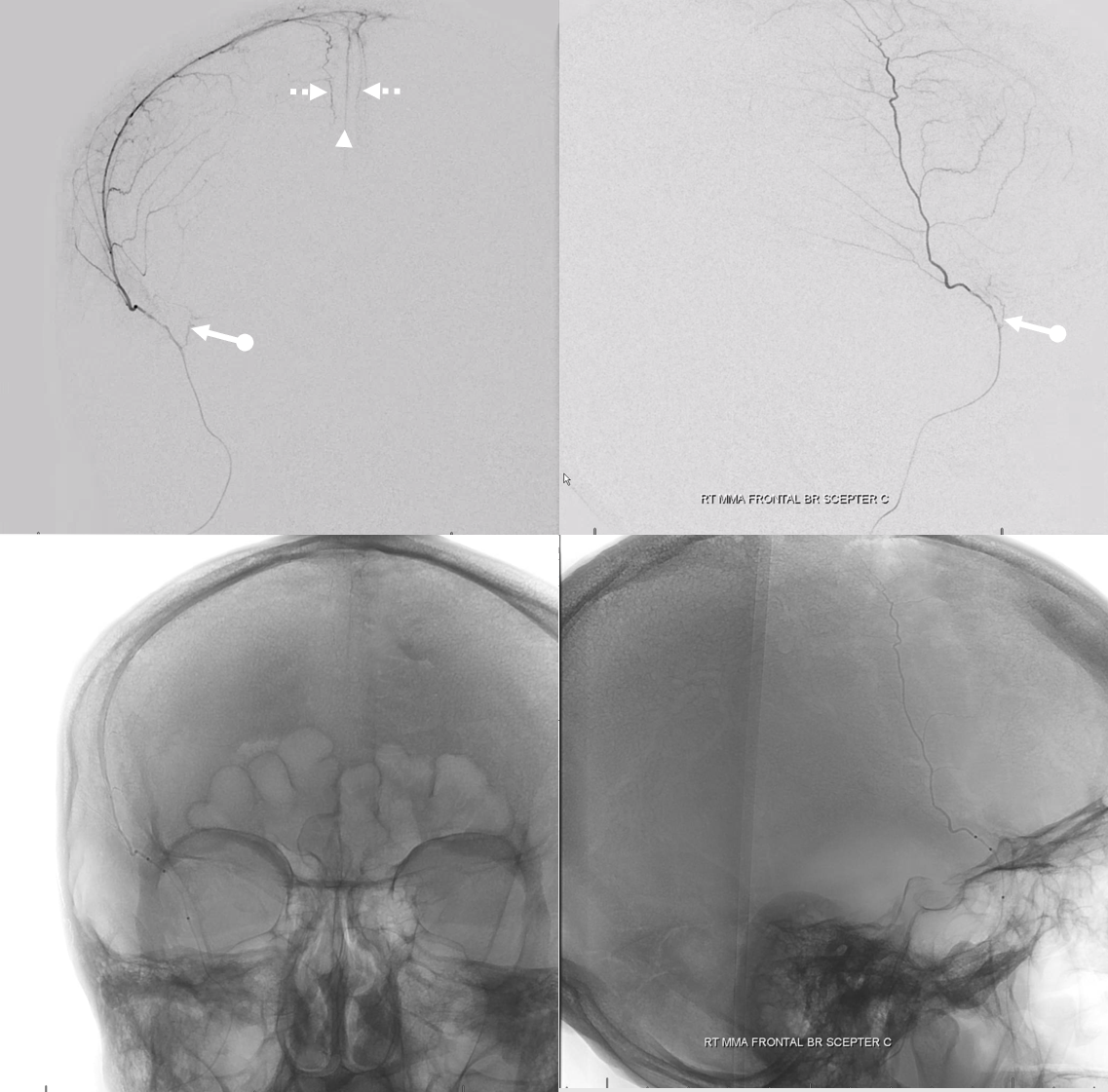
nBCA cast — very good penetration under the circumstances — with excellent penetration into the anterior meningeal system — stopping injection at the ethmoids.
Post — 1 mo later. Not perfect, but smaller, and clinically fine
Below is another educational example! — who wants to see boring stuff anyway?
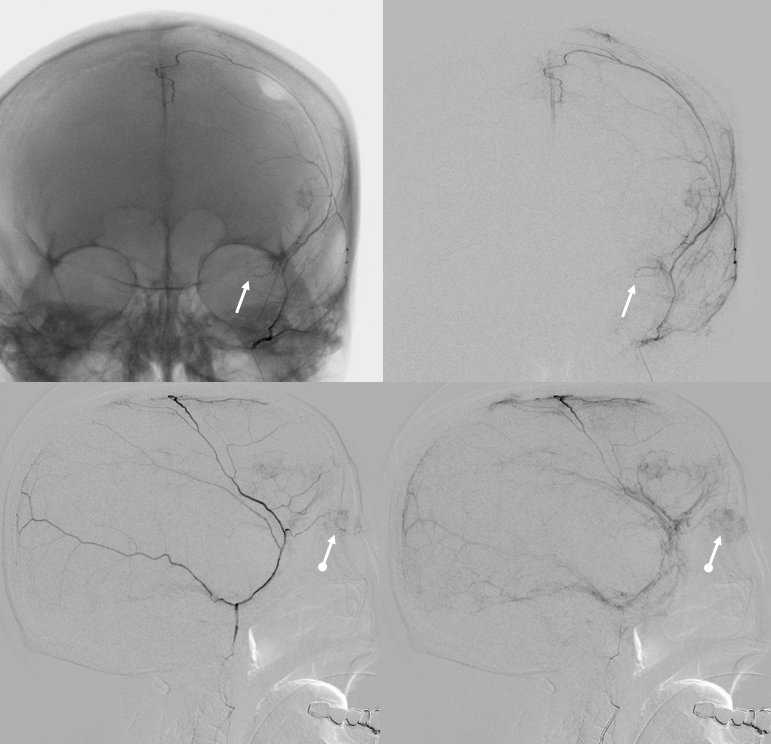
Another meningolacrimal (arrow) — see the lacrimal gland blush (ball arrow)
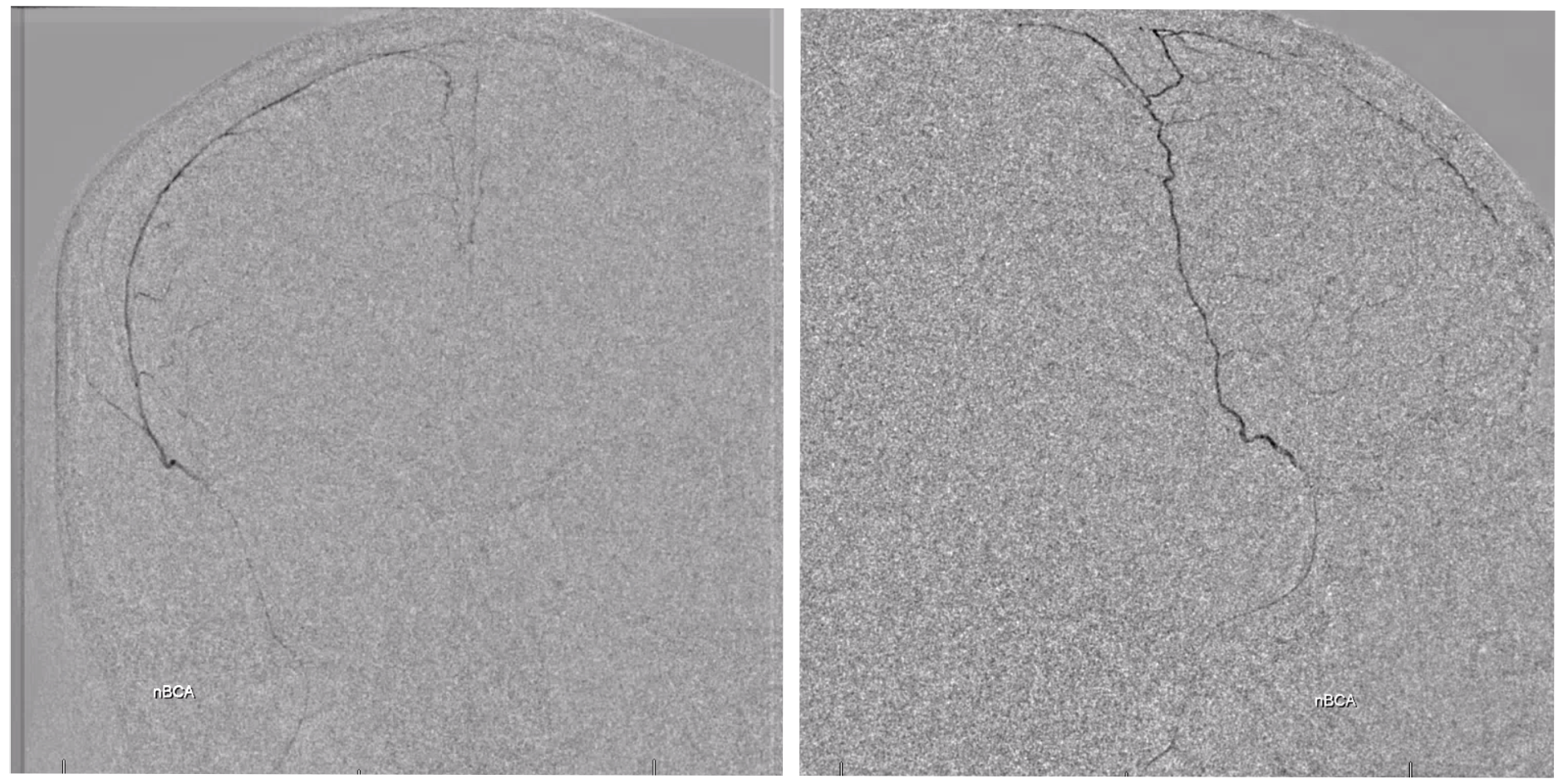
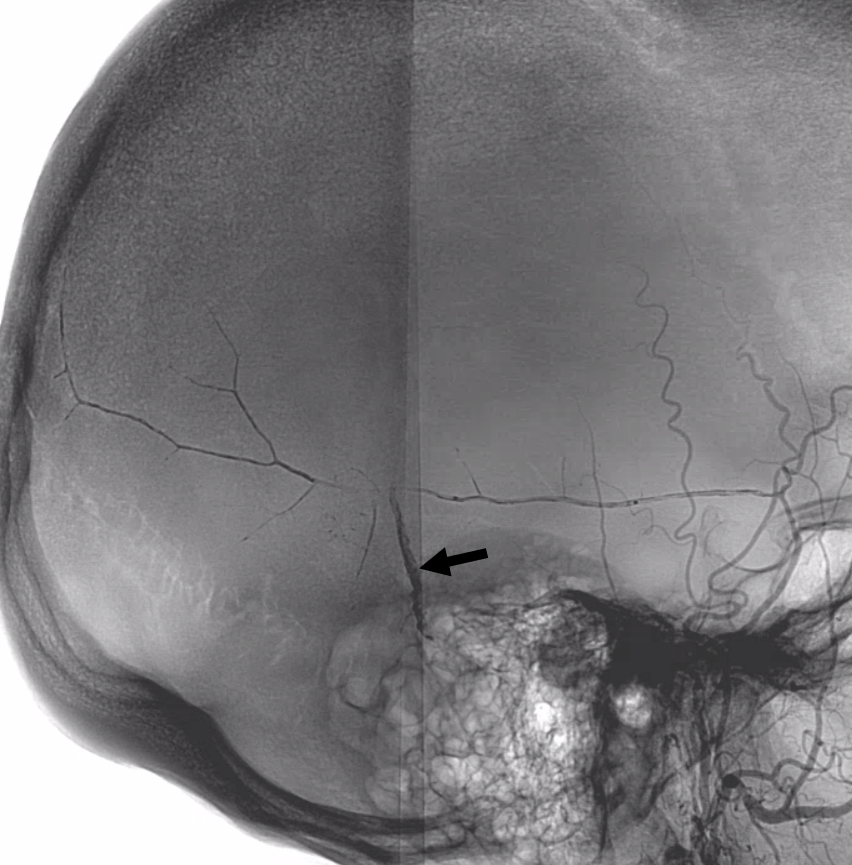
Lateral views — nice venous anatomy here that will come in handy later. There is a big diploic vein channel (black arrows) that can be seen on the native skull view and is later filled in venous phase of MMA injection — remember shared skull and dural arteriovenous supply/drainage. Also notice tram-tracking dural veins (ball arrow) adjacent to MMA branches.
