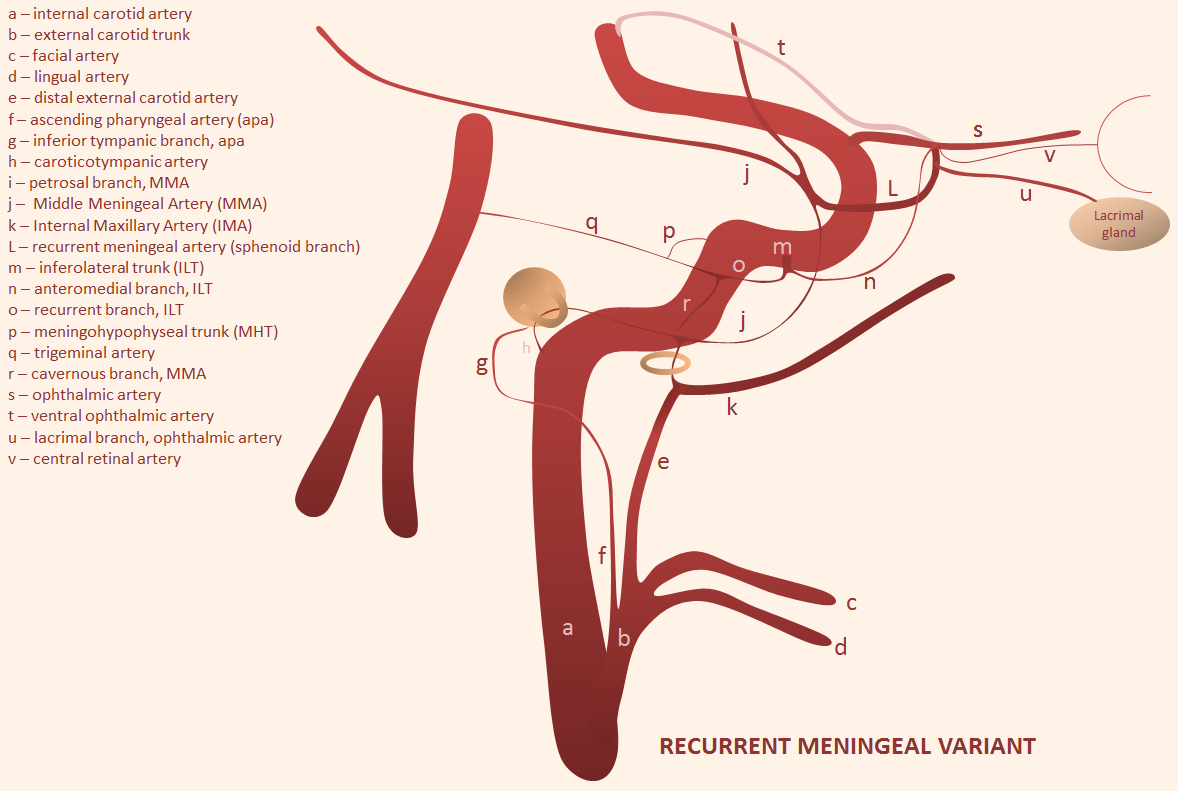
Middle Meningeal Artery
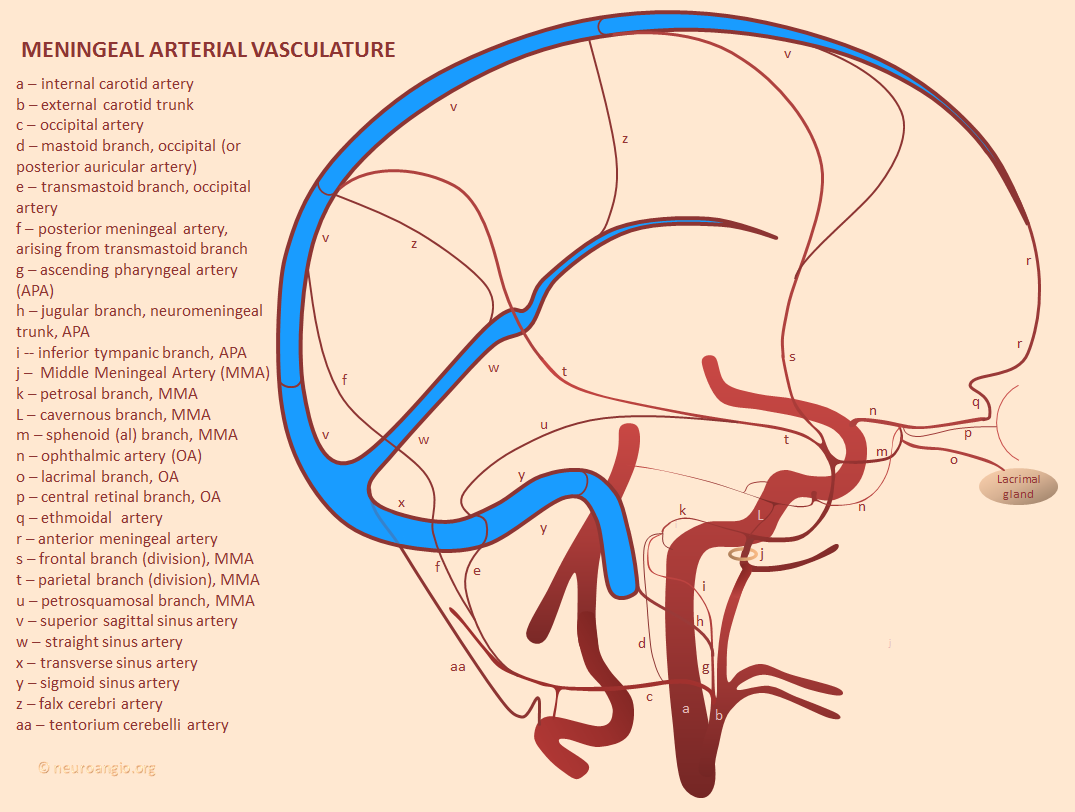
The Middle Meningeal Artery is the largest branch of the Meningeal Arterial Network, by far. Usual origin from the proximal Internal Maxillary Artery (IMAX), with multiple clinically-important variants. Multiple connections to other key vessels, including ophthalmic, internal carotid, MHT, ILT, ascending pharyngeal, occipital — these can be either useful treatment routes or “dangerous