Spinal Dural Fistula (a.k.a. Spinal Dural Arteriovenous Fistula; a.k.a. Spinal Dorsal Intradural Fistula; a.k.a. Type I)
By far most common spinal vascular malformation. Spinal dural fistula consists of a pathological communication between a dural artery and vein. The fistula is typically located in the nerve root dural sleeve, and as such formed by a segmental radicular artery and radicular vein. Some believe that Glomerulus of Manelfe, a ball-like structure (originally described as a “peloton vasculaire”) believed to regulate regional venous and CSF pressures, to be the site of fistula formation. The fistula typically consists of a single hole, even when multiple feeders produce a “busy” appearance suggestive of a nidus. The fistulous connection may drain centrifugally (away from the spinal cord) via the radicular vein into the external venous paraspinal network, in which case the patient may be asymptomatic. However, the fistulous radicular vein is usually connected, via a radiculomedullary vein, with the longitudinal venous network responsible for draining the spinal cord, as rudimentary radiculomedullary veins exist at many more levels that would be visible from an angiogrpaphic injection. Arterialized pressure within the fistulous radiculomedullary vein leads to reversal of normal centrifugal radiculomedullary flow such that the fistula empties, in retrograde fashion, into the longitudinal spinal cord veins. This process congests the spinal cord venous system, which can be angiographically seen as prolongation of normal spinal cord capillary phase. Also, vascular congestion is in itself responsible for patient symptomatology.
The site of the fistula (most often in the thoracic, lumbar, or sacral regions) has no relationship with the patient’s symptomatology, because it is spinal cord vascular congestion, rather than fistula itself, that produces the clinical syndrome. Longitudinal spinal cord veins extent along the entire length of the cord to the region of the conus; thus, regardless of fistula site, the most dependent portion of the spinal cord (conus) is invariably the site of most pronounced congestion and therefore the earliest location of edema, enhancement, and other findings which are visualized on noninvasive imaging. The anatomy of the conal region therefore dictates the clinical presentation. Many patients report a long history of bladder issues or other symptoms related to location of relevant neural networks in the conus. Eventually, however, the disease progresses to produce sensorimotor deficits within the lower extremities which ultimately bring most patients to clinical attention. The length of time between formation of the fistula and clinical decline appears to reflect both size of the fistula and eventual failure of the radiculomedullar venous system.
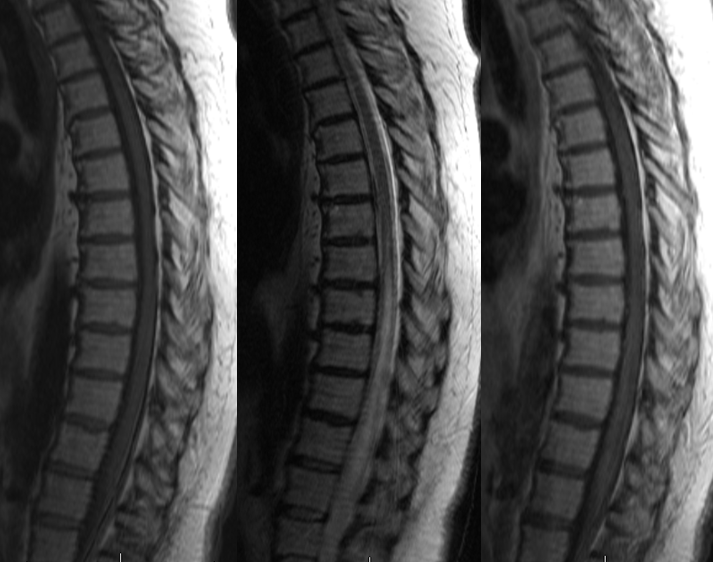
The intrinsic venous system draining the spinal cord, consisting of multiple radiculomedullary veins, is able to accommodate the “extra” fistulous volume for variable length of time. Eventually, however, the radiculomedullary veins tend to fail, probably likely via high flow venopathy and possibly neointimal proliferation related to arterialisation of venous flow. The reasons why the draining veins shut down remains unclear. The sequential closure of these veins, further congesting the spinal cord, and secondary temporary adaptations of the remaining outlets, may be responsible for the classic intermittent symptomatology experienced by the patient. Eventually, enough of the radiculomedullary veins fail, the adaptive capacity of the system is exhausted, and the patient enters a progressive stage of disease, typically characterized by worsening of pre-existing vascillating symptoms (typically genitourinary) and development of sensorymotor lower extremity deficits. An MRI at this time point will reveal the classic picture of dilated spinal veins and cord edema, which is always greatest at the conus for reasons detailed above. MRIs of the same patient prior to the accelerated stage may be quite normal (or at least would require a very high index of suspicion to be read as potentially abnormal). Catheter angiography is typically performed at this point and the fistula is found, sometimes after considerable search (it can be anywhere — off the iliacs, supreme intercostal, whatever) The venous phase of such fistulous level injection will demonstrate dilated spinal cord veins with lack of regional venous radiculomedullary drainage. Typically, by the time the patient is seriously enough symptomatic that most thoracic outlets have closed, and venous phase images show a tortuous enlarged spinal vein emptying into residual radiculomedullary veins at the high thoracic and cervical levels, the remainder having being destroyed. At this time, closure of the fistula will likely prevent disease progression, but will not bring back destroyed radiculomedullary veins and may not produce a meaningful clinical improvement. As with other neurologic conditions, the duration and severity of deficit will be inversely related to extent of eventual recovery. The cord will remain congested even after obliteration of the fistula (to a far lesser extent, for sure) but clinical condition may not normalize. Bladder symptoms are usually last to improve, but may recover after a considrable delay when little hope for normalization is any longer entertained. Rarely, closure of the fistula leads to an unfortunate deterioration rather than improvement, in patient condition. One reason for this decline is posttreatment thrombosis of the spinal veins, which having enlarged far beyond normal capacity and suddenly deprived of high volume fistulous flow, encounter marked venous stasis with subsequent thrombus formation. It may thus be helpful to maintain gentle heparinization of the patient following either endovascular or surgical closure of the fistula.
Treatment: The treatment of spinal dural fistula is usually endovascular. A spinal angiogram is performed and fistula found. A microcatheter is advanced into the region of the fistula and fistulous connection embolized. nBCA is our preferred agent. Coils and particles have no role in permanent occlusion. Under some circumstances the fistula may not be amenable to endovascular treatment — some such considerations include presence of a radiculomedullary artery (or sizable radiculopial artery) giving rise to anteior or posterior spinal artery at the same level as the fistula, insufficient length of fistulous radiculomedullary vein “safety” prior to egress into the spinal veins, inability to achieve an optimal embolization position, etc. In such cases, we typically deposit several coils into the segmental artery to mark the location of the fistula and refer the patient for microsurgical obliteration. The surgeon opens the dura and typically finds the fistula in the region of the nerve root sleeve, where it can be obliterated with cautery. It is important not to decrease fistulous flow during such coiling so as to help the surgeon locate the fistula visually. Needless to say, both angiographic and surgical approaches, roughly sketched above, are quite complex and best undertaken in centers of sufficient case volume and experience.
The following angiographic and diagramatic illustrations provide a better appreciation of the pathology than descriptive language alone.
FIGURE 1: NORMAL ANATOMY

The cord is diagramed to the left, with a zoom in section on the right. A representative segmental artery (A) gives off the radicular artery (B) which supplies the nerve root sheath. The spinal cord drainage is conducted through longitudinal spinal cord veins (D) into , which is drained by a radiculomedullary vein (C) in the direction shown by the blue arrow. Additional radiculomedullary veins are present at other levels, also marked “C”. A radiculomedullary artery (E) is also diargammed, supplying the anterior spinal artery (F)
FIGURE 2: DURAL FISTULA, EARLY STAGE
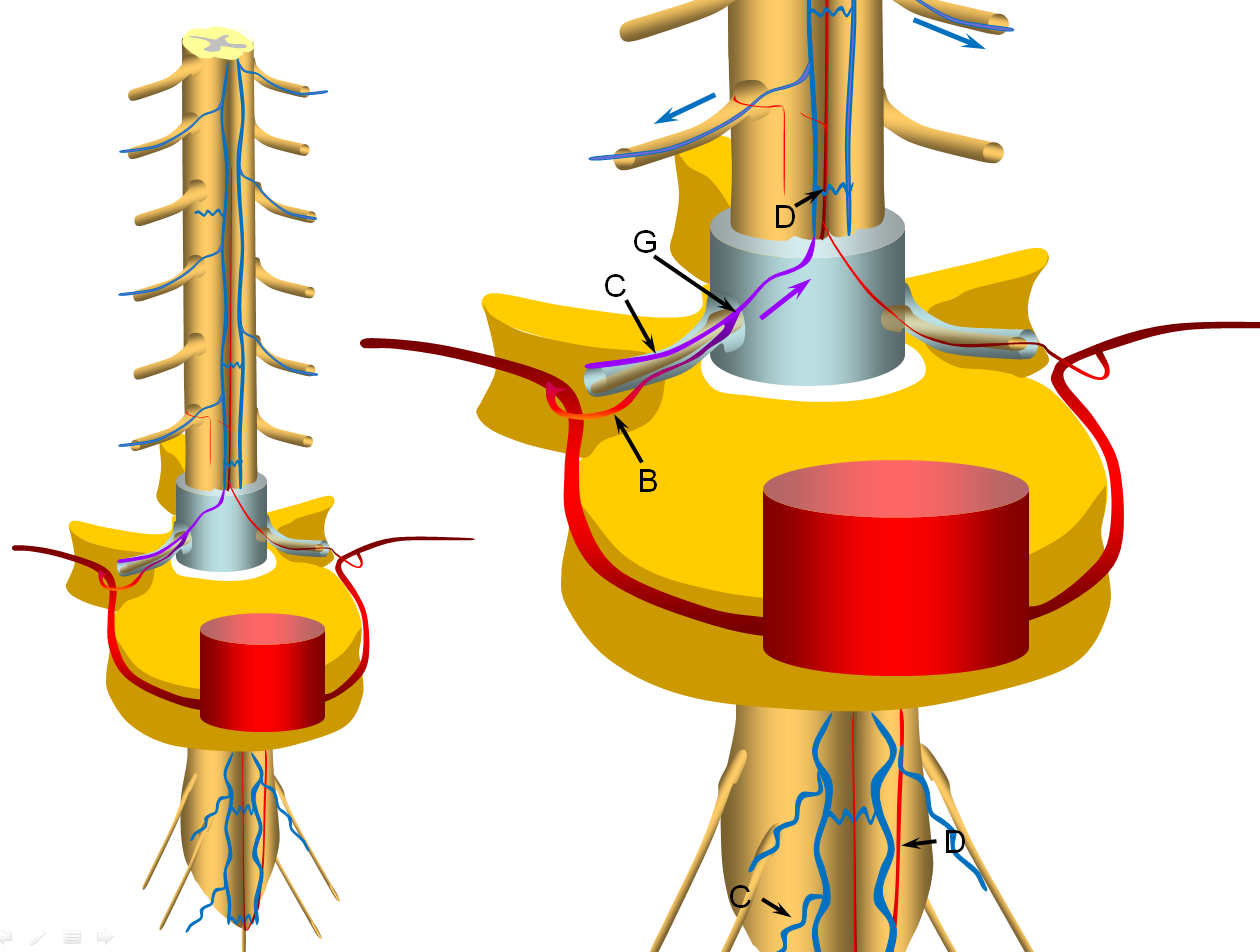
A fistula (G) is formed between the radicular artery (B) and radiculomedullary vein (C), typically within the dural sleeve (dural cover of the radicular nerve, usually in the region of the neural foramen). Other sites of fisula are of course possible, but regardless of fistula site, the clinical picture tends to be similar. Establishment of fistula results in regional venous congestion and reversal of flow within the radiculomedullary vein (purple arrow) which direct fistulous flow into the spinal veins (D) with subsequent egress via other radiculomedullary veins (blue arrows)
FIGURE 3: INTERMEDIATE STAGE (VASCILLATING SYMPTOMATIC STAGE)
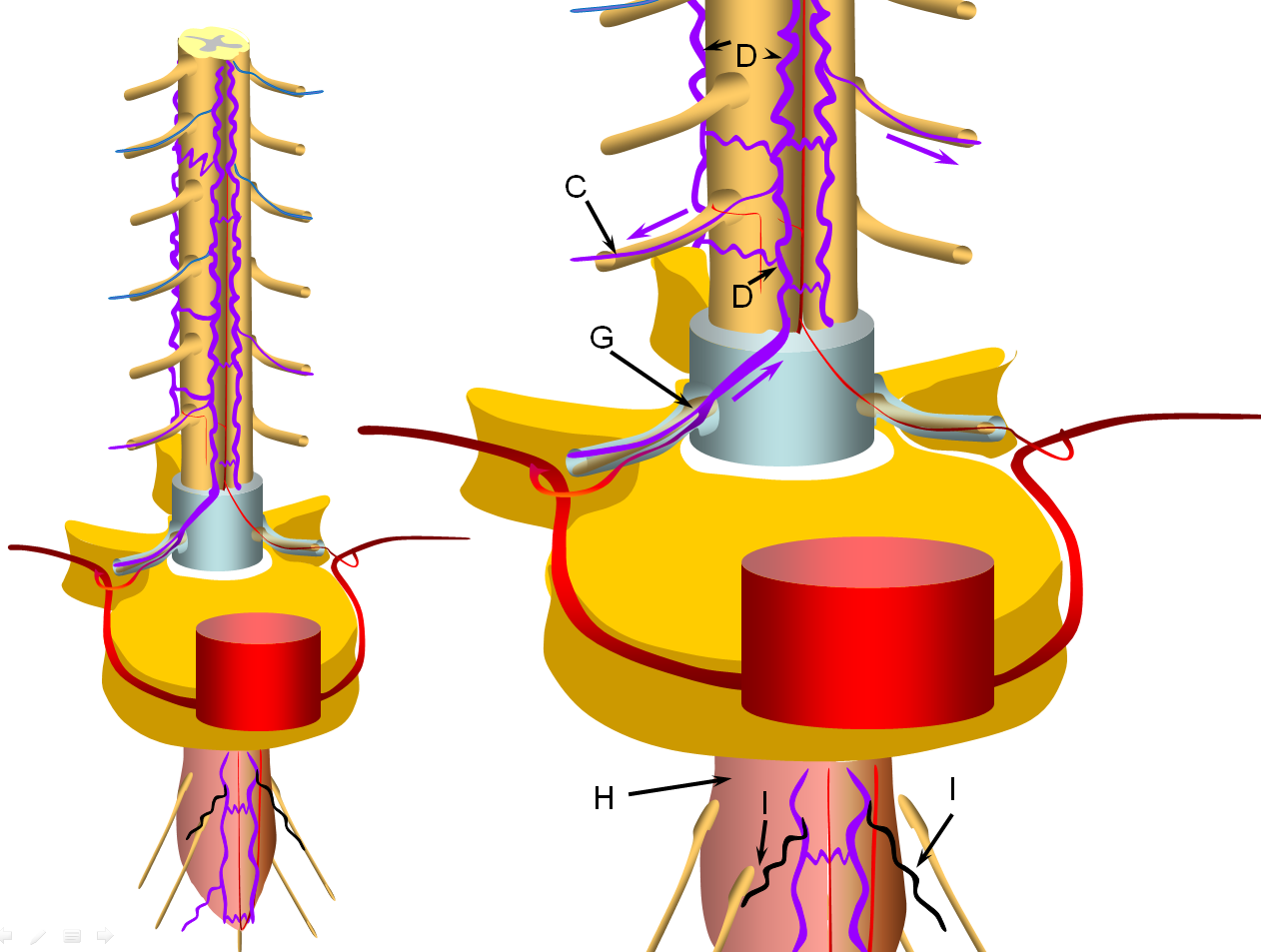
Progressive increase in venous hypertension of the spinal veins (D) result in gradual failure of radiculomedullary veins (I, colored in black) via thrombosis starting with the most dependent location of conus (H, colored in redding hue to depict congestion). The patient typically reports various conus symptoms such as urinanry complaints, which can be vague. Urodynamics are usually abnormal at this stage but there may not yet be enough MRI evidence of congestion to suggest a vascular cause. The symptoms tend to vascillate probably reflecting underlying changes in venous pressures and cord congestion.