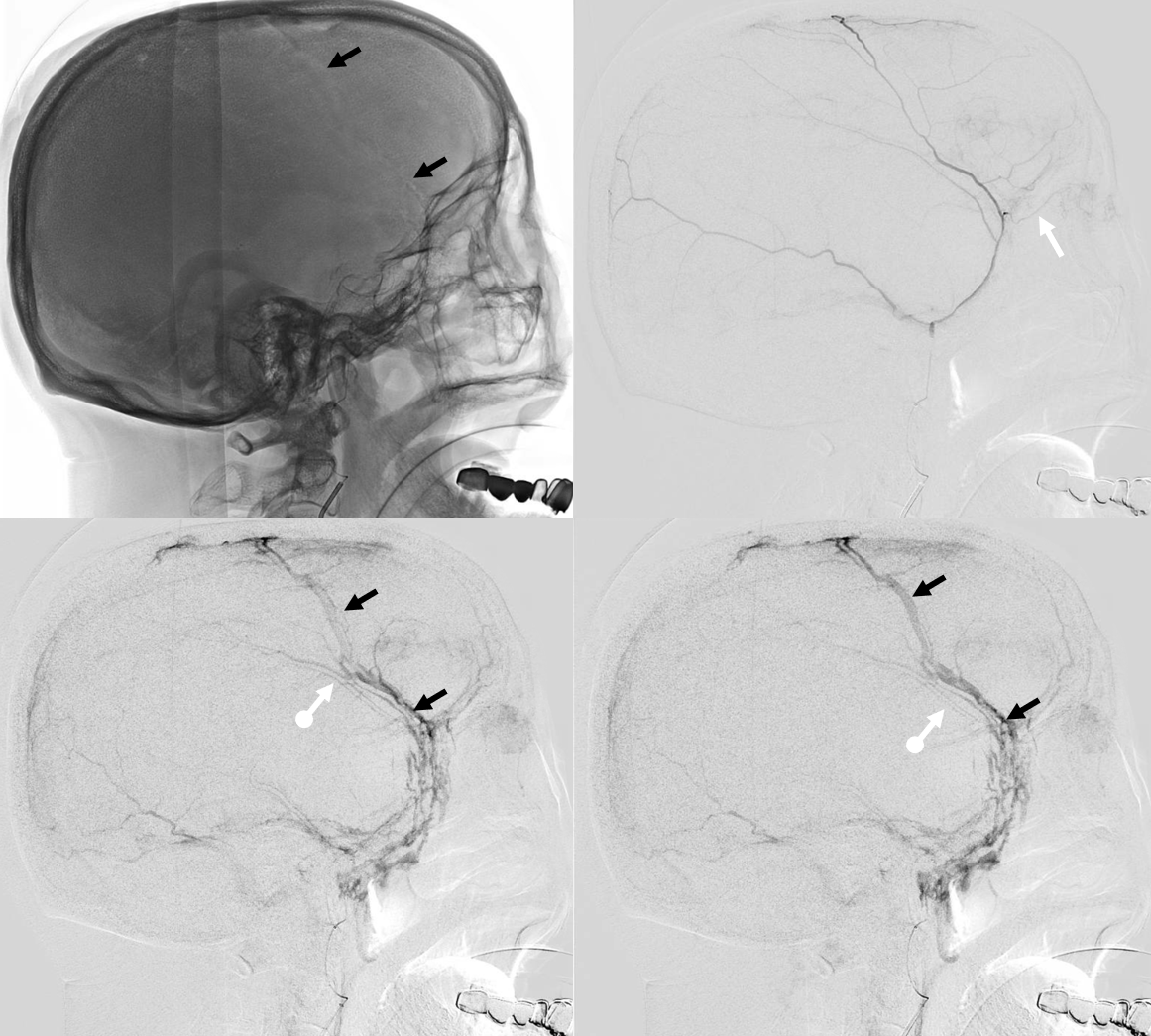
Again, we go distal to the lacrimal (black arrow). There is still flow around the duo – so, we go with particles. Its a single plane machine we are working on today.
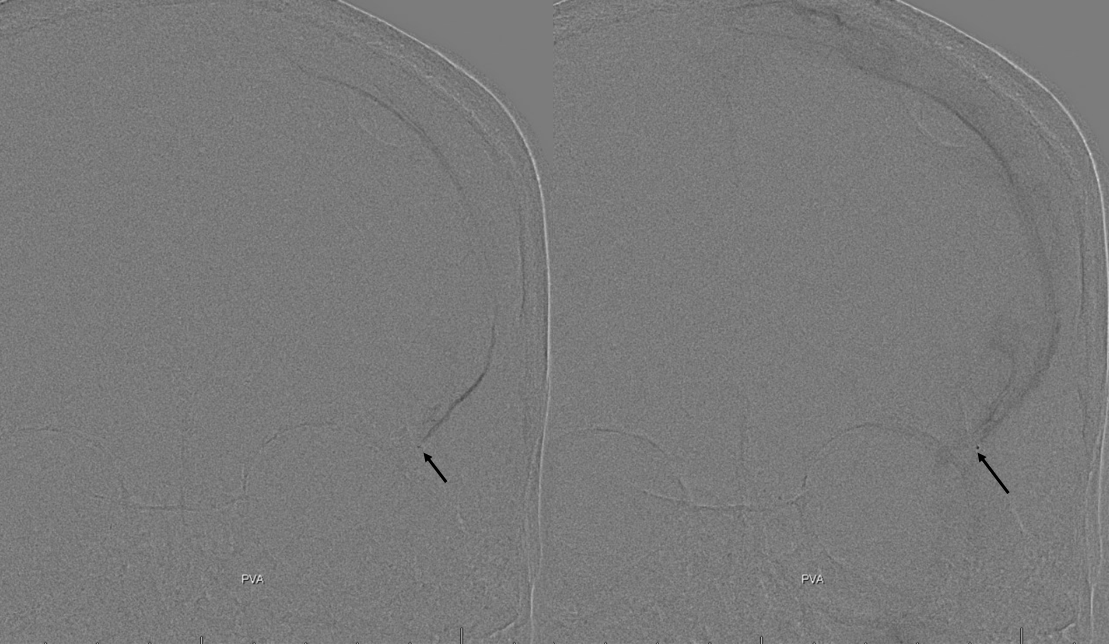
Reflux into the lacrimal is better to see in frontal plane. But, its a lot more radiation. We switch to lateral. Top left image below is fine. Towards end of particle embo however we see some contrast in the same diploic vein channel we saw above (top right image). That happens often with good embo — outflow can be slow — but this was a bit too much. So, we do a DSA (lower left image) — there is a fistula between middle meningeal artery and vein — see the same tram tracking vein (ball arrow) and diploic vein (black arrow).