anastomoses”. Lets keep it simple — the more pictures, the better. For complete information, and cutting edge imaging, check out also Intrinsic Meningeal Arterial and Intrinsic Meningeal Venous Vasculature Pages
Basic Embryology — important for understanding variations and “dangerous anastomoses.” In the early embryo, both MMA and IMAX are actually supplied by the future internal carotid artery, via the “hyostapedial artery” (h, i) — a branch of the petrous ICA that goes through the crura of the stapes bone. The external carotid, which is of different embryonic origin, primarily supplies the other stuff — facial, lingual, occipital, ascending pharyngeal. Below is a picture of this early (16 mm according to Streeter/Padget) embryo arrangement
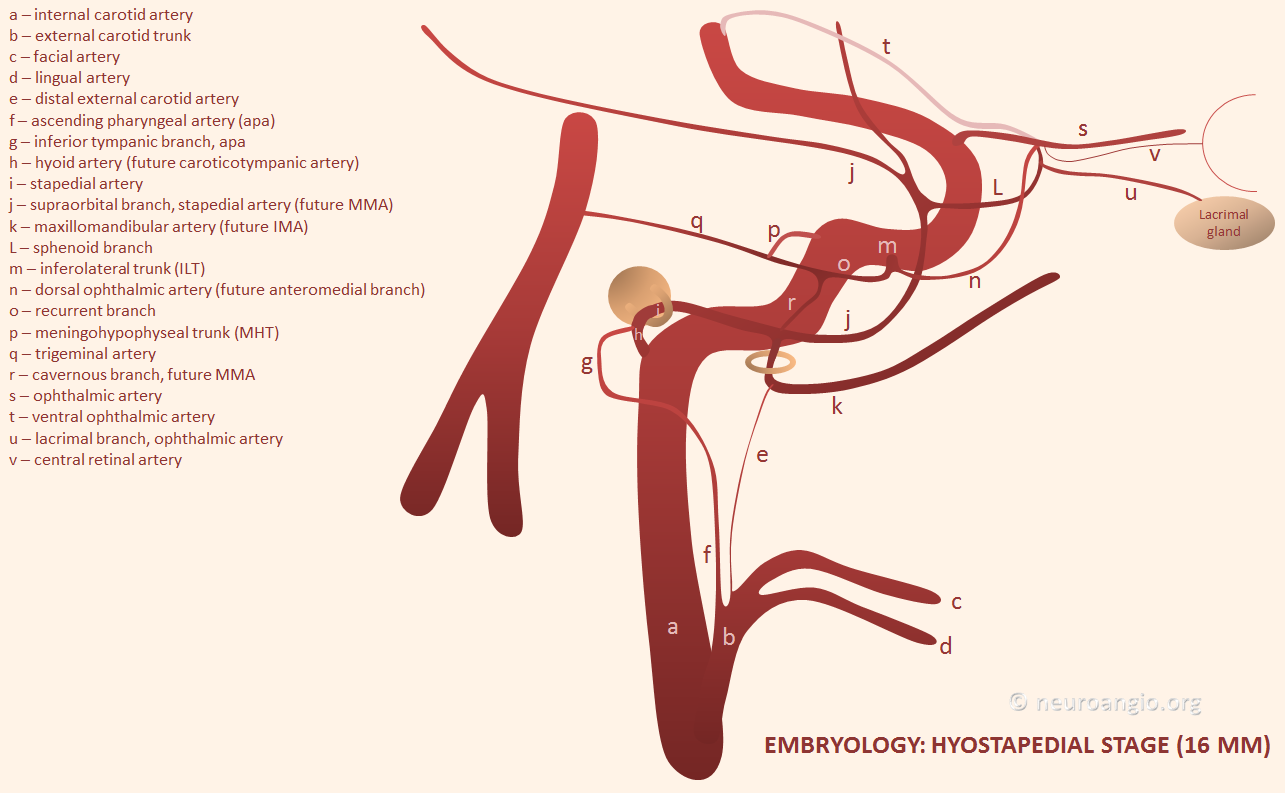
The theory is that demand of the growing cerebrum leads to loss of MMA and IMAX to the enlarging territory of the ECA. Supposedly, for the same reason the ICA usually looses its posterior cerebral and upper basilar (SCA) territories to the enlarging vertebrobasilar system (see neurovascular embryology page). In any case, in the adult both MMA and IMAX typically belong to the ECA, like this
Complete Hyostapedial Spectrum Variant

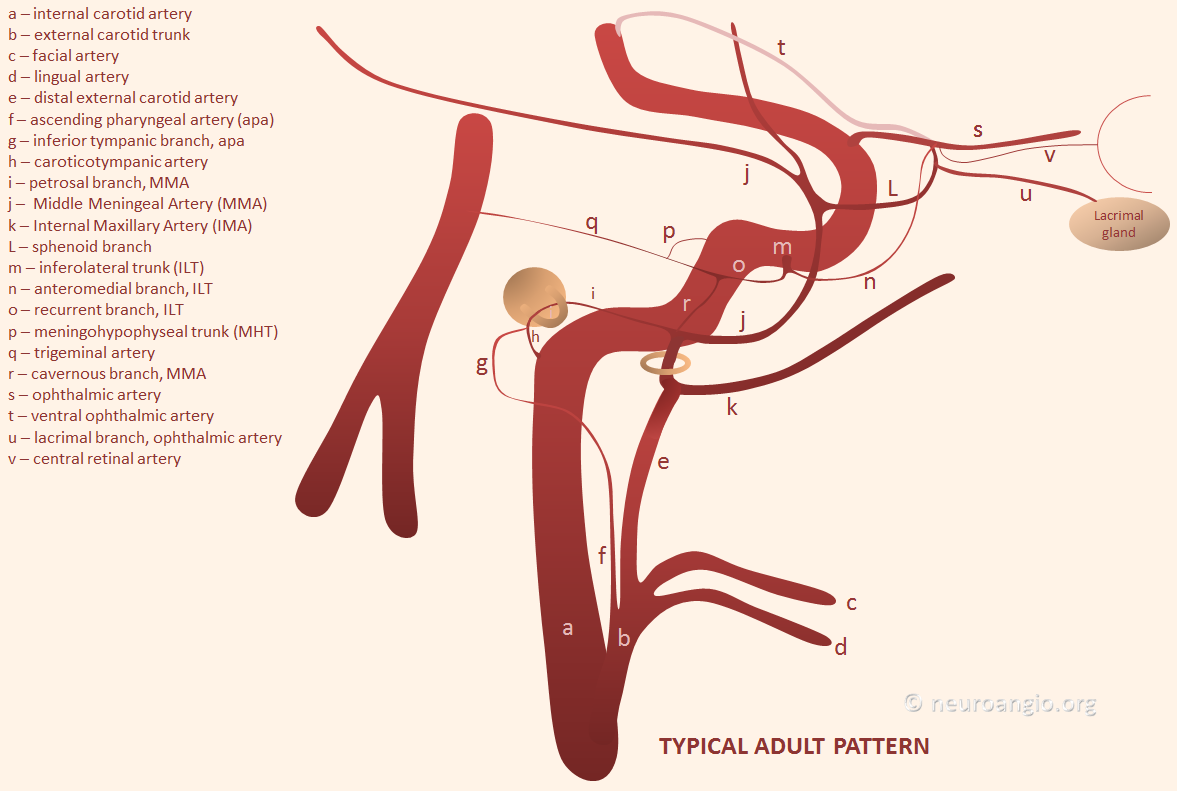
A supremely rare variant, supplied by Dr. Jonathan D. Clemente. Both MMA and IMAX are supplied by the vidian artery — yes, its not hyostapedial, but is on the spectrum of the same variant. The only case we’ve ever come across. Makes one wonder if the embryology theory is really valid, but here it is.