FIGURE 4: LATE STAGE (PROGRESSIVE FAILURE OF RADICULOMEDULLARY VEINS)
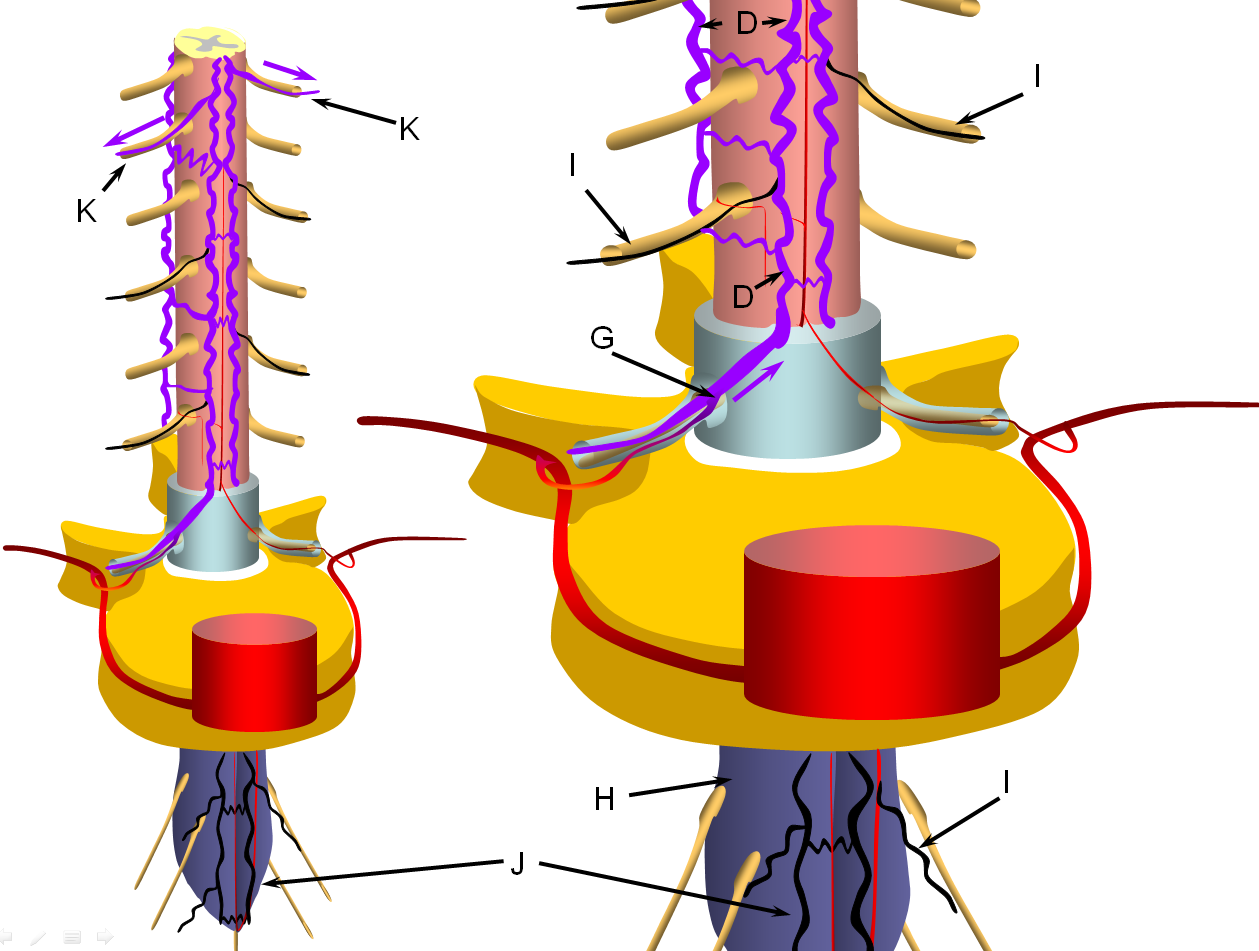
The stepwise process of venous outflow failure and subsequent partial adaptation to find progressively higher drainage routes continues until enough radiculomedullary veins fail to enter a more rapidly progressive stage of disease, depicted here. The fistula drainage is conducted via surviving rostral radiculomedullary veins (K), a large portion of the cord is congested, whereas the lower conal regions may undergo irreversible damage (J, diagrammed in blue). Most patients present somewhere between the intermediate and late stages.
The following MRI and catheter images illustrate the above concepts.
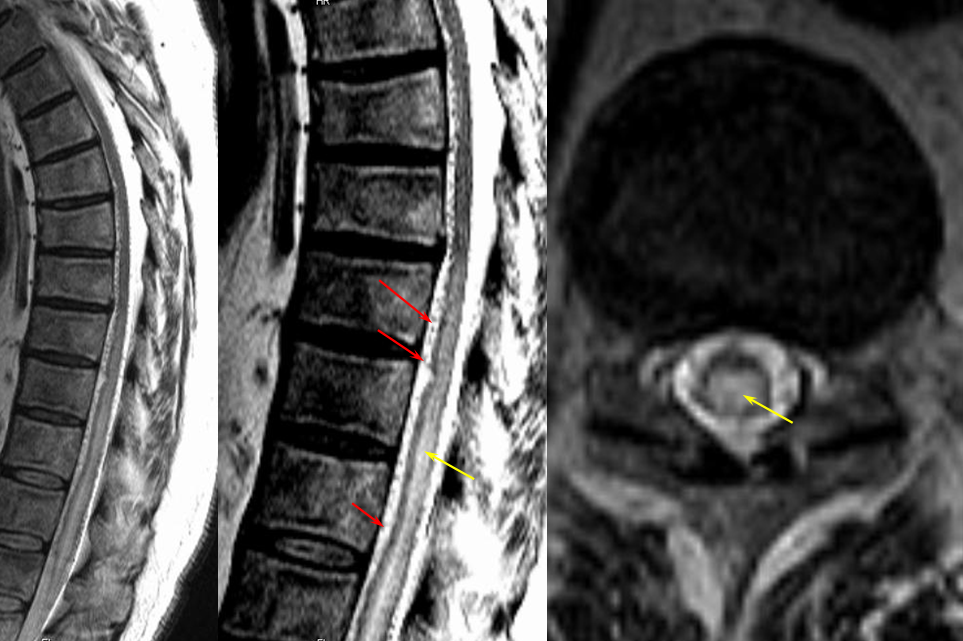
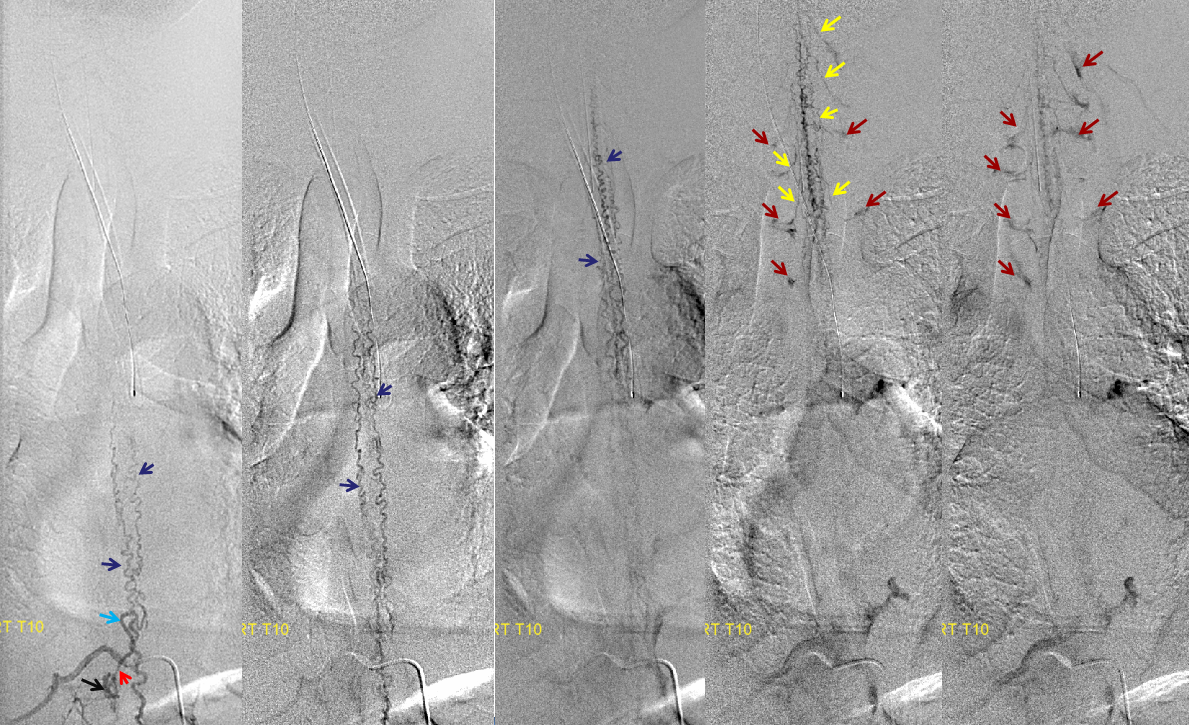
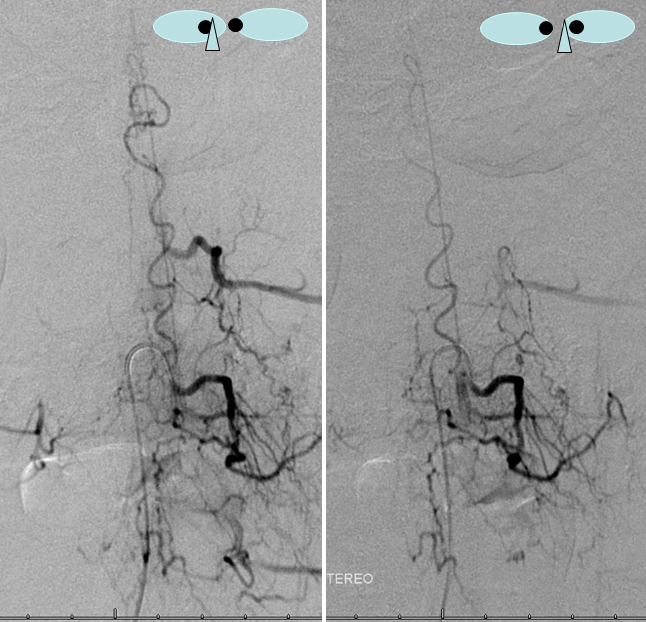
Same level injection visualizing the entire thoracic and lower cervical cord (the carina shadow is at T5 level in this case). The fisula (red) drains via a dilated radiculomedullary vein (light blue) into congested spinal veins (dark blue). Five snapshots are taken of the successive time points, the latest at t+40 seconds from start of injection. The first image from left is arterial phase. Successive images (left to right) demonstrate marked venous congestion with no evidence of radiculomedullary outflow in the mid and lower thoracic sections. These veins have thrombosed secondary to high-flow venopathy. The first venous drainge of the fisula is at T4 level (above the carina), where multiple radiculomedullary veins (yellow) can be seen draining the fistula into the venous plexus surrounding the neural foramen (brown).