Why does this happen? Probably combination of fragile arteries and injection pressure. It is a very frequent occurrence with Onyx, which is often injected under a lot more pressure than either contrast or nBCA. However, happens with contrast also, as below. Its important to recognize this for what it is — a technical issue — and not seek some kind of physiologic explanation like sudden opening of a pre-existing physiologic shunt. These fistulas also happen often after trauma with skull fractures — see case here. Nothing to get too excited about — just put some Onyx or glue there and seal it — image on bottom right shows Onyx cast in the tram-tracking veins and diploic channel — the actual hole is marked by black arrowhead.
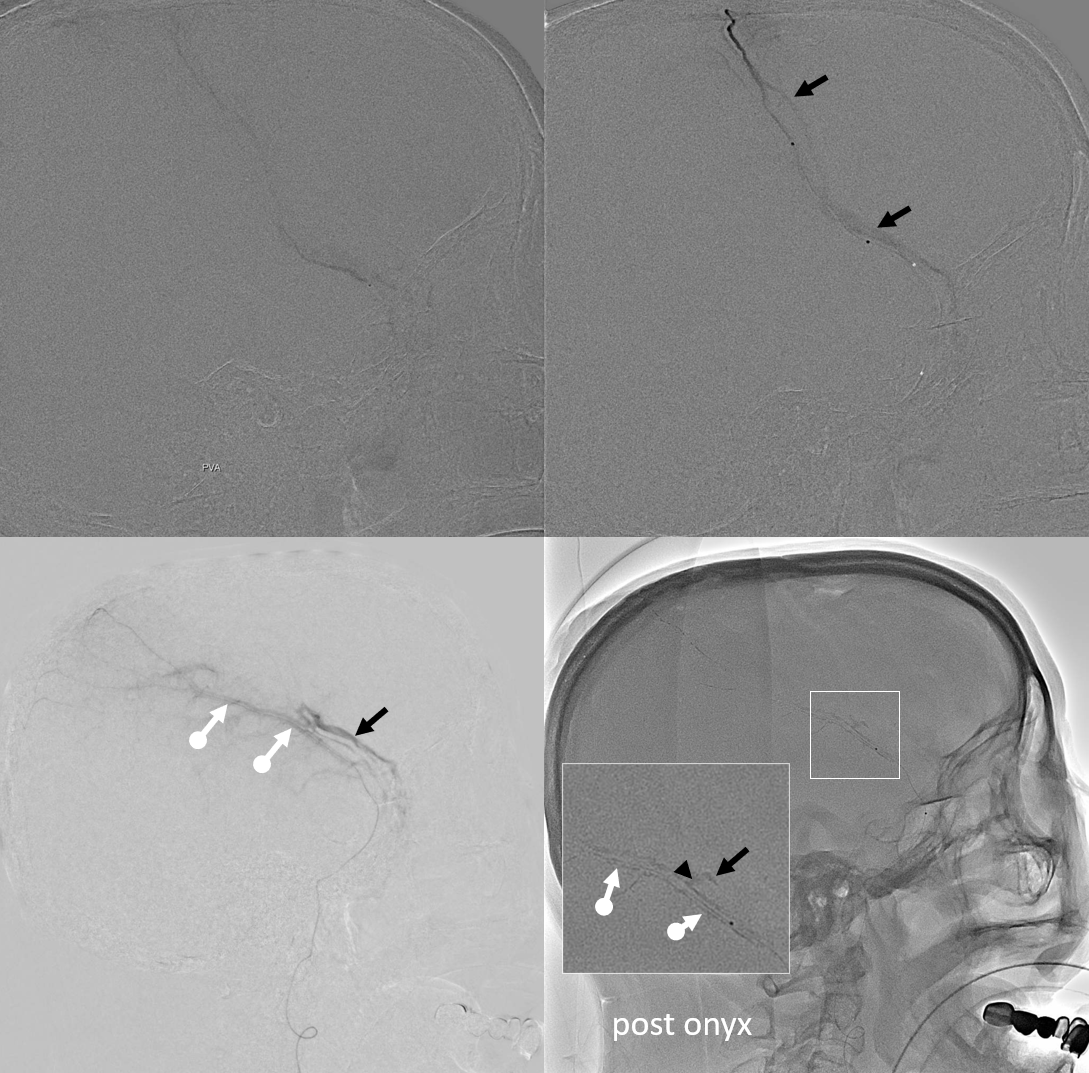
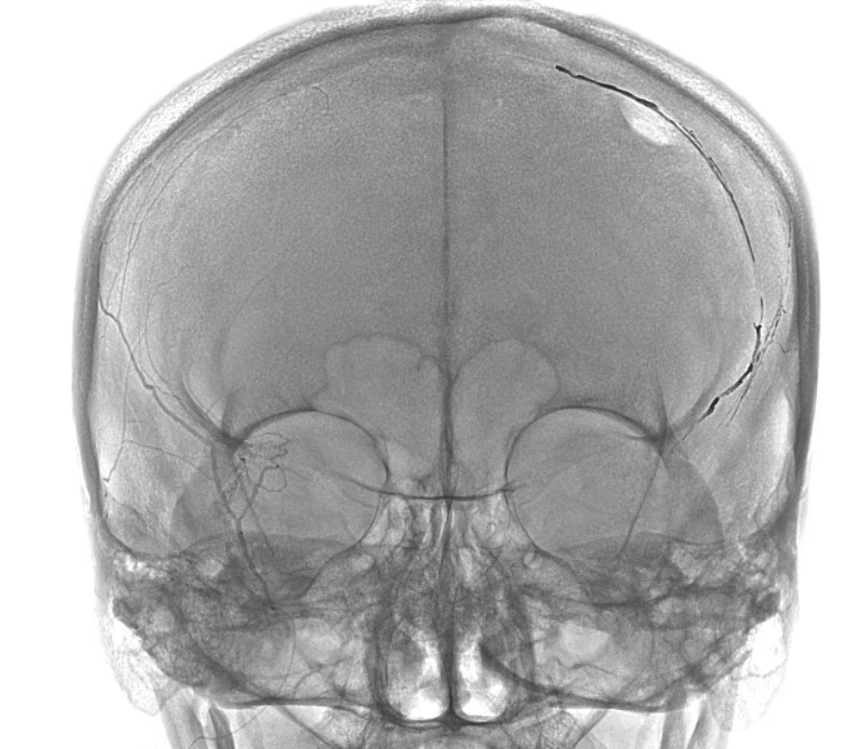
Final frontal — notice contralateral meningolacrimal disposition also
Particle Use as Embolic Agent of Choice
This is the only intraprocedural fistula we saw with particles, which is our preferred method of cSDH embolization. We use 45-150 Contours — they are aspherical and we believe thus more sparing on nerves than spherical types. The primary outer network anastomoses between major MMA branches are 100-300 microns so the smallest spherical particles (Embozenes or Embospheres for example) will penetrate these also if that’s your choice. However, using anything over 300 microns is suboptimal.
There are several reasons to prefer small particles. One is that most early studies on MMA embo used particles, so far with reportedly excellent results. Particles are also way cheaper than liquid embolics. Finally, and most importantly, small particles are best at overall tissue penetration — better than liquid embolics. Did I get it backwards? Aren’t liquids better penetrators? Not really. Yes, of course liquids can go further than particles — much further, as we saw in several examples above. However, they do so very unevenly, penetrating far (for some complex hemodynamic reasons) into some territories and not at all into others. Sometimes, it works great, but usually equidistant penetration is lacking. Particles, on the other hand, are nondiscriminatory, and will go as far as vessel size will allow into all available areas. Of course, controlled studies are under way for liquid embolics, and they will likely succeed. Particles will be studied as well.
So, back to those fistulas. They happen often with Onyx, where sequential depositions of nonadhesive polymer can generate intravascular pressures much higher than physiologic. This, combined with manifest fragility of vessels in subdural collections, leads to extravasation quite often. Of course, this is a self-sealing, asymptomatic event, of no clinical significance in every case we have seen so far.
Here are a bunch of examples (again all asymptomatic) — each row is a frontal and lateral of three different cases. This is like a study of dural venous anatomy — we see all the different drainage types — into venous lakes in walls of major venous sinuses (arrowheads), tram-tracking veins adjacent to major arteries (arrows), and diploic veins (black arrows)
Diploic vein extrav example
Sequential images of Onyx injection into distal petrosquamosal branch, extravasating into venous pouch in wall of sigmoid sinus

Cranial Nerve Issues (other than ophthalmic)
The two most commonly encountered cranial nerve arteries are the always discussed petrous branch to the facial (and greater petrosal) nerves, and rarely mentioned cavernous branch (not often present, is in balance with anastomoses with the middle meningeal branch of the ILT) possibly supplying the Meckel cave and its associated nerves. Since it can connect with the ILT, it is also a possible “dangerous anastomosis” with the ICA. Finally, the extremely rarely seen connection with the ILTand ICA can also happen via a very long (and thus rare) route of the sphenoid branch connection to the recurrent meningeal artery (see below).