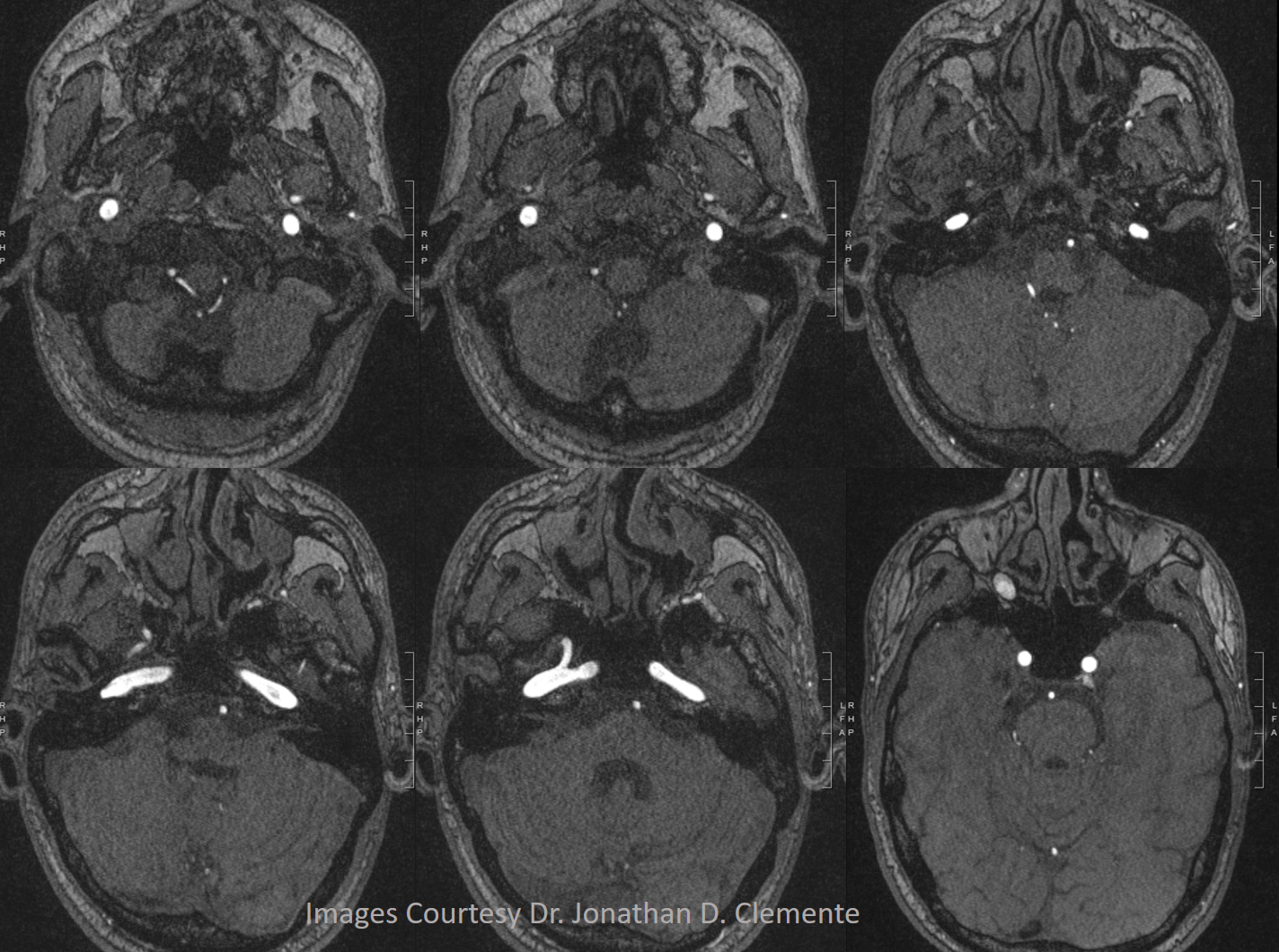
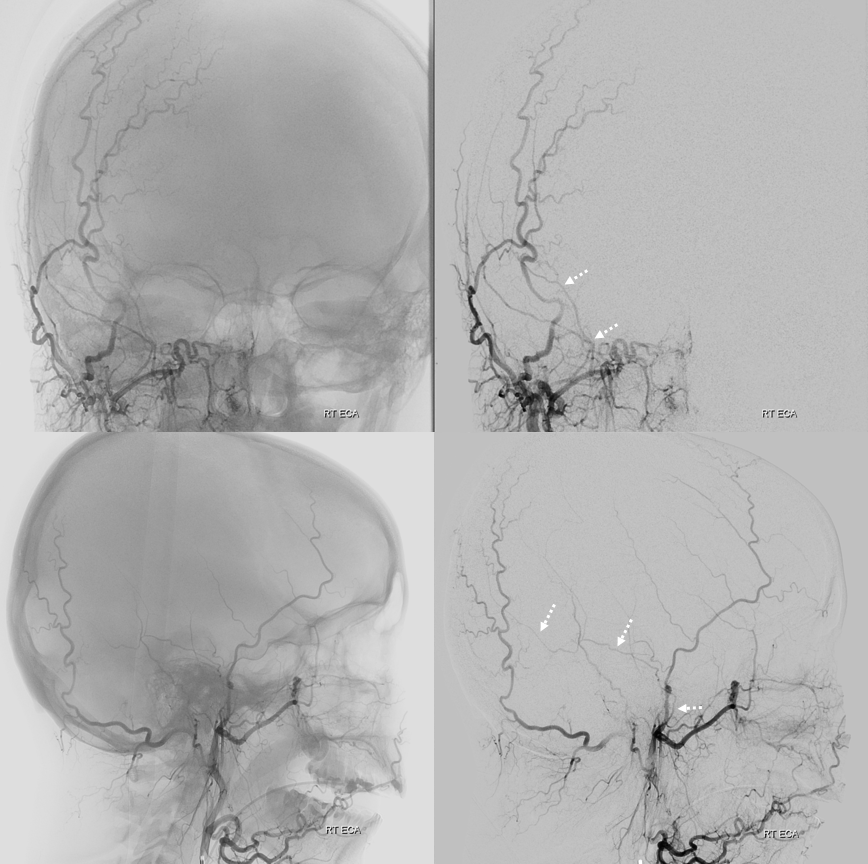
Below is one with arrows. On the normal left side, the IMAX (black arrow), STA (black arrowhead), and MMA (dashed black arrow) are all in usual places. On the right, the vidian artery (white arrow) supplies both proximal (white ball arrow) and distal (open white arrow) IMAX. The IMAX “gives rise” to both MMA (dashed white arrow) and STA (white arrowhead). Not bad, huh!