Anterior Spinal Artery Injection in case of Dural AV Fistula
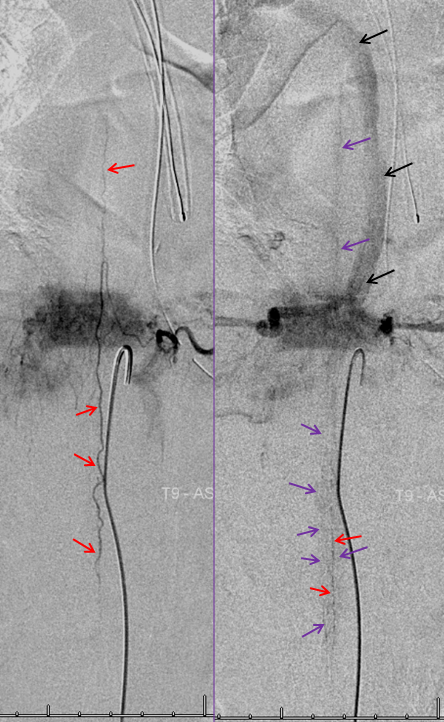
The same patient. The anterior spinal artery (red) is visualized from left T9 level. The spinal cord is congested (purple) with no visualization of a normal spinal veinous network at the expected time of the venous pase (evidenced by visualization of the azygous vein (black) draining the vertebral body and paraspinal elements. The spinal cord veins are not seen as they are congested by the fistula.
REFERENCE NORMAL APPEARANCE OF ANTERIOR SPINAL ARTERY/VEIN COMPLEX

Normal apperance, for reference. Left-sided image in arterial phase of radiculomedullary artery (brown) supplying the anterior spinal artery (black), and visualizing via transient reversal of flow another radiculomedullary contributor (yellow) and, via retrocorporeal anastomoses (not shown) a poterior spinal artery (red). Later normal venous phase images show a normal spinal vein (dark blue) with an associated regional patent normal radiculomedullary vein (light blue)
SAME PATIENT, A DIFFERENT REPRESENTATION
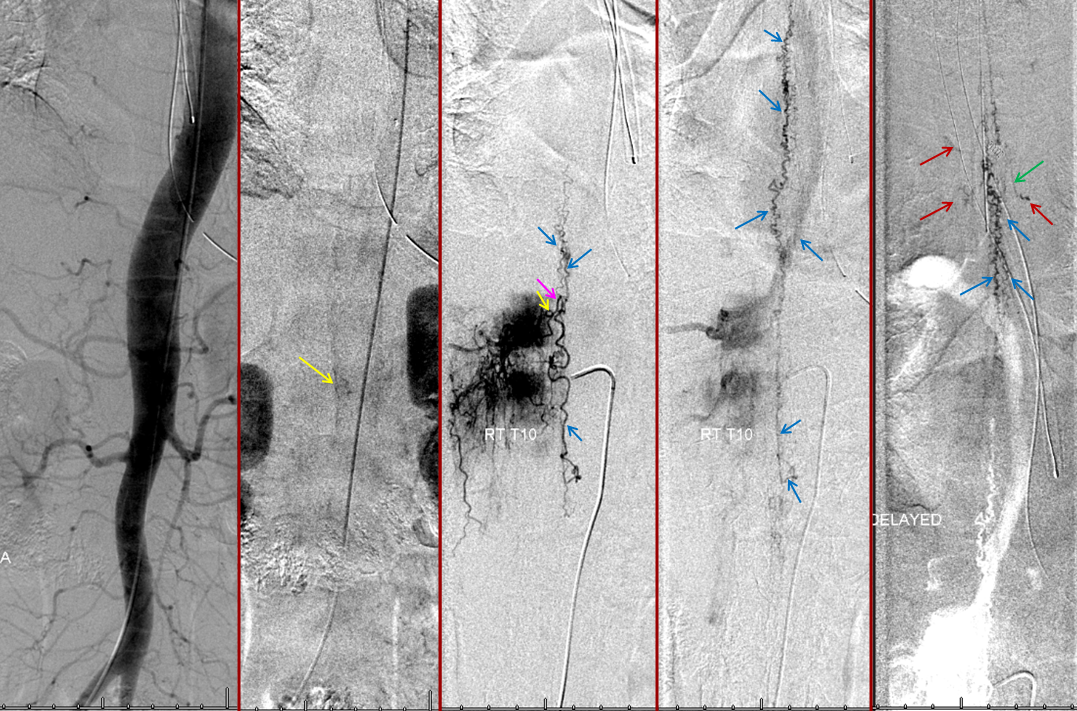
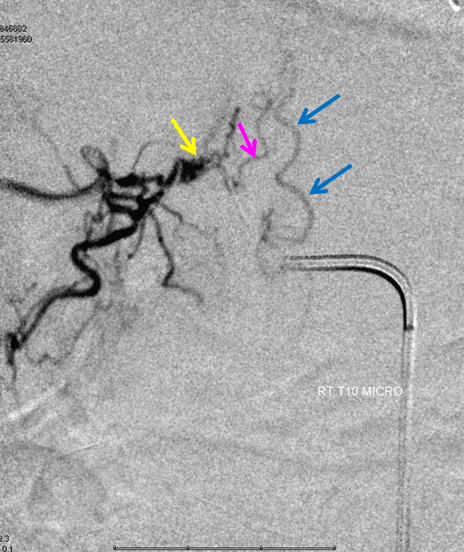
Thoracic aortogram arterial phase (leftmost) and late arterial phase (second from left) demonstrating a region of suspicion (yellow). Right T10 injection arterial phase (middle) demonstrating the fistula (yellow) congesting the radiculomedullary vein (pink) and spinal veins (blue). Late venous phase image (second from right) still shows no radiculomedullary veins. Very late (50 second from injection) image (rightmost) showing radiculomedullary veins above the carina (green) draining into the foraminal venous plexus (brown)
Dural Fistula with EXTREME venous congestion — an apt illustration of how far things can go. This patient was completely plegic by the time the following study was aquired — a fairly good predictor of extreme venous hypertension. The fistula, at T10 level, has no identifiable thoracic or cervical drainage, finally decompressing into the cavernous and straight sinuses.
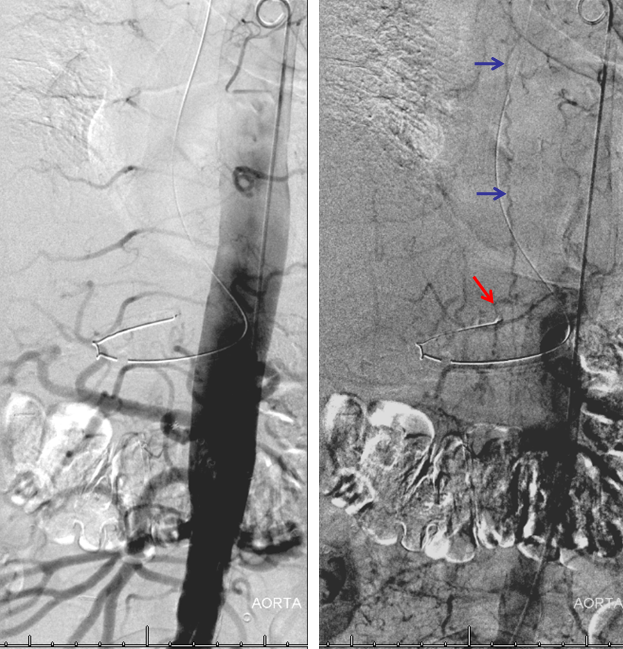
Aortogram, with late arterial phase imaging (right) demonstrating a large fistula (red) and a dilated spinal vein (blue). To see a fistula this well on an aortogram is a bad sign.
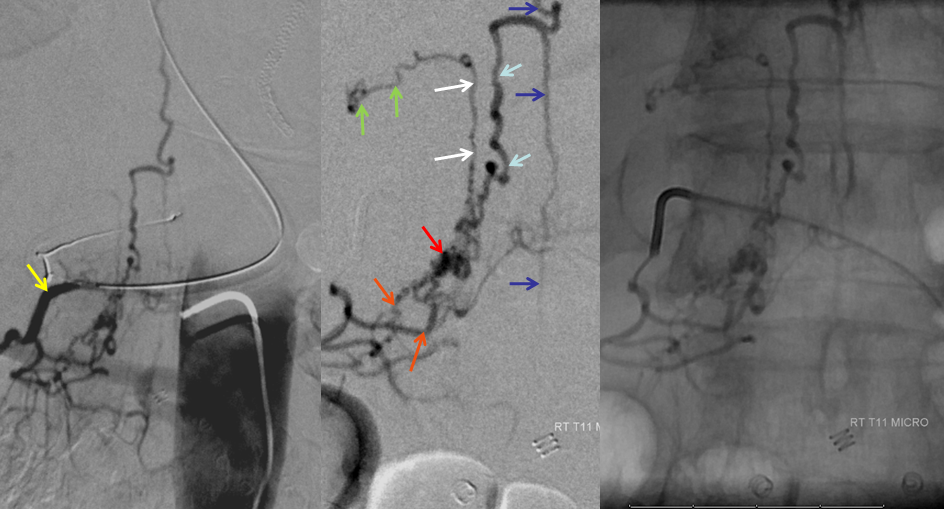
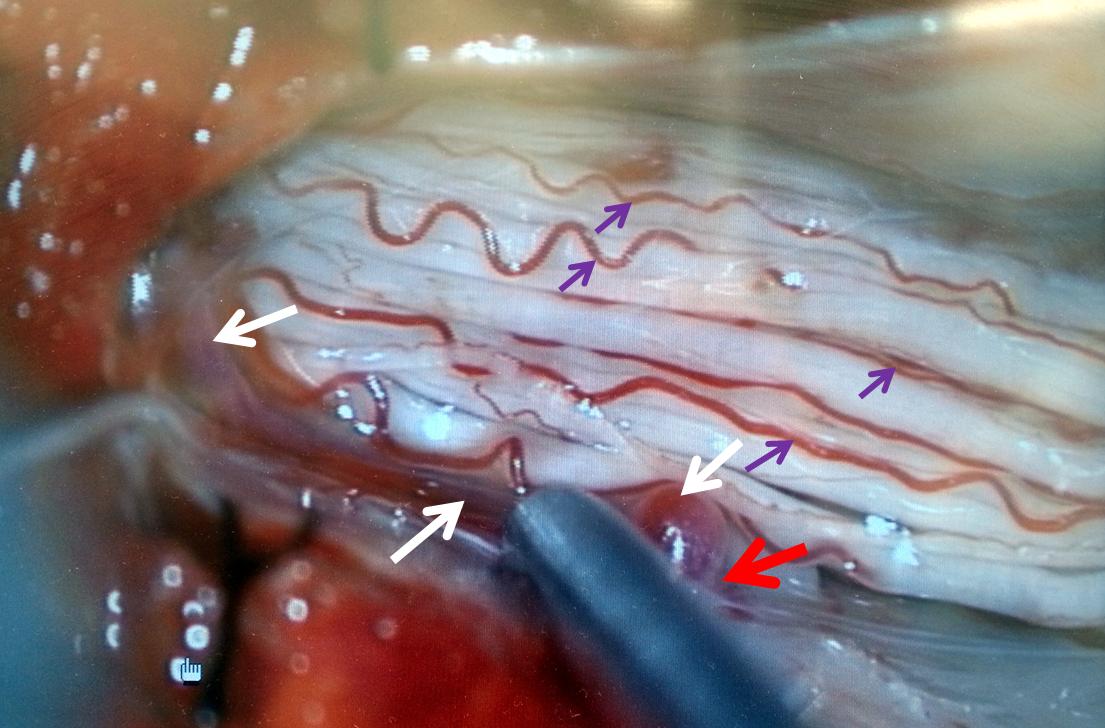
AP image on left side shows the T10 segmental artery (yellow). Microinjections of same level (center and right, unsubtracted) obtained after wedging the guide considerably far down the segmental artery, demonstrate two radicular arterial channels (orange) leading up to the fistula (red), from which a radicular vein (light blue) retrogradely decompresses into and congests the spinal vein (blue). Notice also a parallel vein (white), probably extradural, decompressing the fistula into the right T10 radicular vein (light green).