Since the recurrent meningeal anastomosis is very rare, and both petrous and cavernous branches are located at the very proximal MMA — just beyond the Spinosum — avoiding injury to these nerves is simple. Don’t embolize from a Spinosum or very proximal position.
Petrous Branch
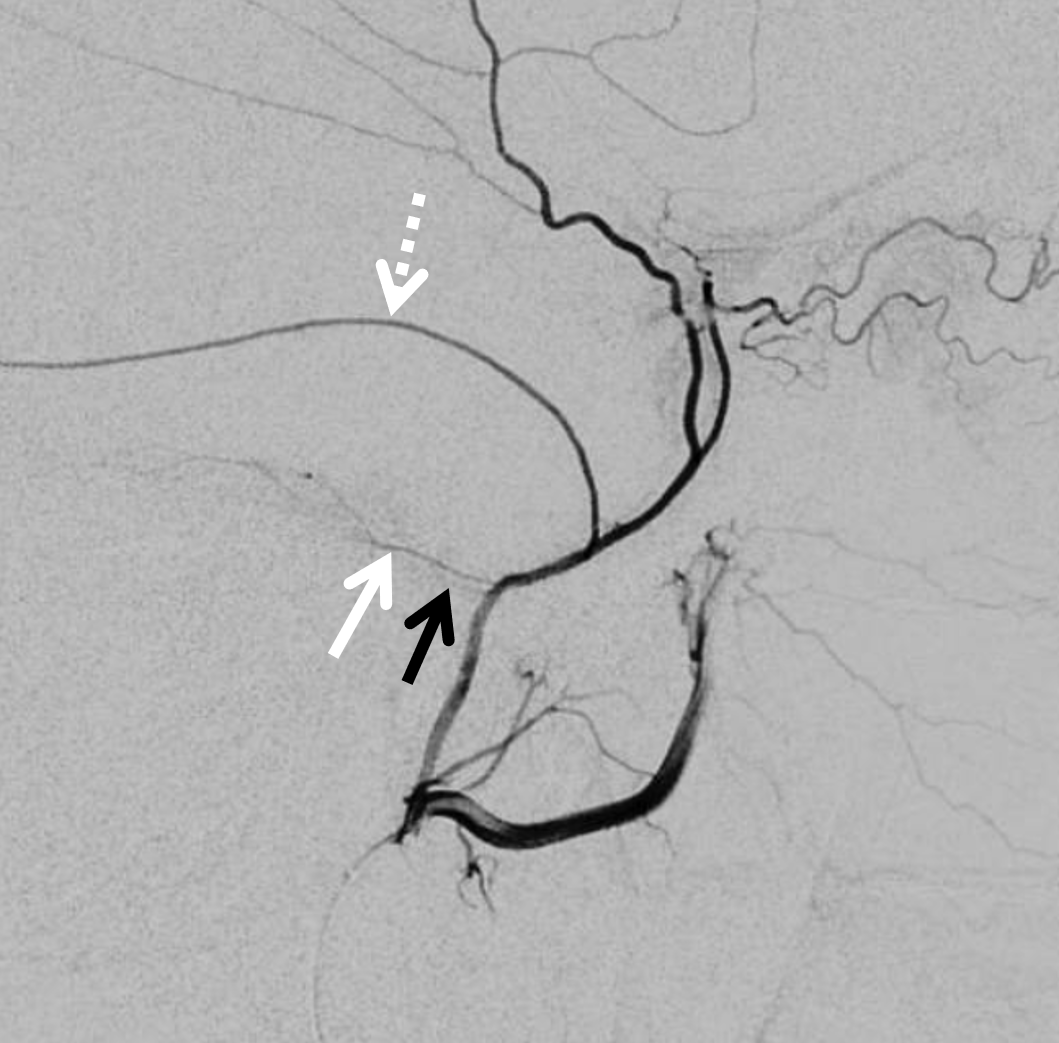
Arises from the very proximal intracranial MMA, just after it emerges from spinosum. Projects posteriorly in lateral views and slightly superiorly in frontal views. It is a VERY small branch usually, and frequently obscured by the dense temporal bone in lateral views. Frontal views are better. Also, don’t mistake the big petrosquamosal branch for the petrous one. The petrosquamosal one will project over petrous bone on lateral views (white arrow below), however it curves sharply laterally on frontal views whereas petrous one (black arrow) does not. Whatever the case, just dont embolize from a proximal MMA position.
Below is a good example of fakeout — a “petrosquamosal” branch is labeled by dashed arrow — higher than usual, right? There is a small branch that might look like the petrous one (white arrows) — but it is not. It is the real petrosquamosal branch — look at it on frontal views — projecting laterally. The real petrous branch is a tiny black arrow one, and almost invisible on lateral views.
Detail view — here you see the real petrous branch — black arrow — tiny indeed
DYNA images, particularly when obtained and reconstructed by Dr. Raz, can be amazing here — again, the branch is usually tiny, and runs INSIDE the petrous bone, by definition, so good luck seeing it on lateral views. Especially in a moving target under conscious sedation.
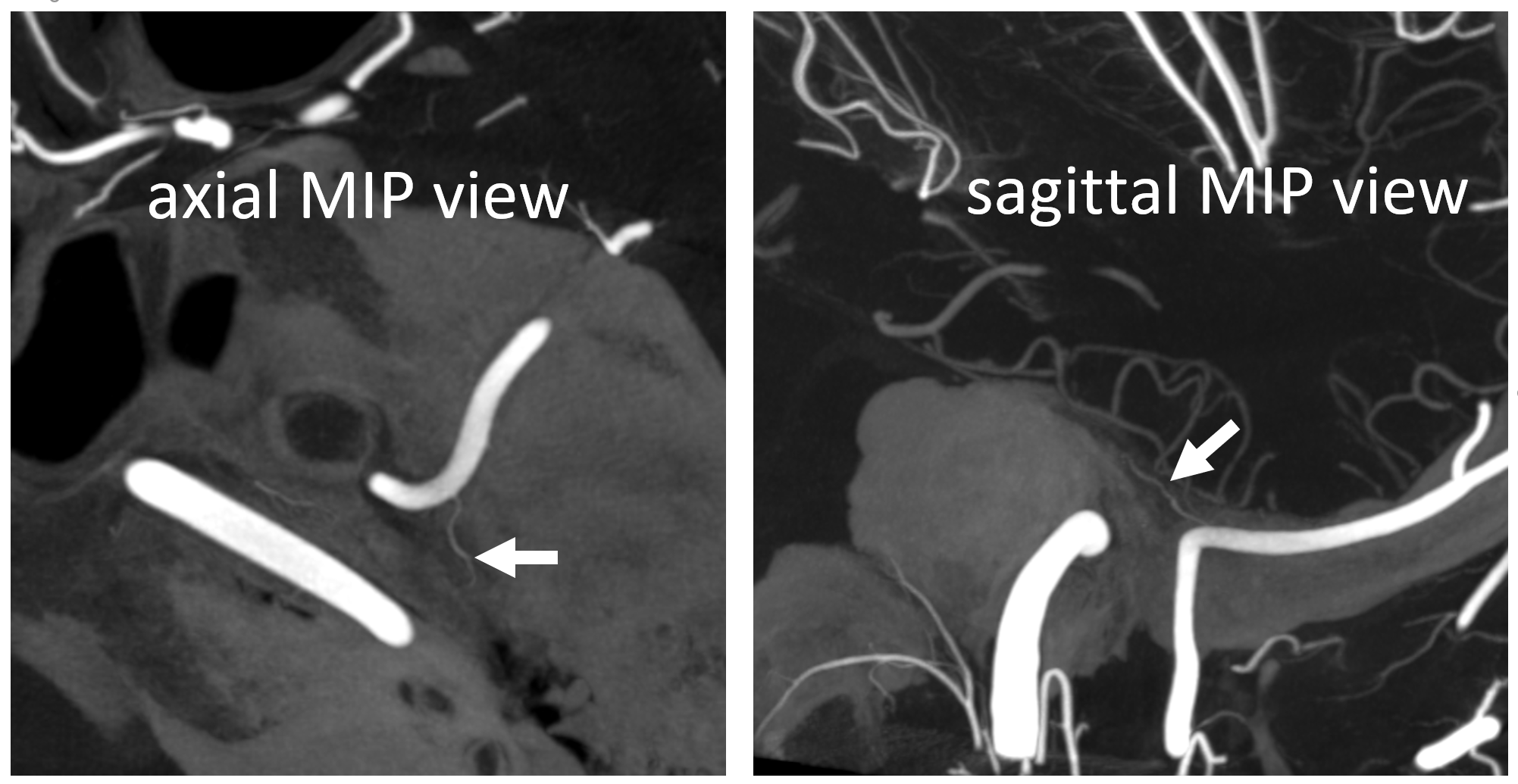
Below is a re-show of the DYNA we have above — petrous branch (white arrows) off that frontal Ovale origin one.