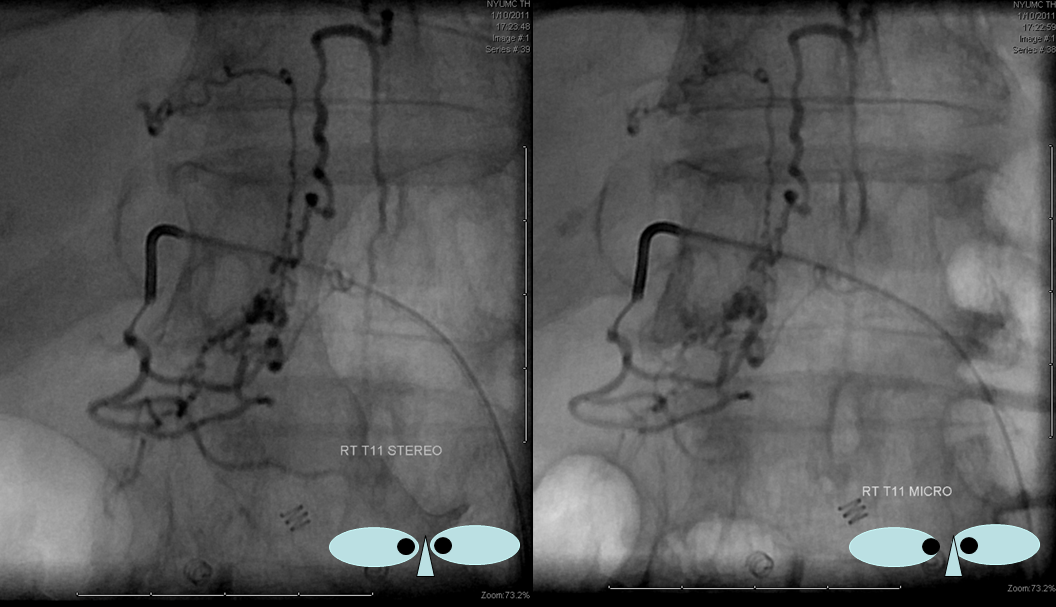
Unsubtracted stereo pair of the fistula — a very cool shot, where one can surmize the point where the draining vein becomes intradural: no labels, have to stereo :-), or if not, it is the point where the vein makes a small zigzag going down (and posterior through the dura) before heading back north again. The parallel extradural vein remains relatively ventral.
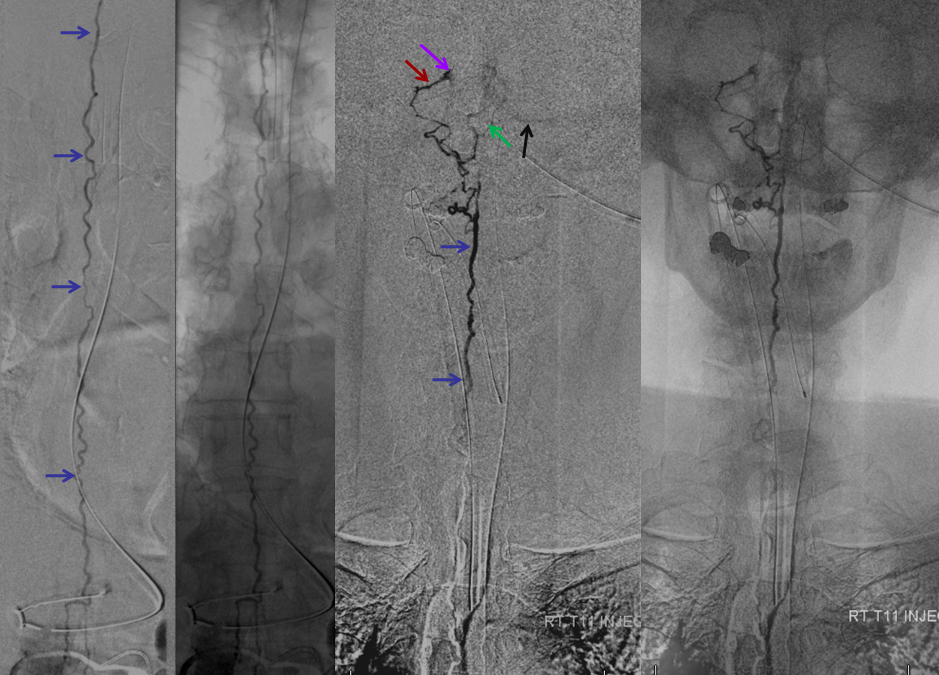
The incredible venous congestion, illustrated by T11 injections of the fistula, whereby the spinal vein (blue) can be visualized draining superiorly along the entire length of the cord, finally decompressing via the superior petrosal sinus (brown) and a posterior fossa vein (green) into the cavernous sinus (purple) and the sigmoid sinus (black)
T11 injection, imaged in the lateral plane of the craniocervical region, again showing the spinal vein (blue) retrogradely draining via the superior petrosal sinus (brown) into the cavernous sinus (purple), and additionally via a posterior fossa dural vein (green) into the straight/transverse/sigmoid sinus systems (black).
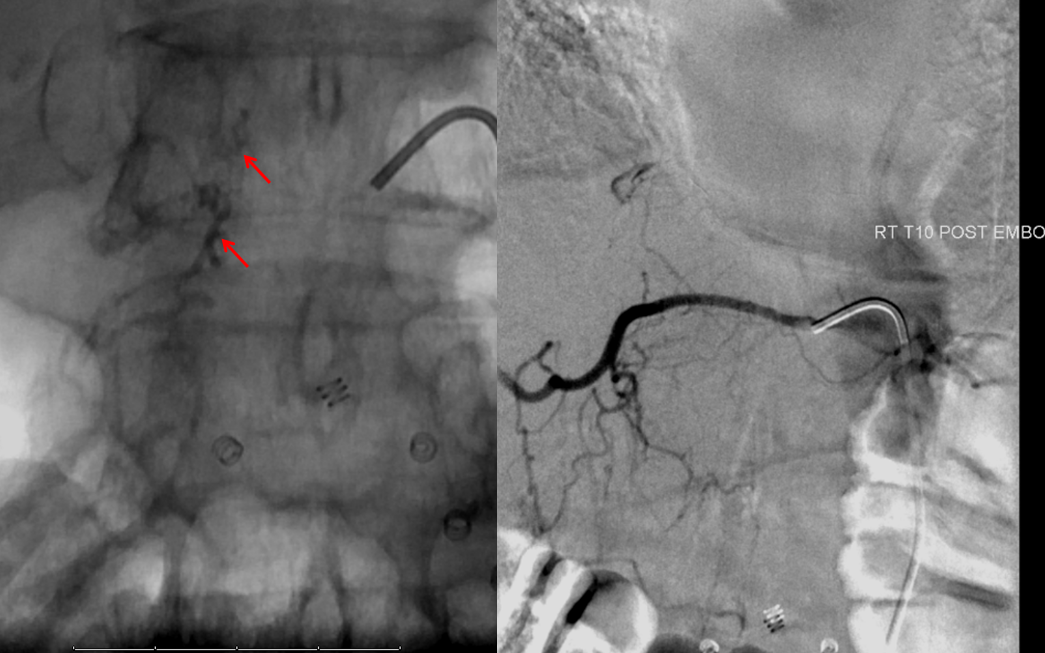
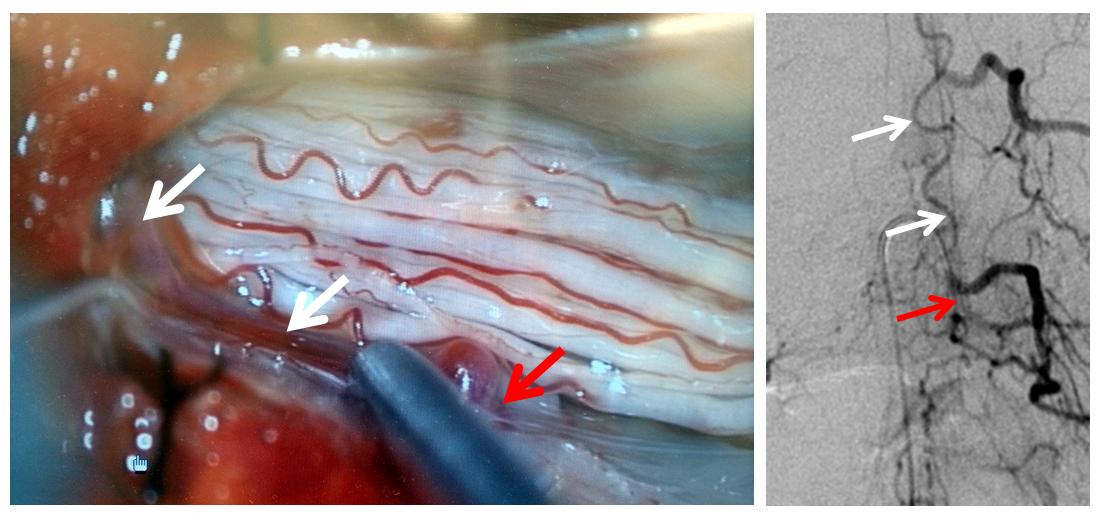
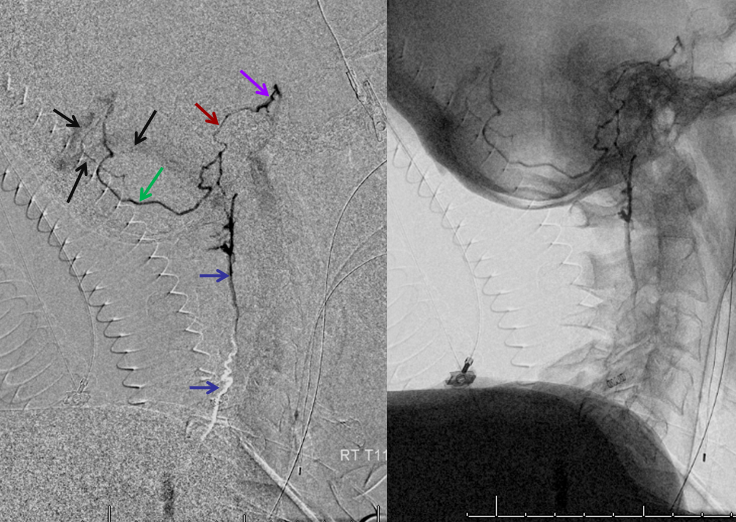
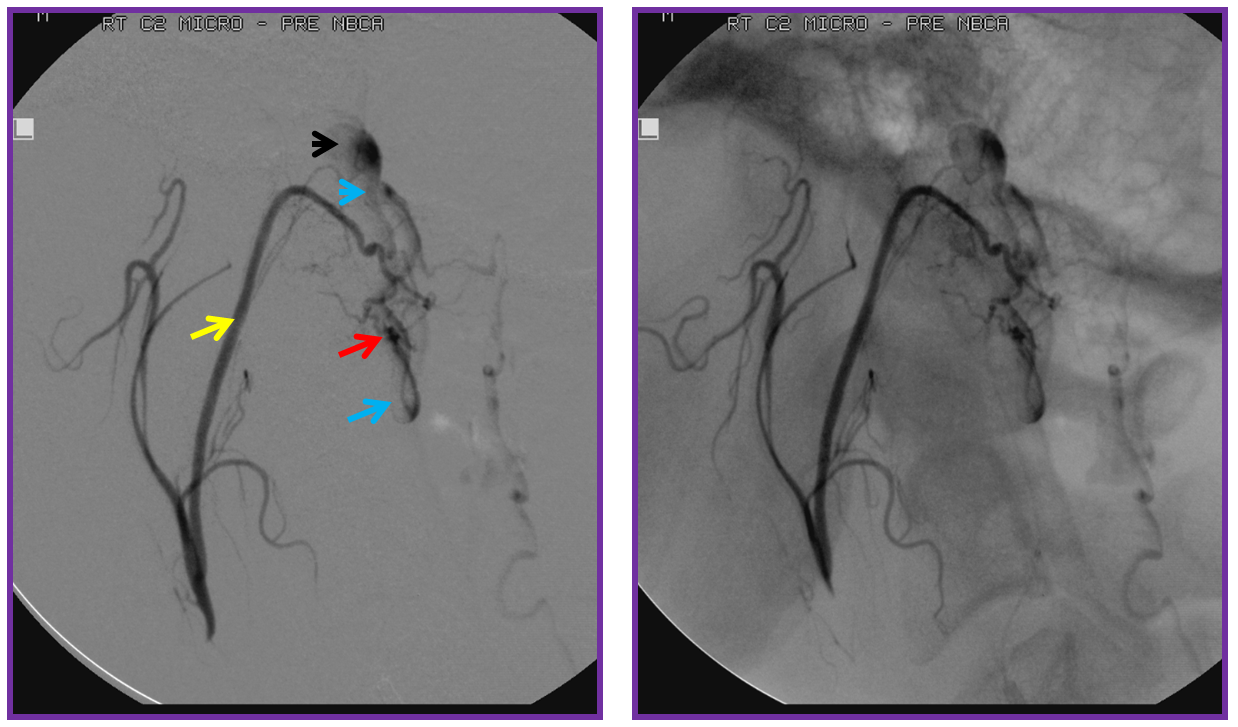
Finally, a post-embolization AP image on the left demonstrating n-BCA glue cast in the fistula (bottom red arrow) and its draining vein (top red arrow). No residual fistula is seen (right image). The fistula and its draining RADICULAR vein must be permeated with embolic material to effect a permanent cure. Every attempt is made not to spill glue into the spinal vein (blue arrows on above images) since it may still be used by the spinal cord. Notice spasm of the segmental artery where the guidecatheter was previously wedged in (not shown). The guide is usually pulled, along with the microcatheter, after a glue shot.
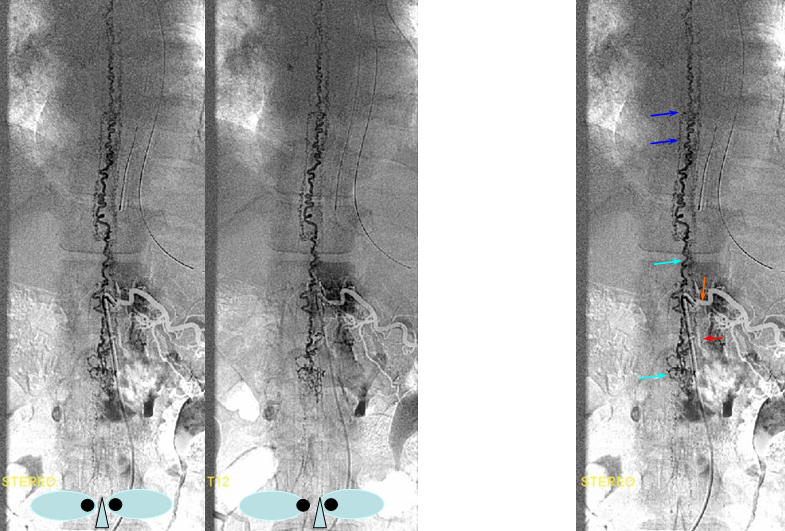
DIFFERENT PATIENT, STEREO IMAGE
Dural Fistula angio, stereo and labels: — a little different view of a fistula obtained by adjusting the mask to “white out” the arterial phase. A T12 segmental artery (orange, B) gives rise to a dural branch (red, M). A fistula (yellow) proximal to a dilated vein (light blue) is present. The spinal cord veins are congested. The dark blue veins constitute the dorsal venous network, while the larger light blue channel is ventral to the cord). The upper left pictures are a stereo pair (with the eyes), obtained with the AP camera 4 degrees apart while keeping everything else constant. Crossing the eyes or using special glasses can help you see it in stereo (where you can readily tell the difference between ventral and dorsal venous networks)