Of course — there is variation in everything here — the petrous branch can be larger or smaller — or rarely not there at all, with facial arcade supplied by occipital or posterior auricular origin stylomastoid branch, or by the middle meningeal branch of the ILT.
Cavernous branch
As above — less common than petrous one, but no less troublesome. Maybe more so, as it is a possible connection to the ICA via the ILT, in addition to supply of Meckel cave. Same proximal origin as petrous branch. Contour particles are much less likely to hurt nerves than liquid embolics. We have over 50 skull base meningioma Contour embos with no cranial nerve problems — and this includes patients with partial palsies, who are especially susceptible. For cSDH embo though, proximal embo is completely unnecessary and should be avoided.
The cavernous branch will project very medially on frontal views along skull base. It will be “down the barrel” in lateral views and further obscured by the proximal MMA itself — for these two reasons concentrate on frontal views.
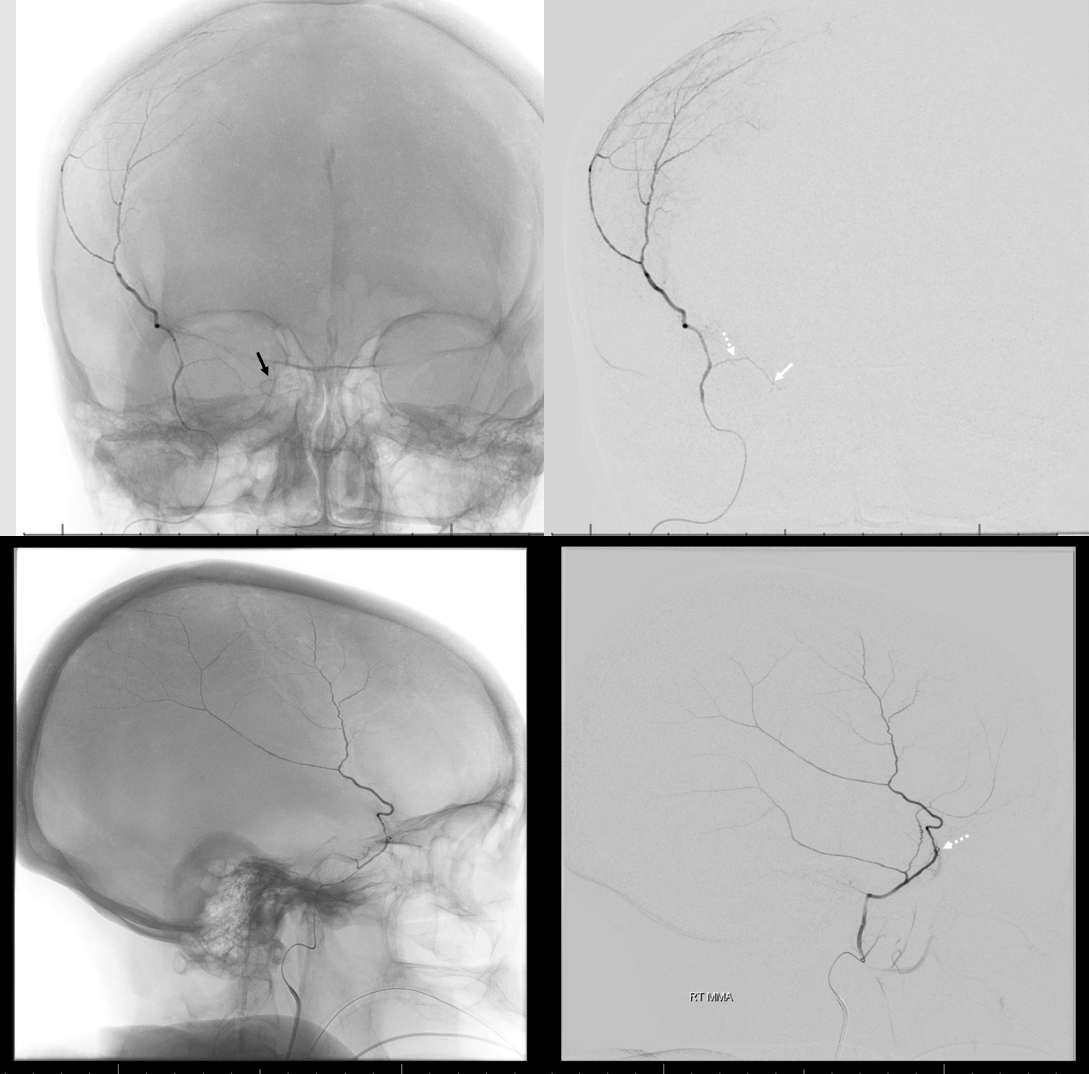
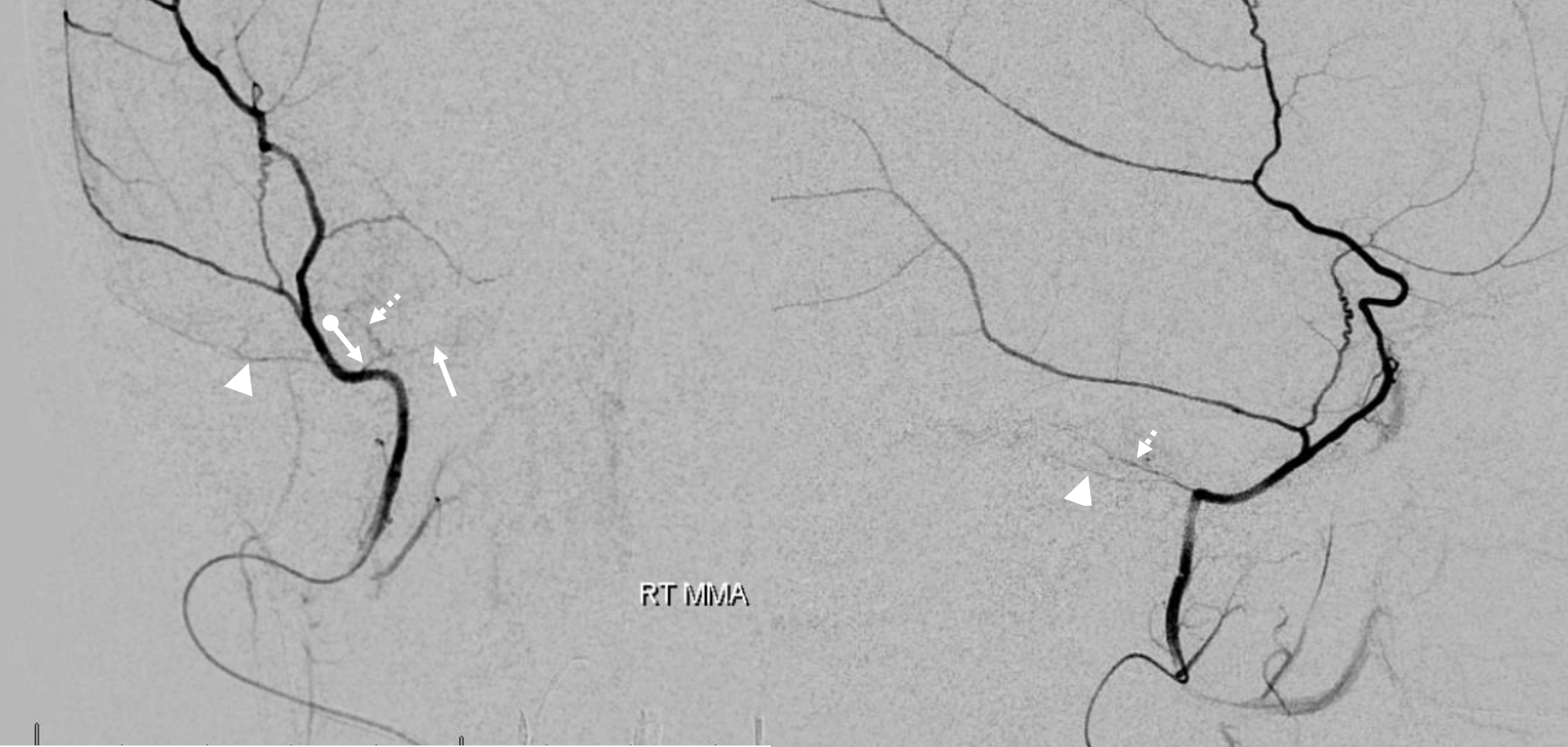
In this excellent example, there are both cavernous (arrow) and petrous (dashed arrow) branches present. Neither is well seen on the lateral — however there is again a small true petrosquamosal branch (arrowhead) pretending to be the petrous one
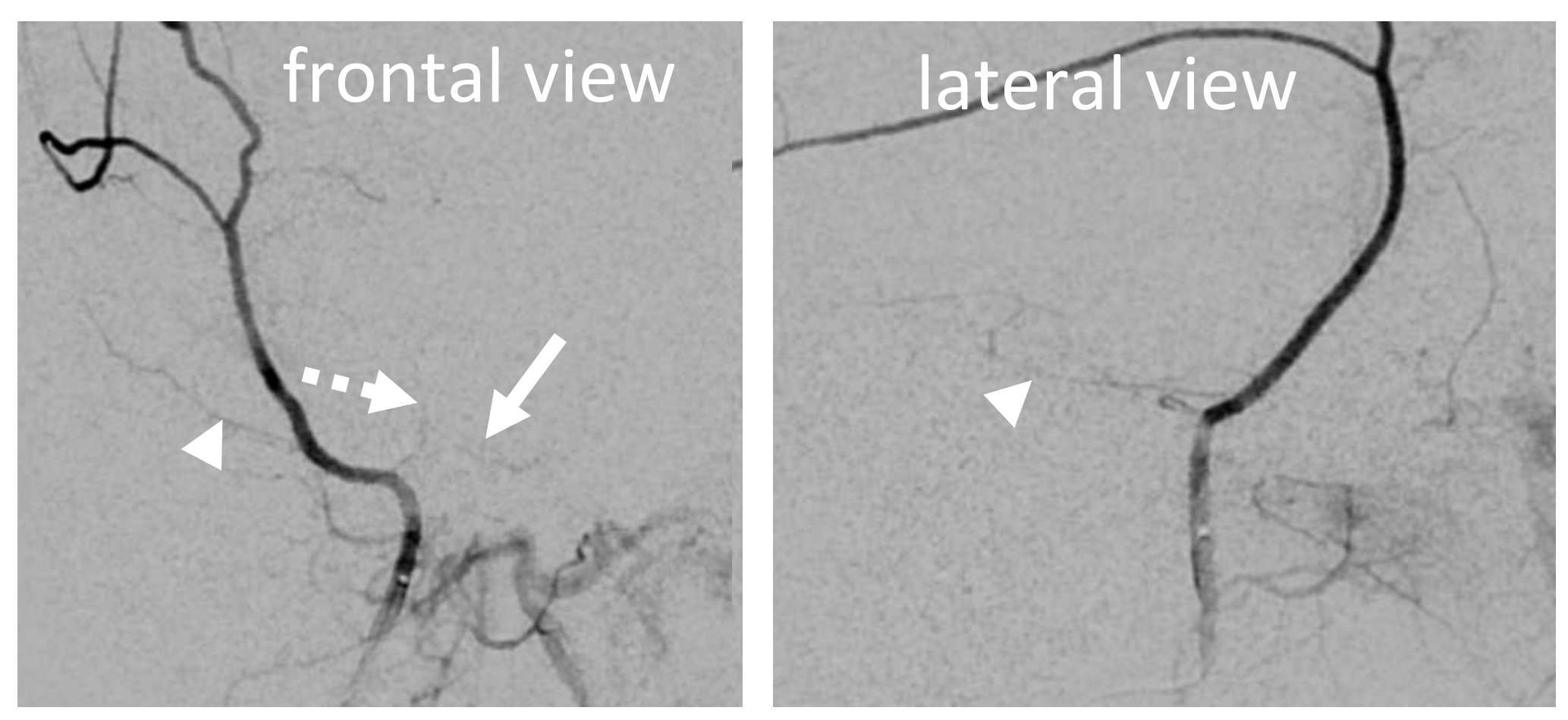