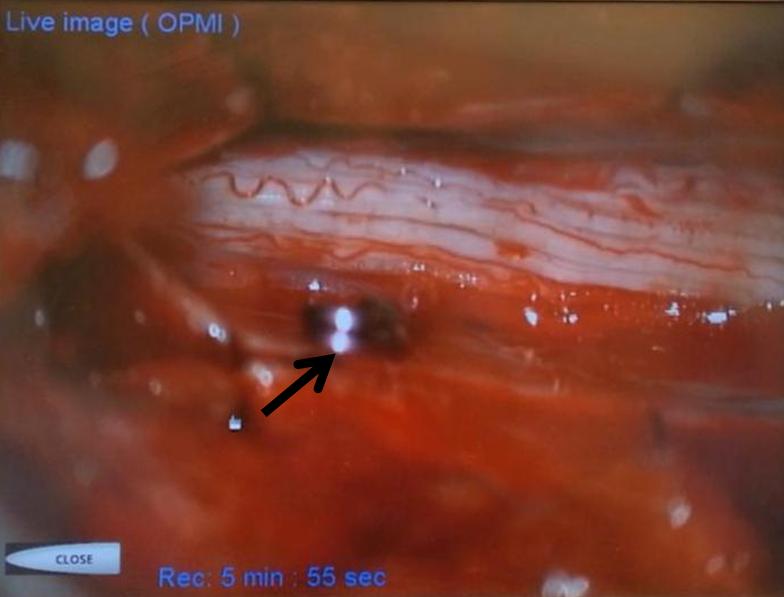
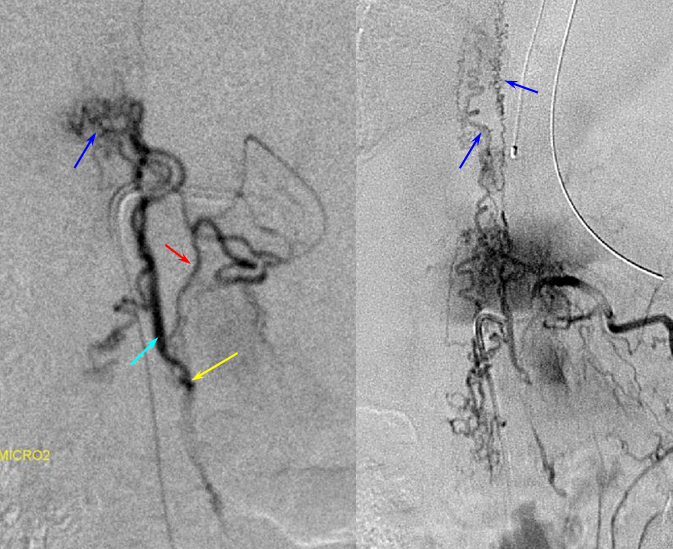
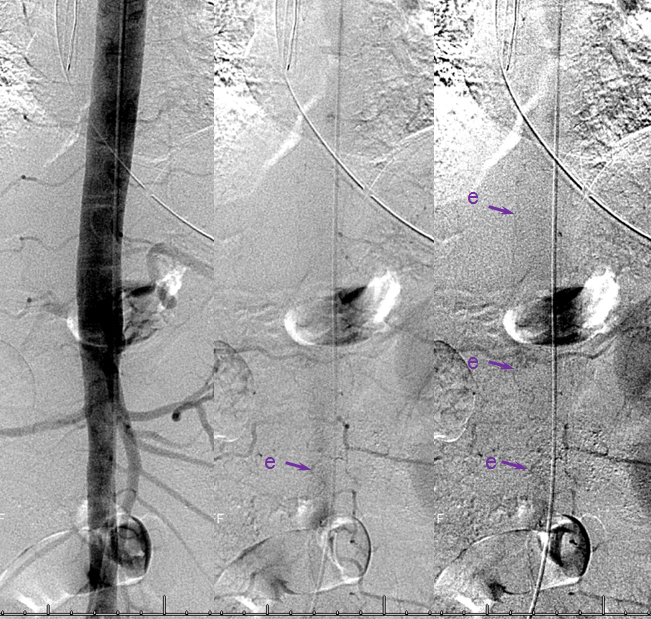
A microscopic injection of another dural fistula
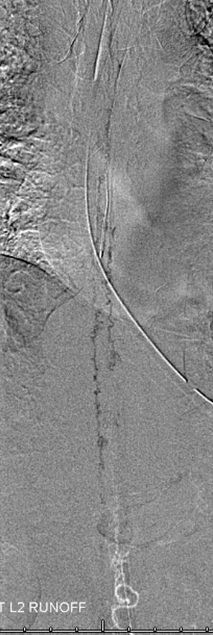
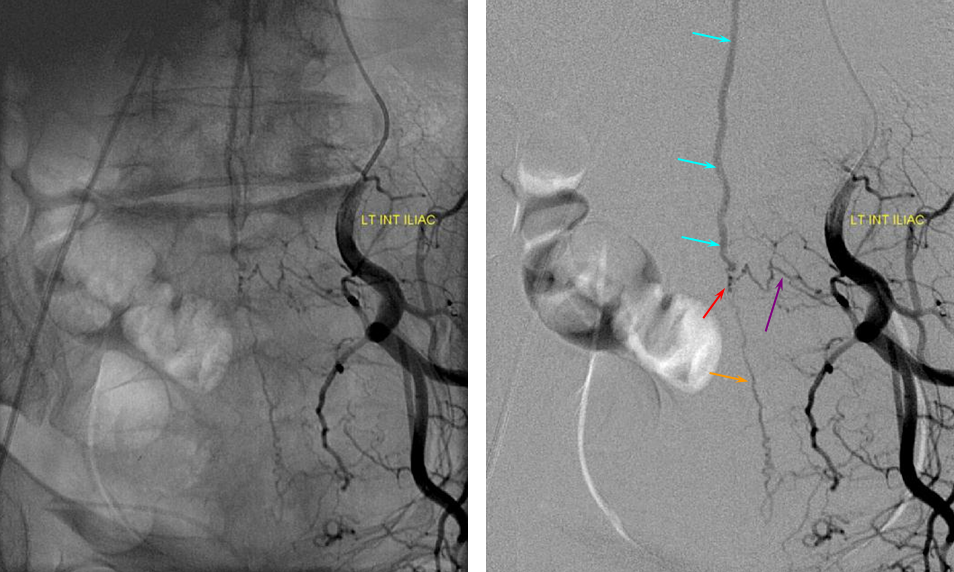
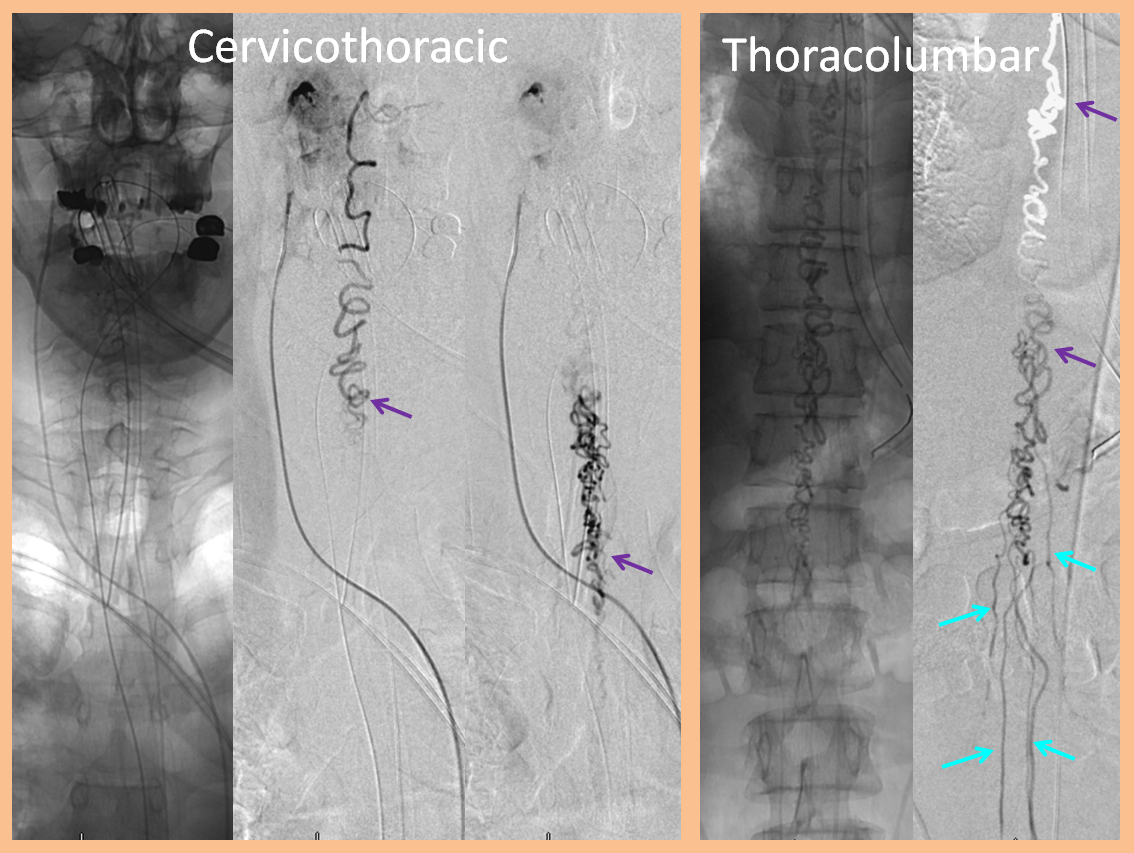
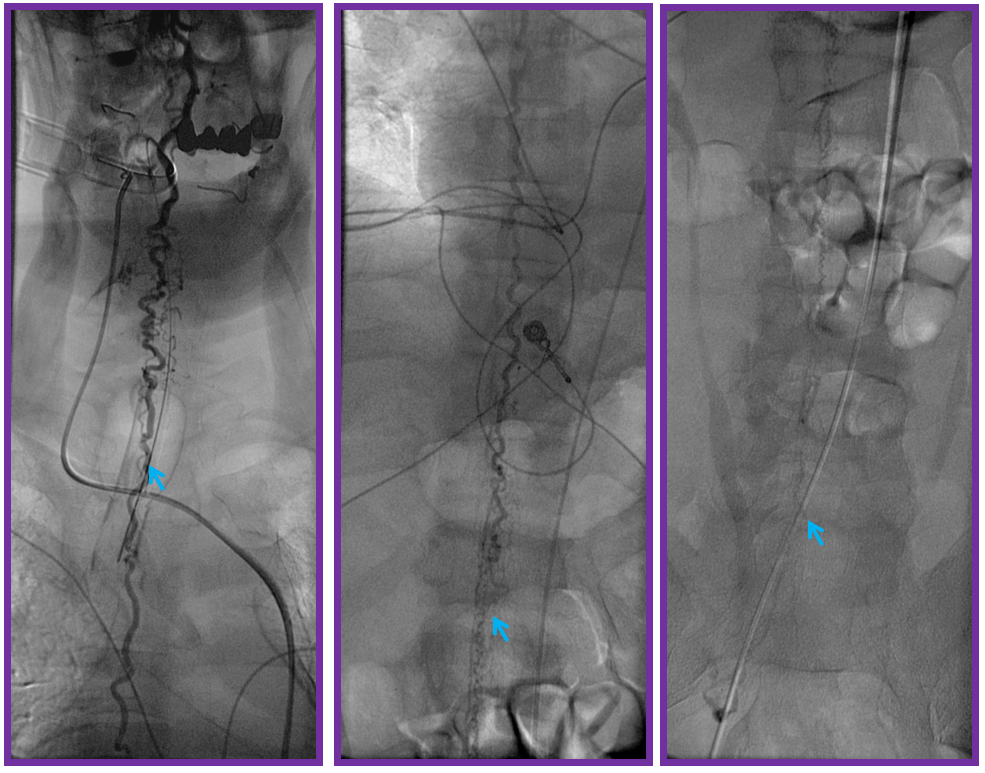
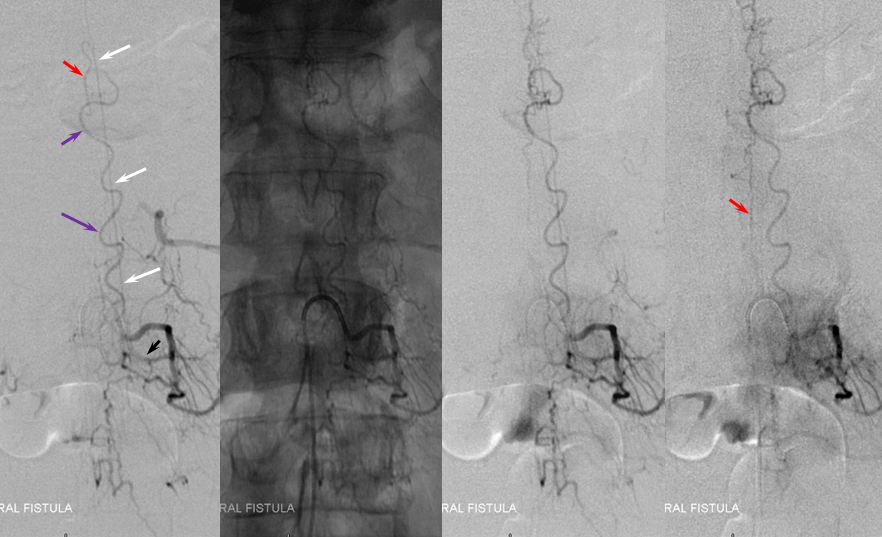
ILIAC ARTERY ORIGIN OF AV FISTULA

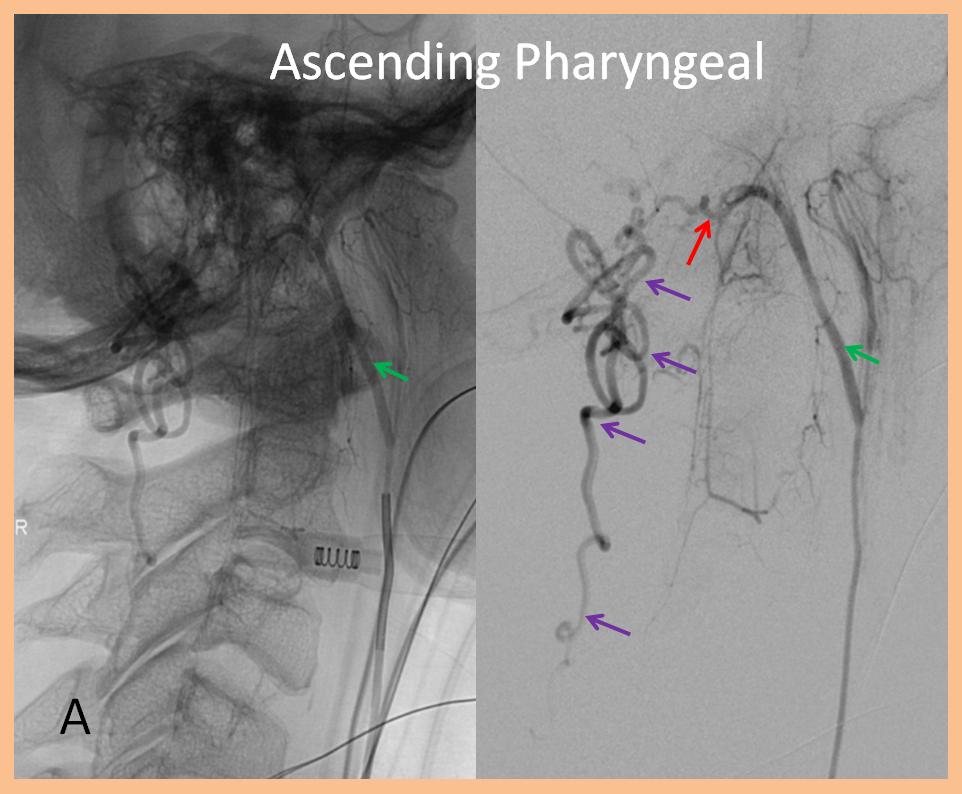
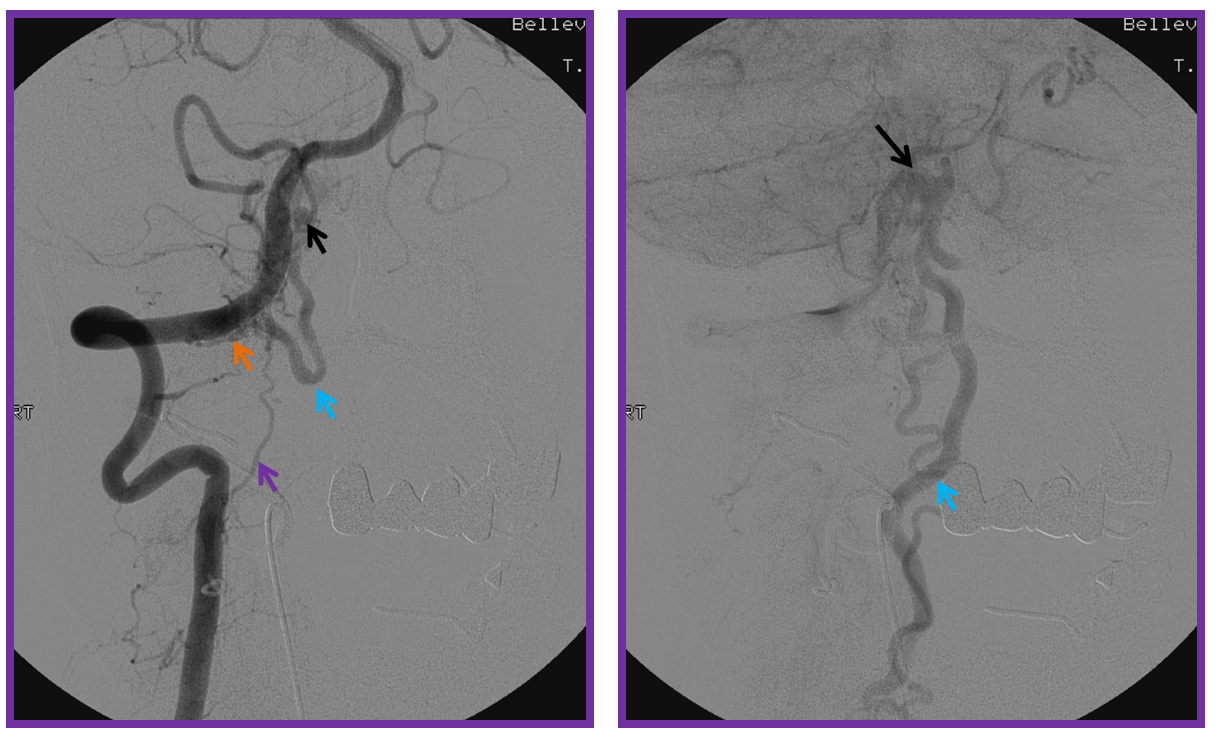
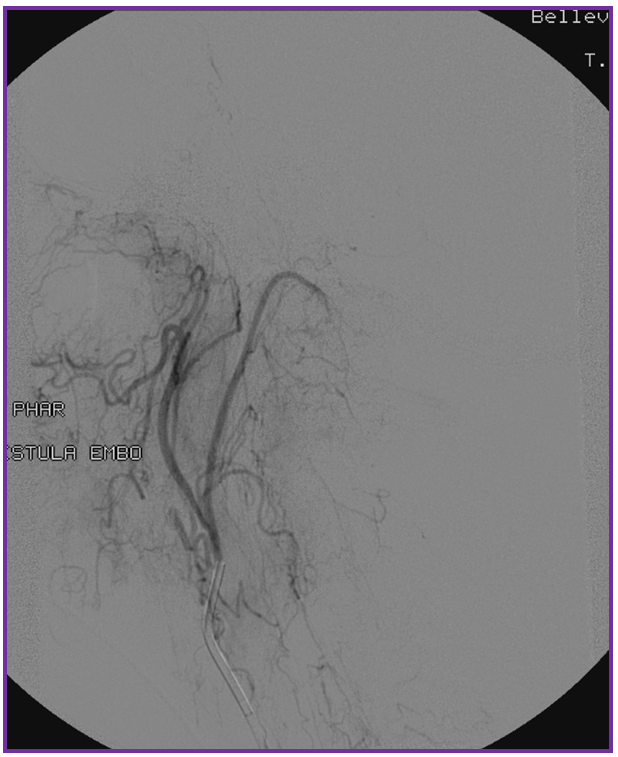
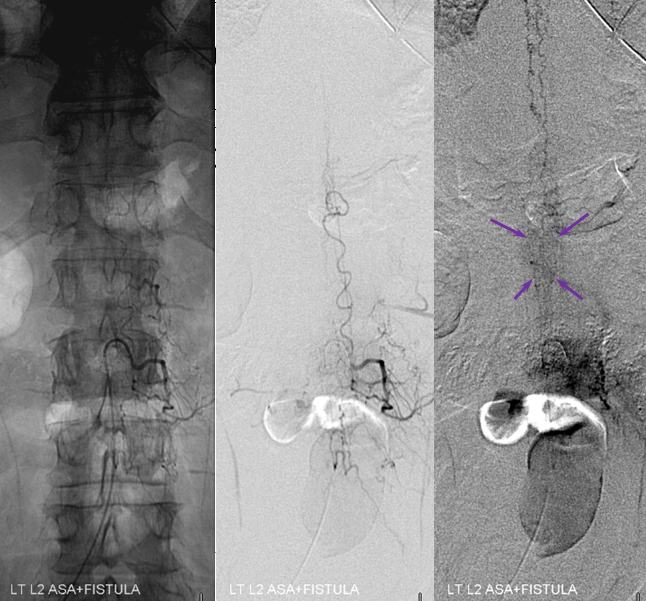
3. Ascending pharyngeal artery origin of intradural fistula.
At the other end of the spinal canal is a fistula supplied by the ascending pharyngeal artery.
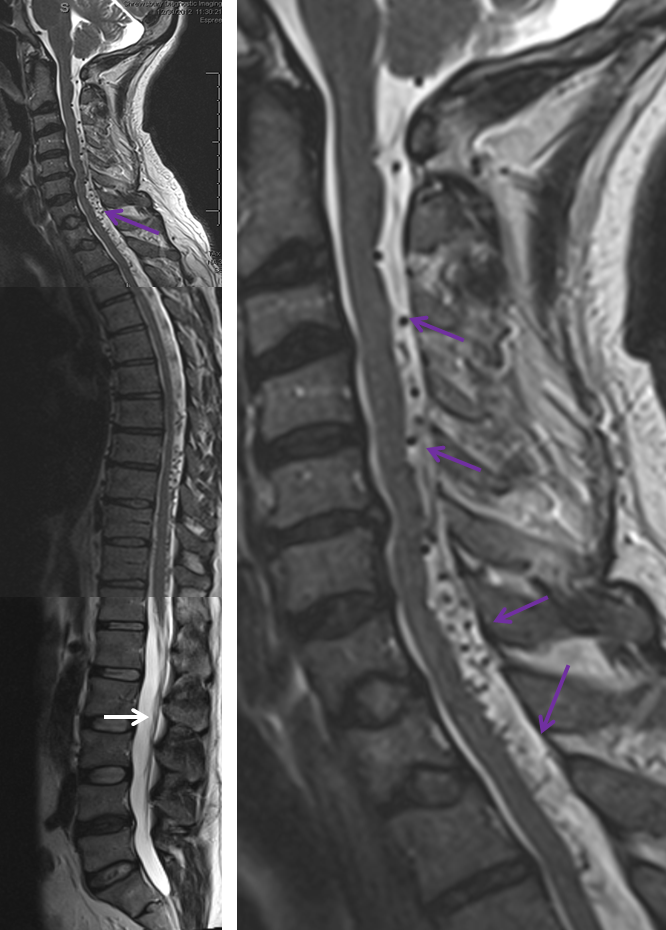
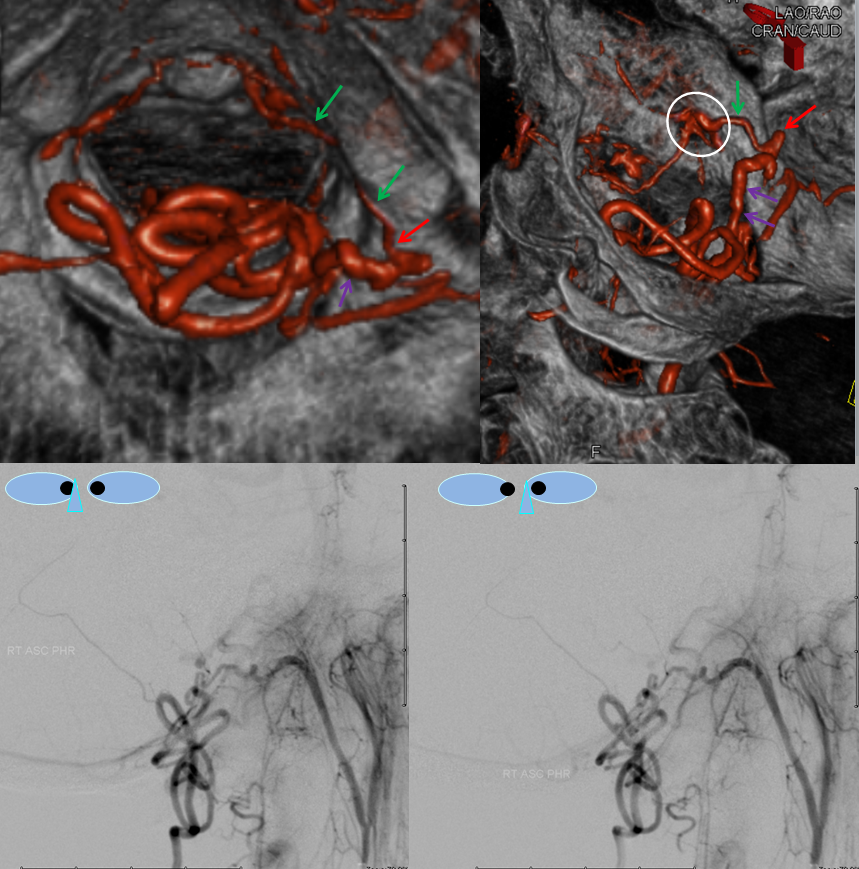
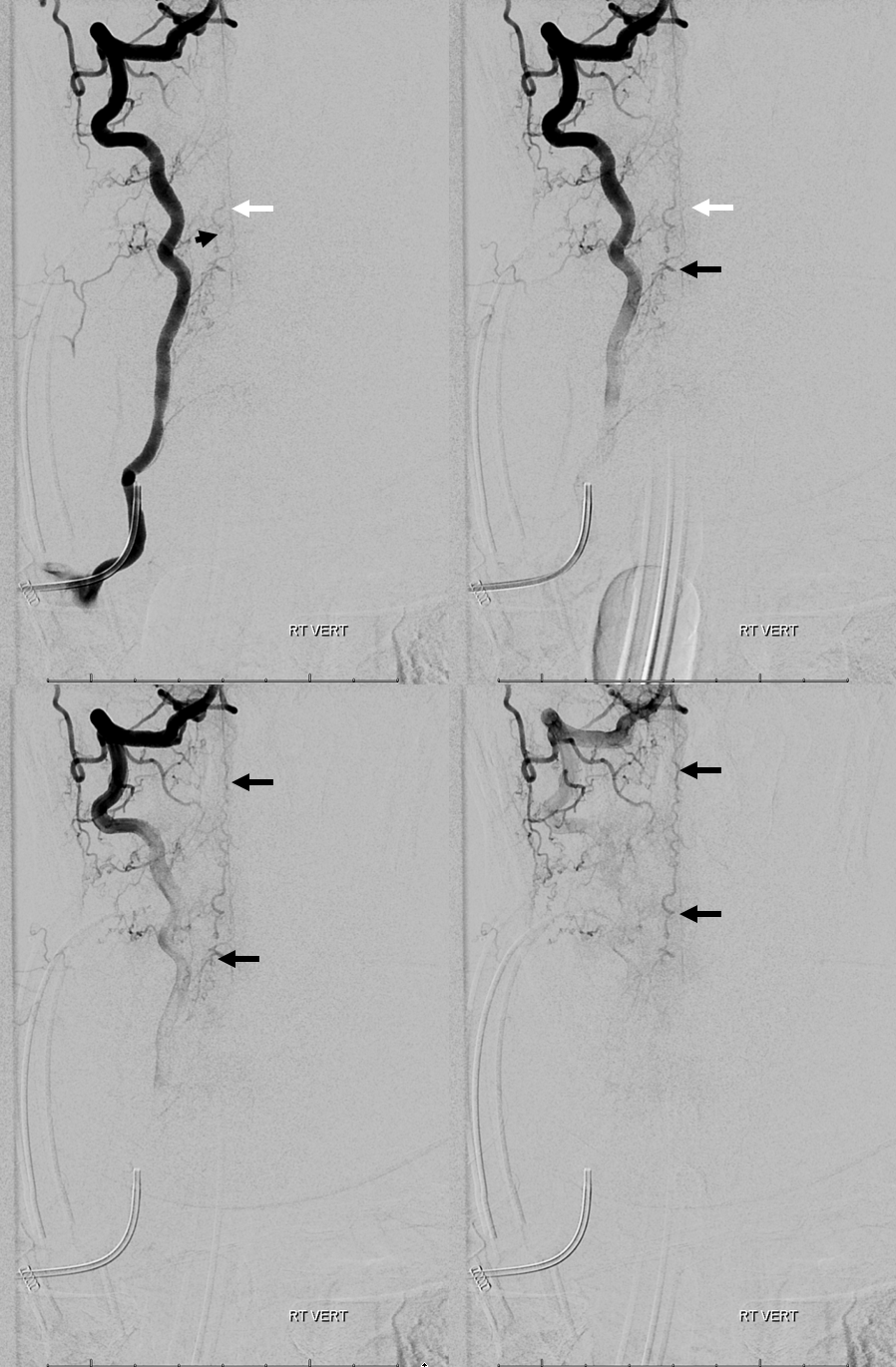
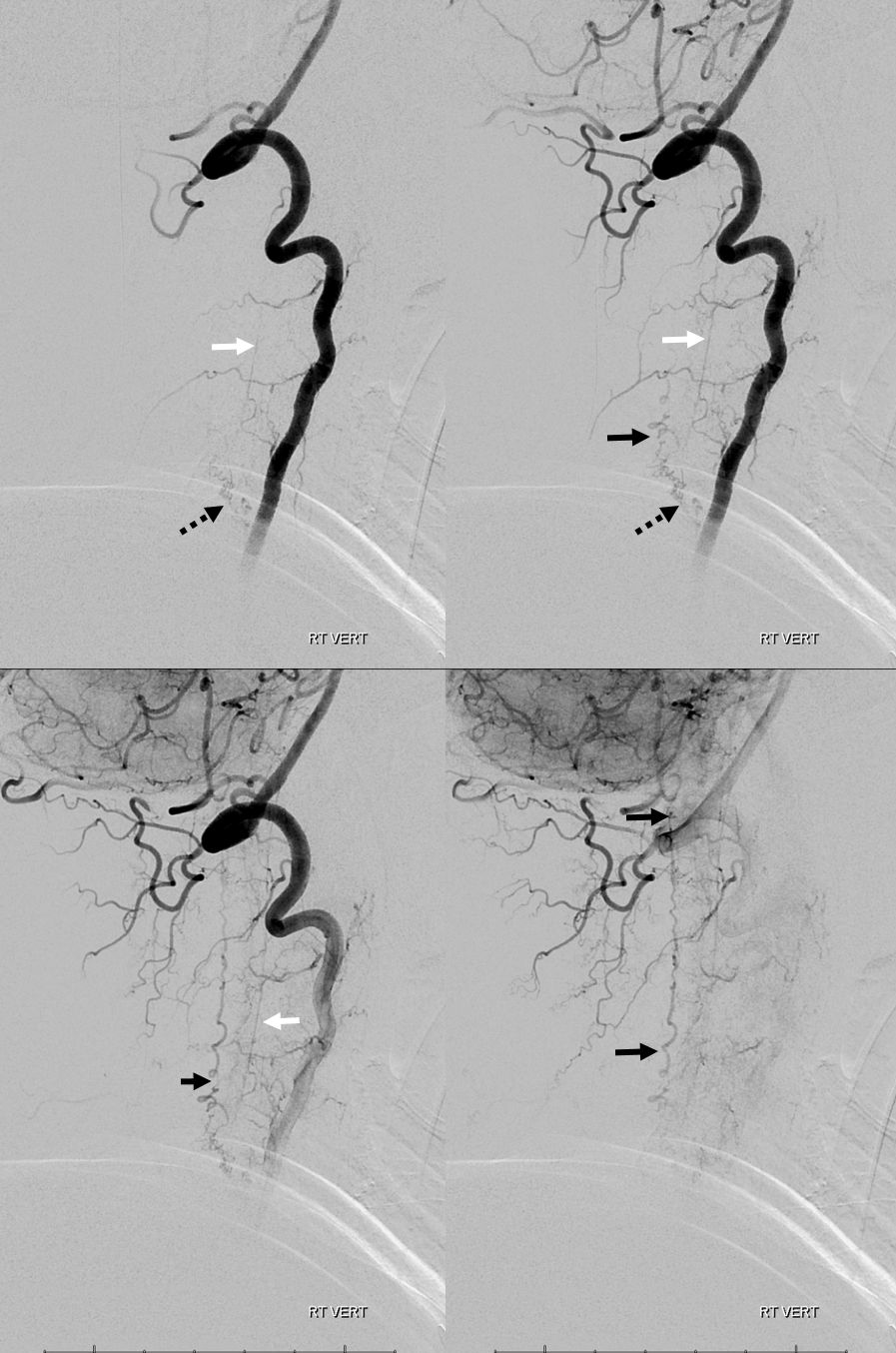
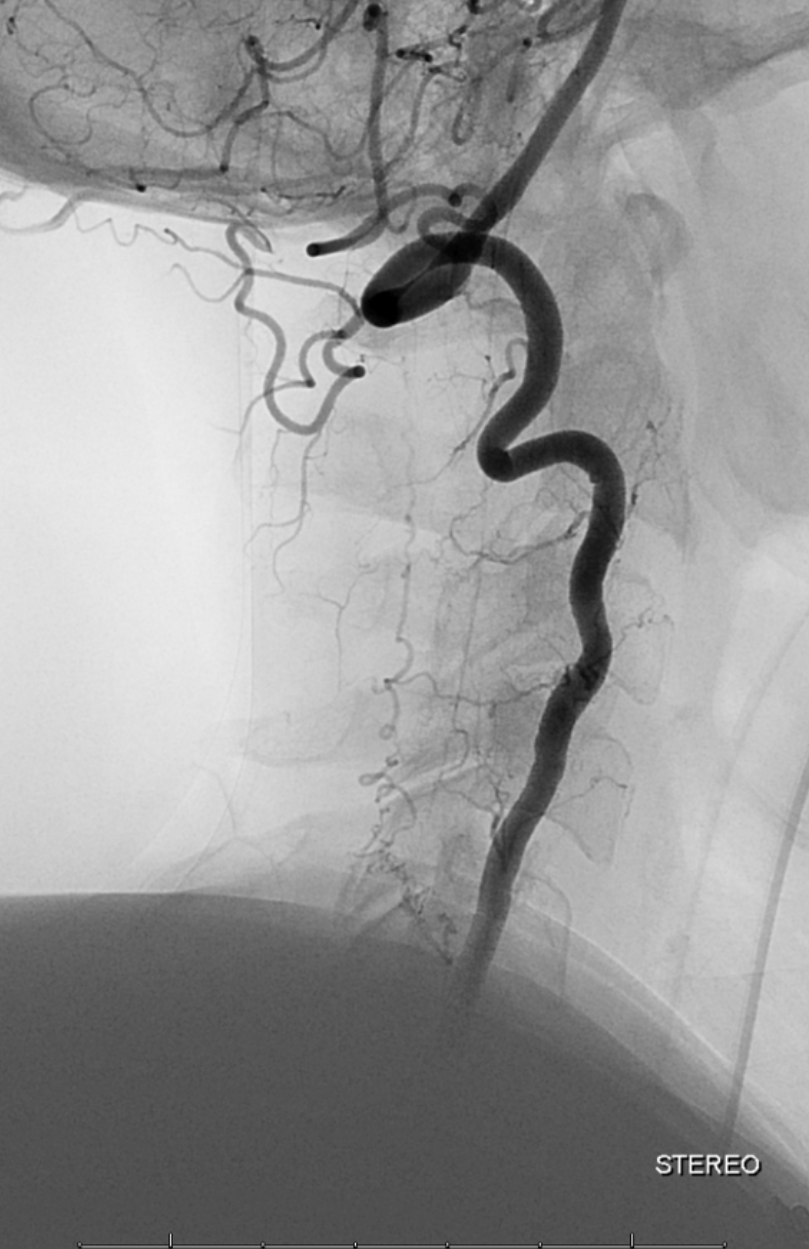
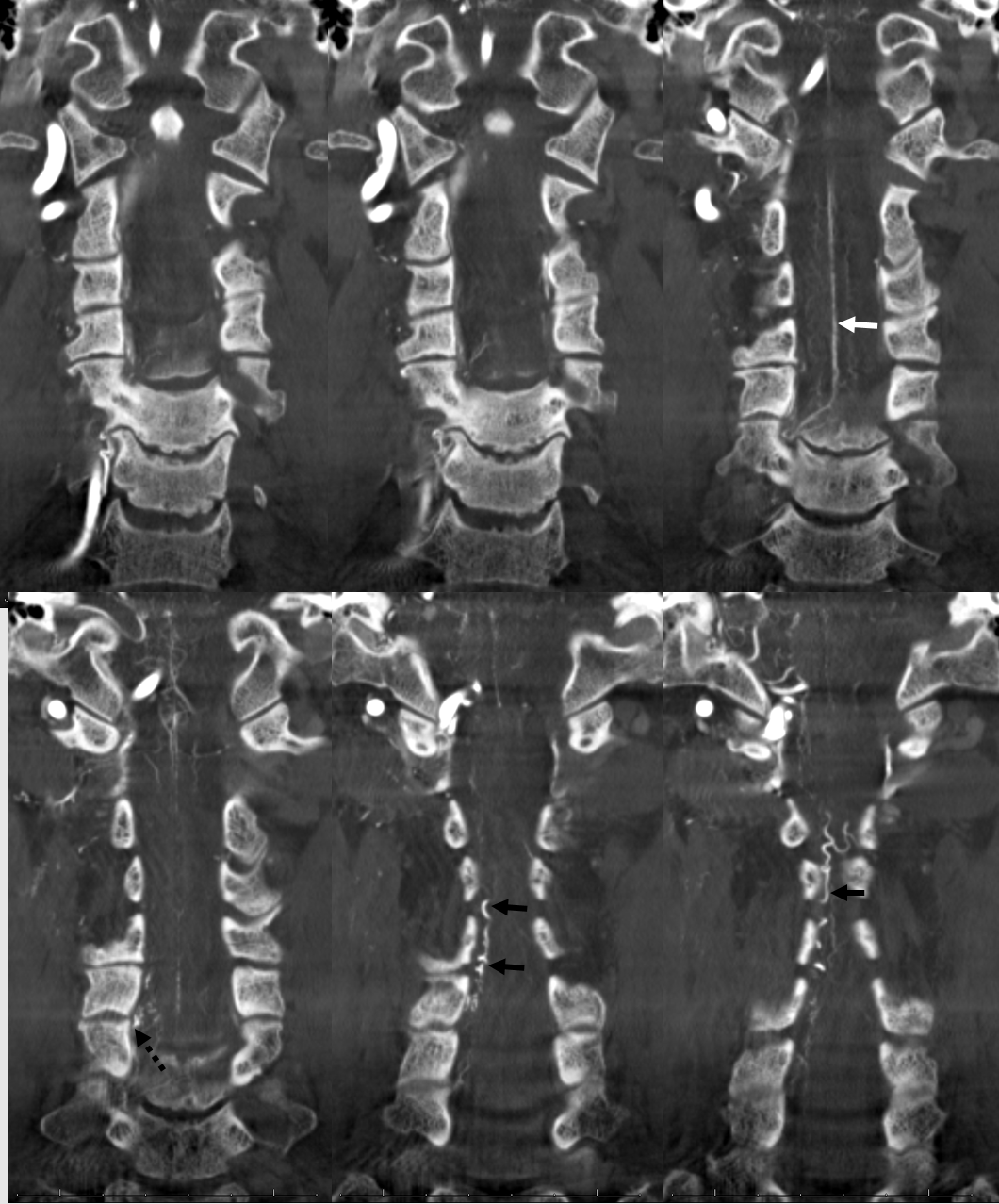
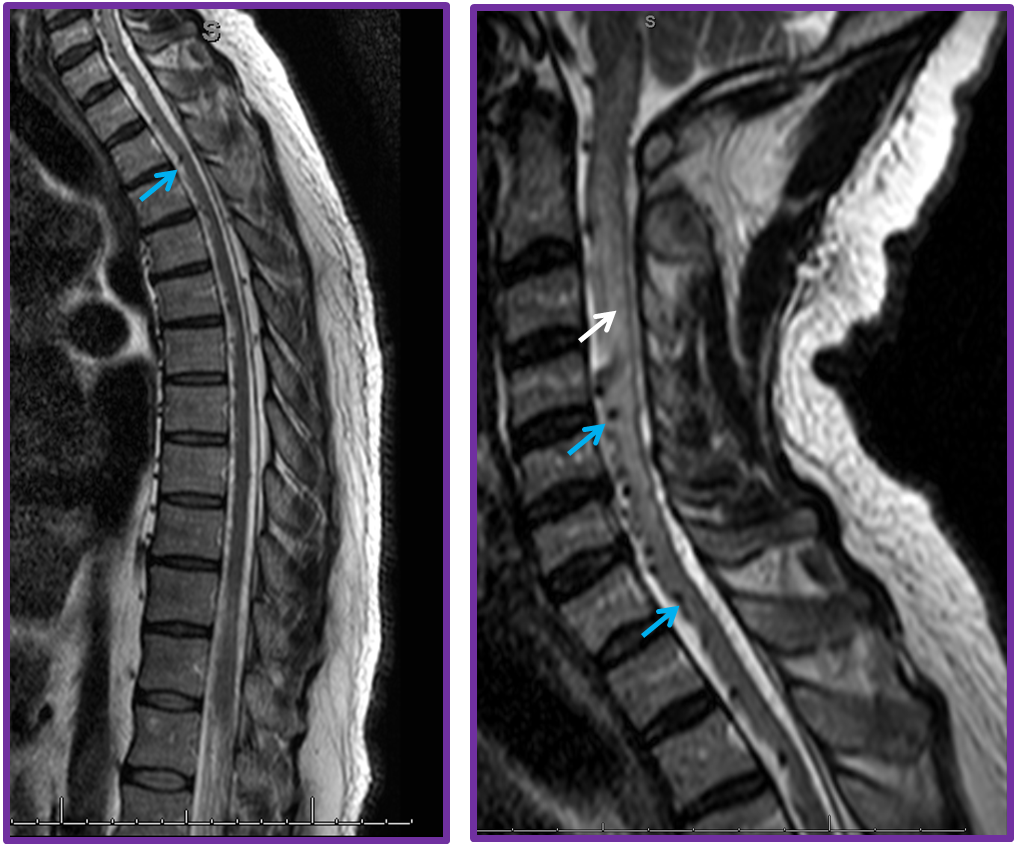
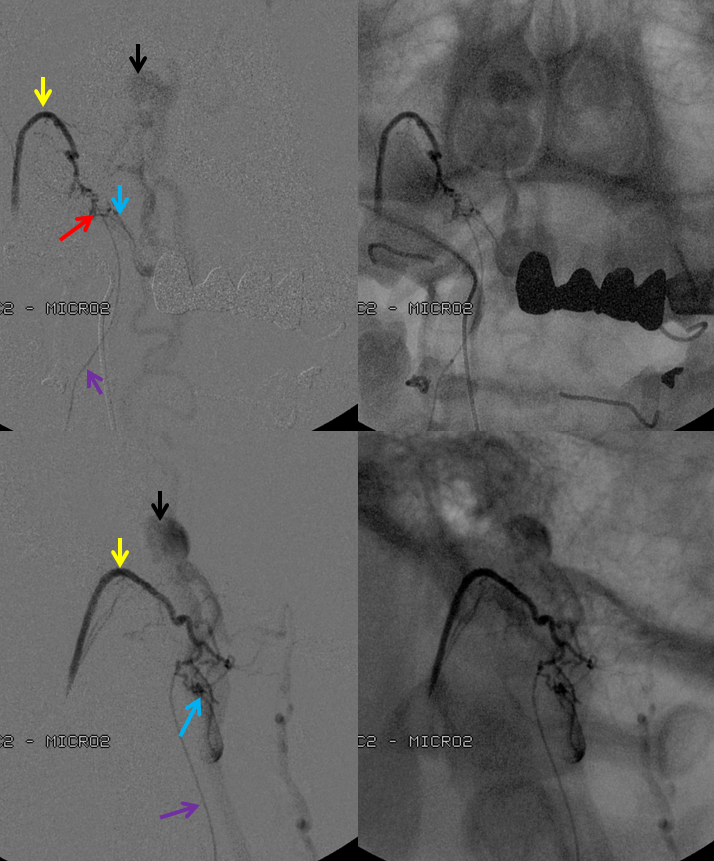
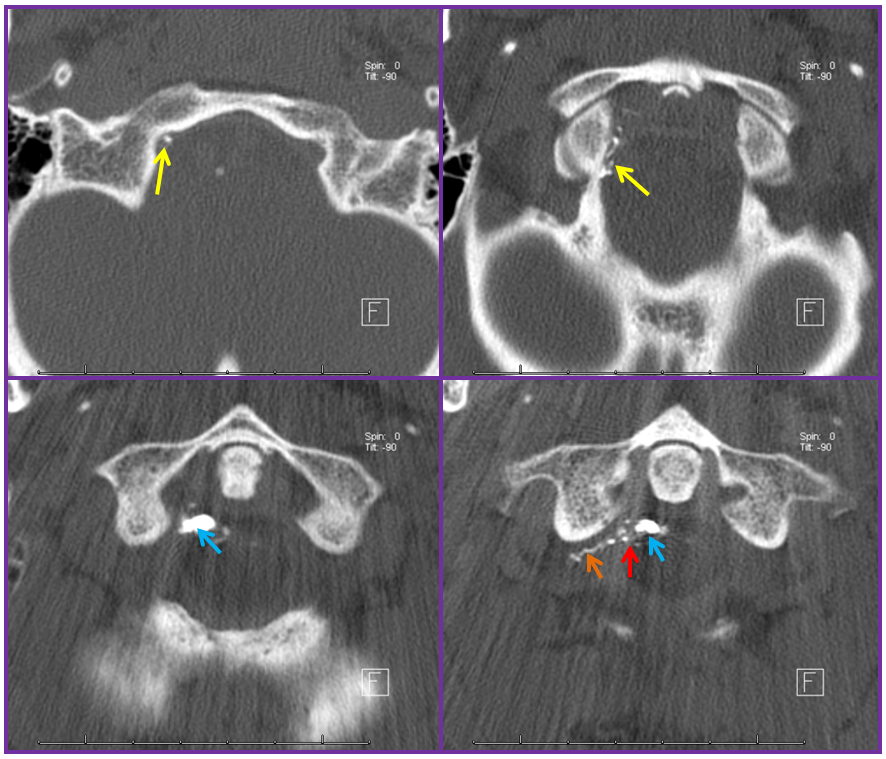
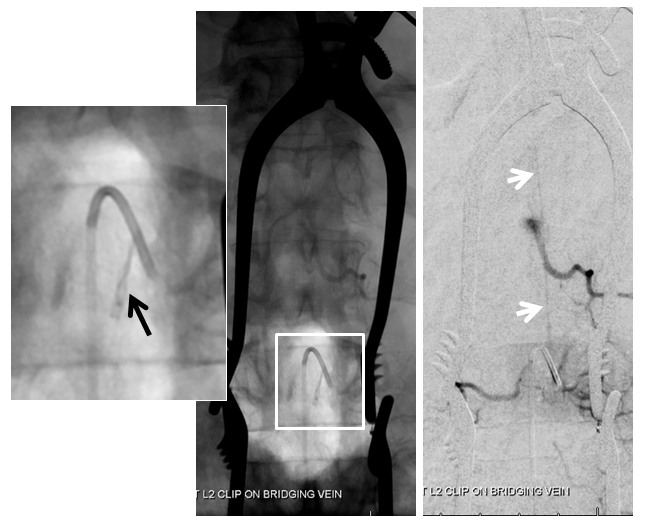
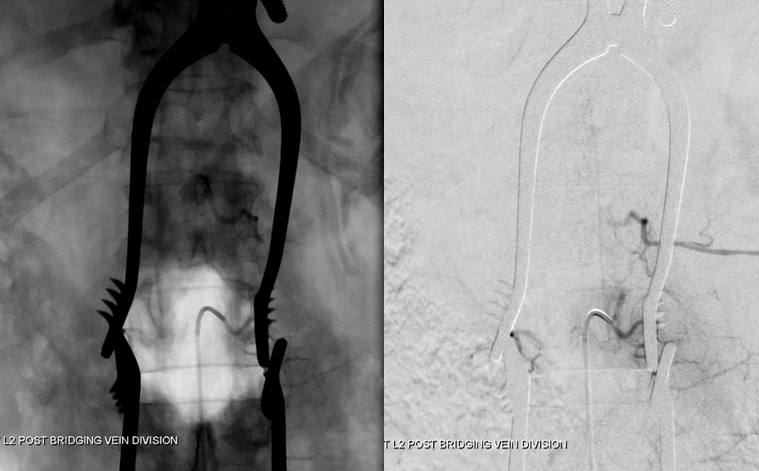
Stereo views are not simply cute. The are very useful in confirming that the long bridging vein undulates along the left posterolateral surface of the cord, with the anterior spinal artery serving as a stereo landmark of the ventral surface. This helps determine optimal surgical approach, which we choose in such cases instead of risking inadvertent radiculomedullary artery occlusion in an attempt at endovascular disconnection.