Rotation angio MIP view (NOT DYNA) of the same case — see that cavernous branch (arrow) in Meckel cave — and real petrosquamosal (arrowhead) nowhere near the facial canal (dashed arrow)
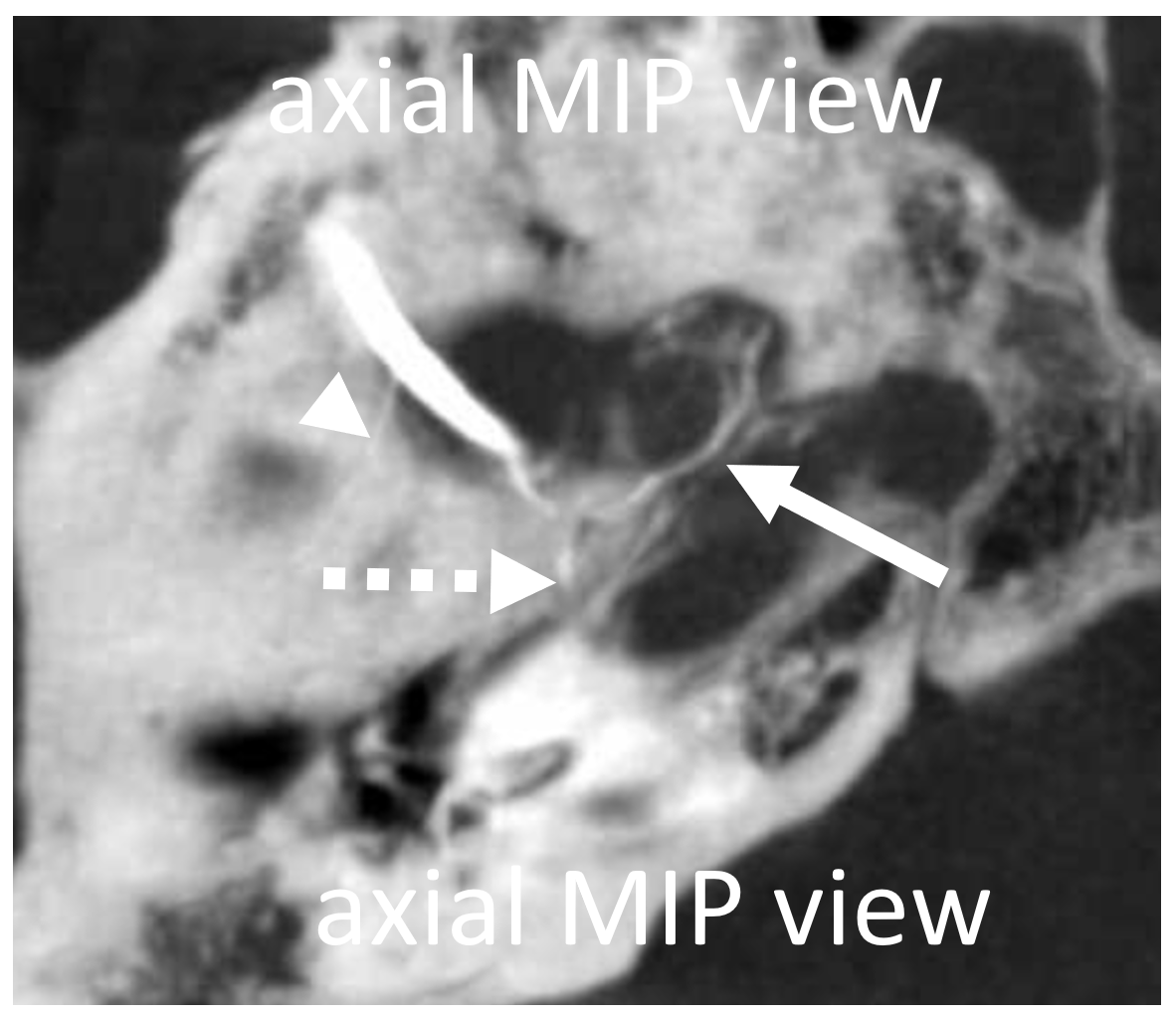
Different patient — Eytan made these super DYNA MIP recons, with excellent visualization of cavernous branch
Recurrent Meningeal Branch
This is a nice opportunity, one last time on this page, to circle back to the spectrum of variations. We talked about the more common meningo-lacrimal variant and the less common meningo-ophthalmic. The arterial connection between both and the MMA is via the sphenoid ridge artery. Think of it as a railroad. Traveling on the MMA, if you take the sphenoid ridge branch, the first stop is the lacrimal branch, the next one is ophthalmic. But, the road does not stop there. There is a connection at the end with the recurrent tentorial artery, connecting the sphenoid branch with the ILT. So, we circle back — from a more direct MMA to ILT connection via the cavernous branch to the rare and very circuitous, but ever more dangerous one, via the recurrent tentorial branch — placing in danger the ophthalmic system, cranial nerves of cavernous sinus, and ILT connection with the ICA. Not bad, huh. How to avoid all of this, in a moving patient, will all kinds of other uncertainties? Go distal and don’t reflux — embolization position above orbital roof is very safe.
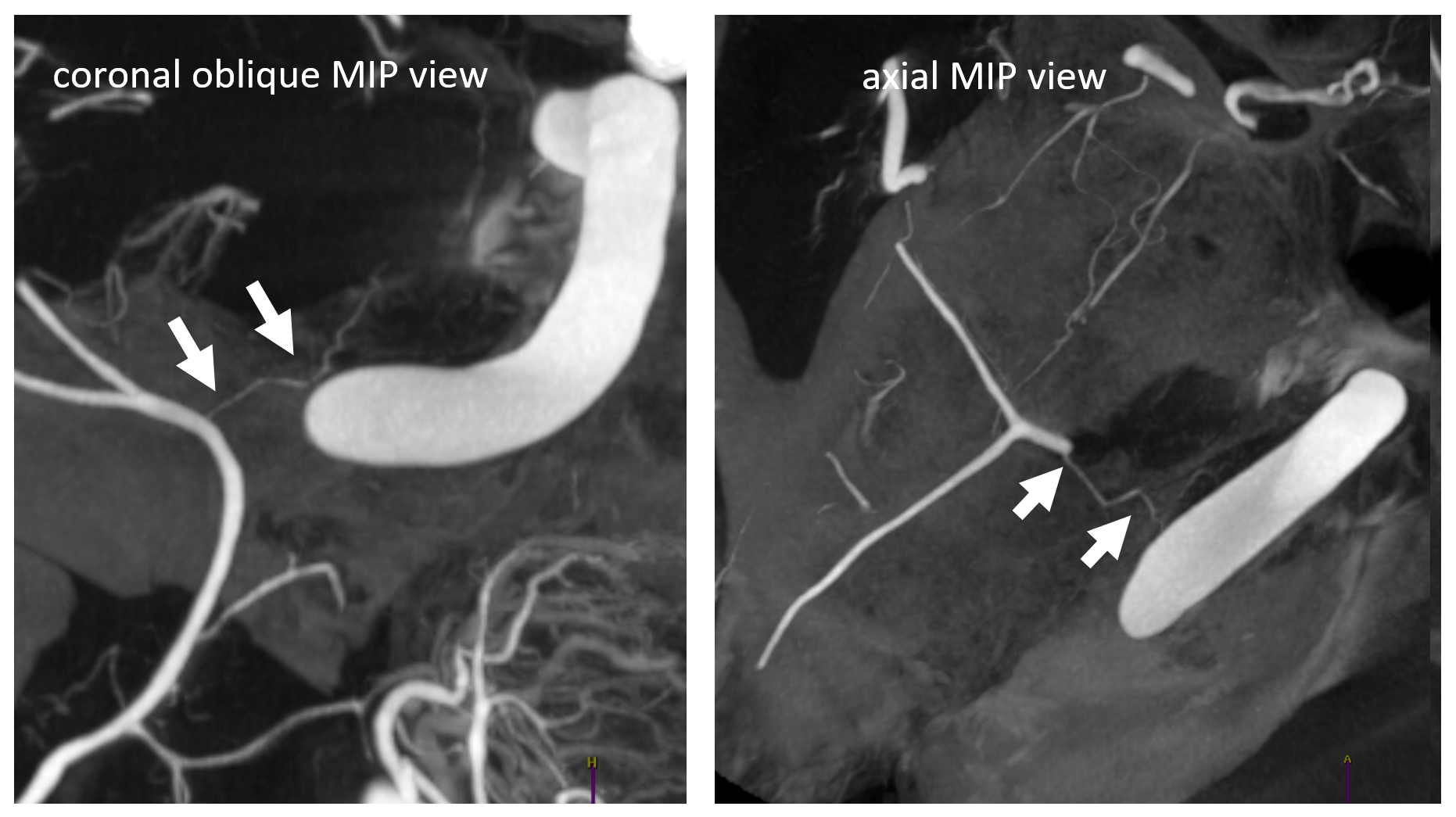
In one of Eytan’s DYNA CTs, the sphenoid ridge (dashed arrows) connects with the recurrent tentorial branch (arrow) — its location accentuated by dense ICA calcifications. There are two small branches also, to the Meckel cave and ophthalmic region (not labeled)
On DSA, this is seen as very medial extension of sphenoid ridge branch (dashed arrow), all the way to those ICA calcs (black arrow). Despite very medial extension, there is no major connection to the ophthalmic system. Lateral is, as usual, not very helpful. As you can see, the tendency to overly rely on the lateral is not a good idea.
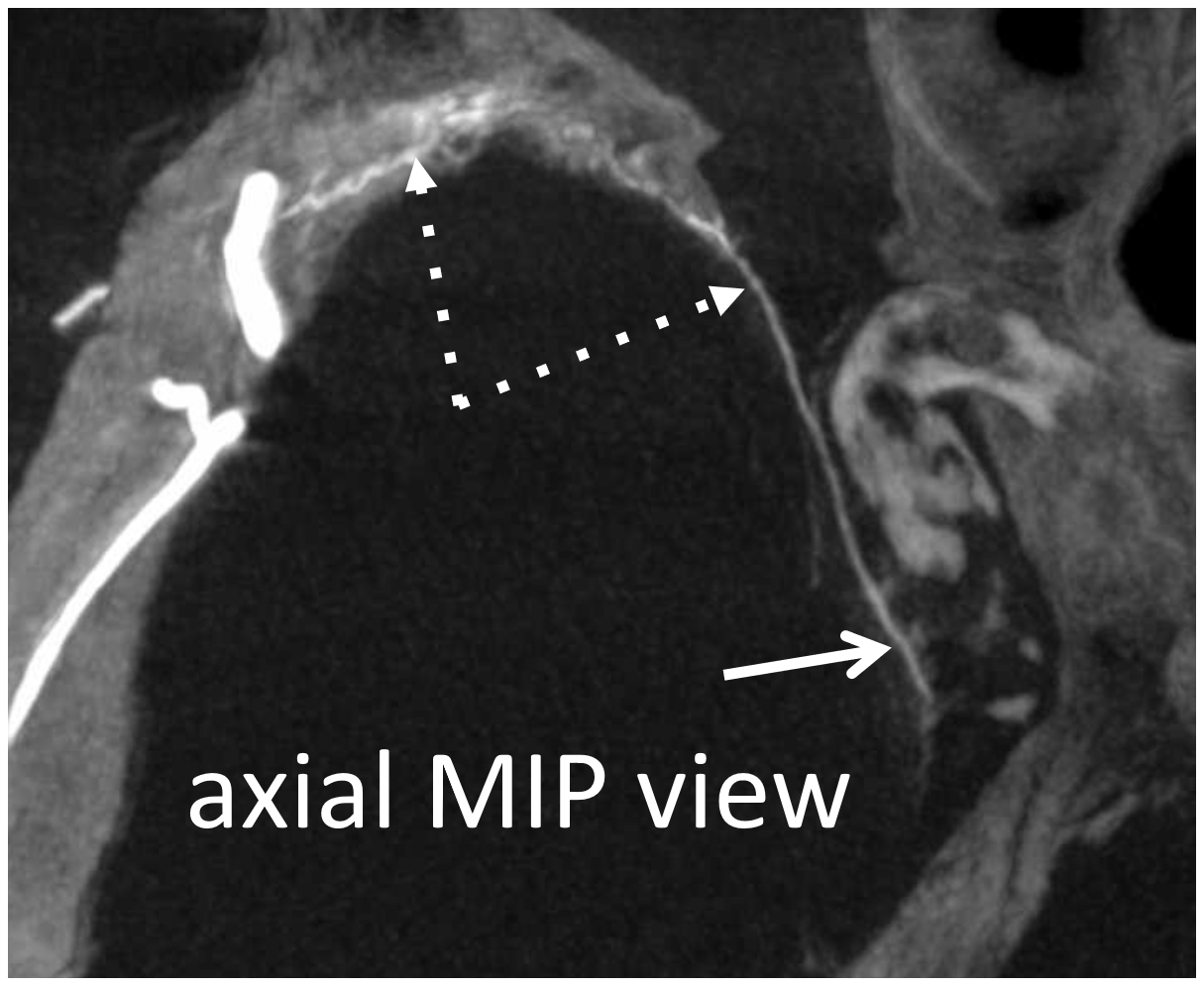
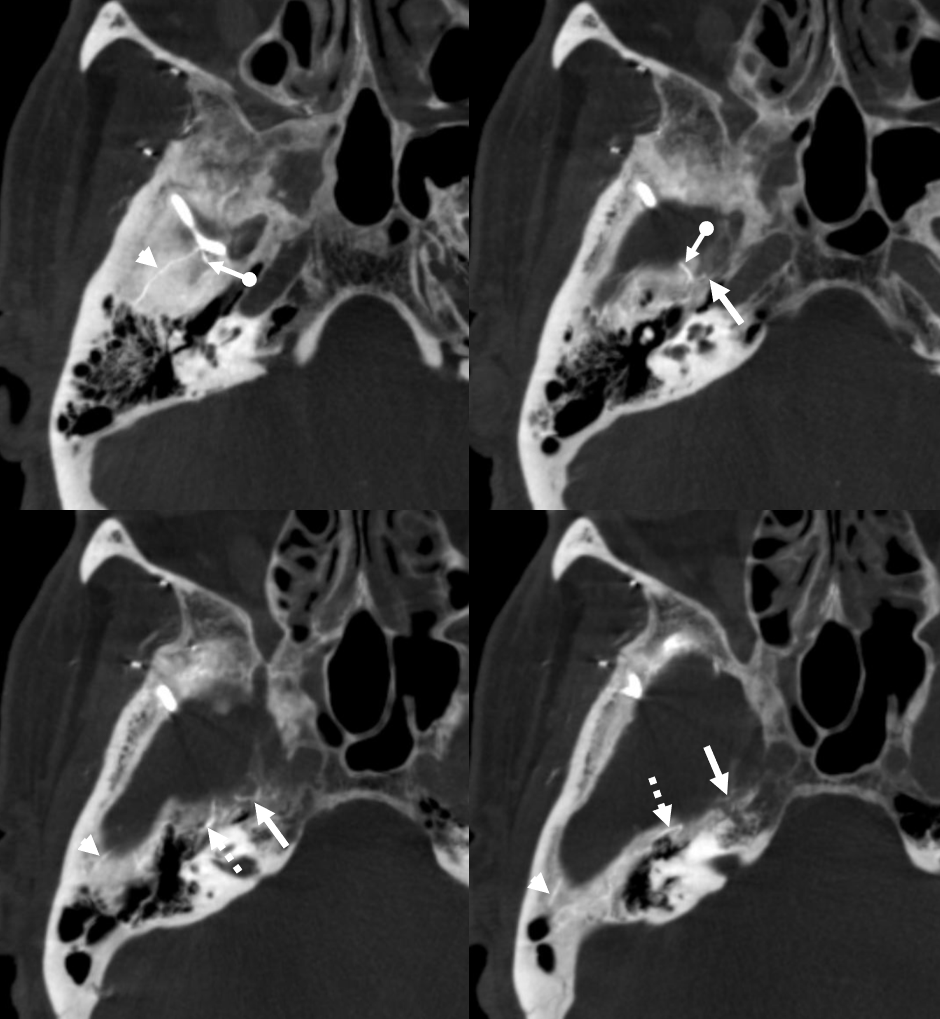
But wait! There is more 🙂 Same patient, a more proximal MMA injection at the spinosum. Look — common stem (ball arrow) of petrous (dashed arrows) and cavernous (arrow) branches. The petrosquamosal branch — you should recognize than by now is arrowhead.
Rotational MIPs — not DYNAs — good enough to show these also (same arrows)
Not freaked out yet? Well, for a final digestif, complements of Drs. David Gordon and Eytan Raz, a sip of the exquisitely rare…
Autosynangiosis
You might think that presence of a dural collection barrier between dura and pia would make an autosynangiosis impossible. Life mocks our theories, usually at our expense… Here is a patient with an old MCA infarct, and a cSDH

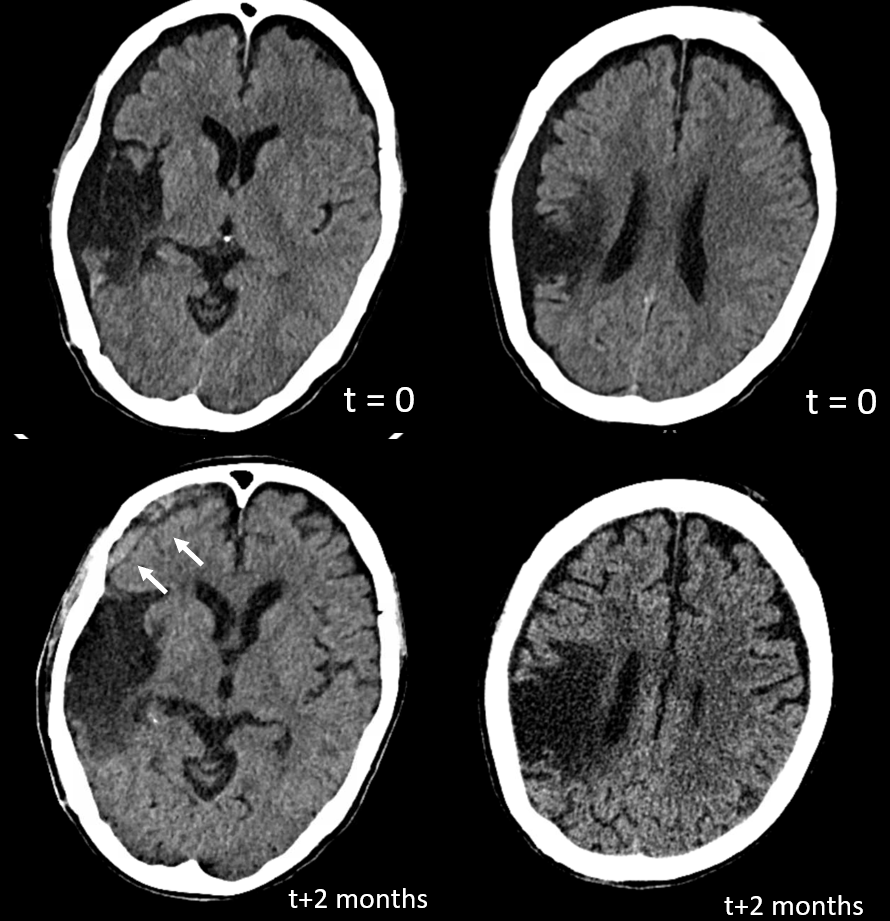
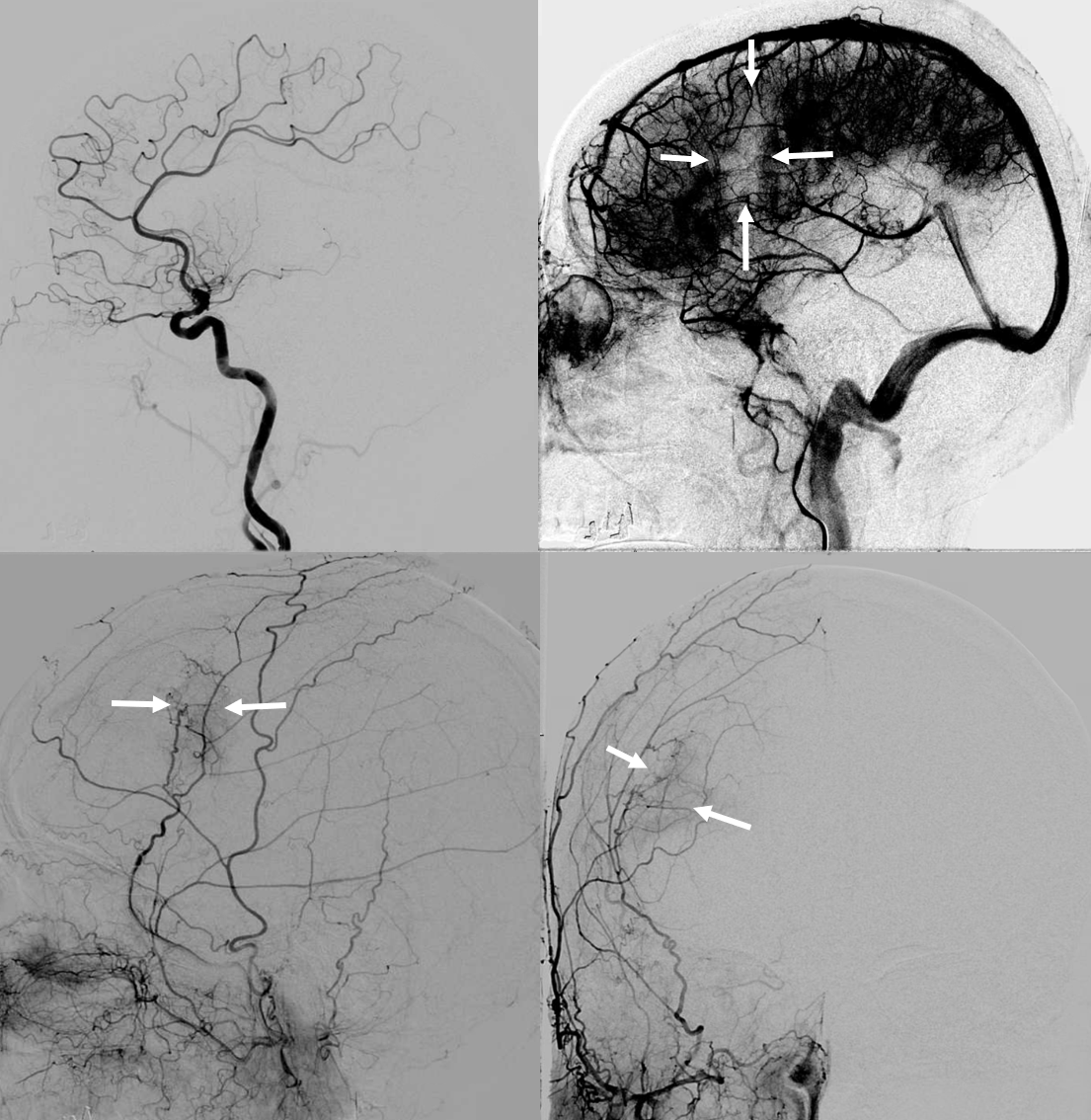
ICA injection — still missing M1, lots of leptomeningeal reconstitution, and a curious light patch (upper right image arrows), probably just in front of all that CT encephalomalacia. ECA injection shows the same area supplied by the MMA — an autosynagiosis — probably just behind the subdural that is (at t+2 months) limited to anterior frontal region. Needless to say, Drs. Gordon and Raz, possessed of The Right Stuff, did not bite. Given that a dedicated internal injection is not always done prior to embo, yet another trap to be mindful of.
Conclusions
The efficacy of MMA embolization, while apparently very high, awaits confirmation in randomized trials. Questions re embo timing, technique, optimal agents remain, among others.
Standard protocol of MMA embolization
DSA of CCA, with specific attention to ophthalmic artery
DSA of ECA, with specific attention to ophthalmic artery
DSA of MMA from Spinosum position, with specific attention to ophthalmic artery, nerve supply, and other “dangerous anastomoses”
If no ophthalmic or other issues, careful 45-150 um Contour particle embo from position beyond petrous and cavernous branches, followed by pushable coils
If there is orbital involvement, distal catheterization of frontal branch beyond orbital connection, followed by particle and coil embo (if not wedged) or liquid embolic if wedged
Remaining MMA branches are embolized by particles and coils
If frontal or frontoparietal recurrent meningeal branch is present, the microcatheter is wedged in an accessible MMA branch next to recurrent meningeal territory, followed by particle or liquid embolic embo to attempt penetration into recurrent meningeal
Final post CCA DSA
Questions / comments: Click here