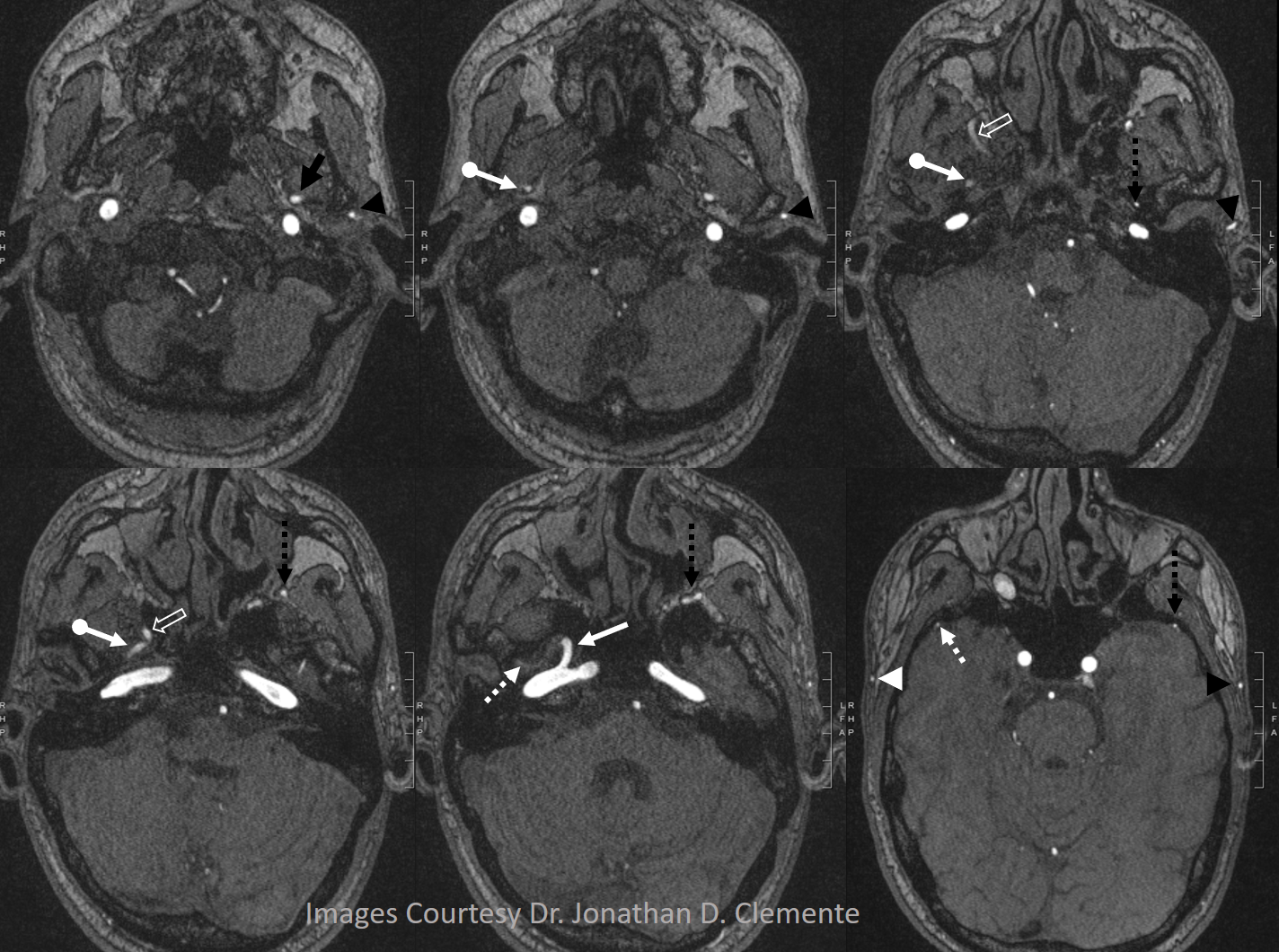
Volume rendered image of same
Usual Hyostapedial variant — a.k.a. persistent stapedial artery
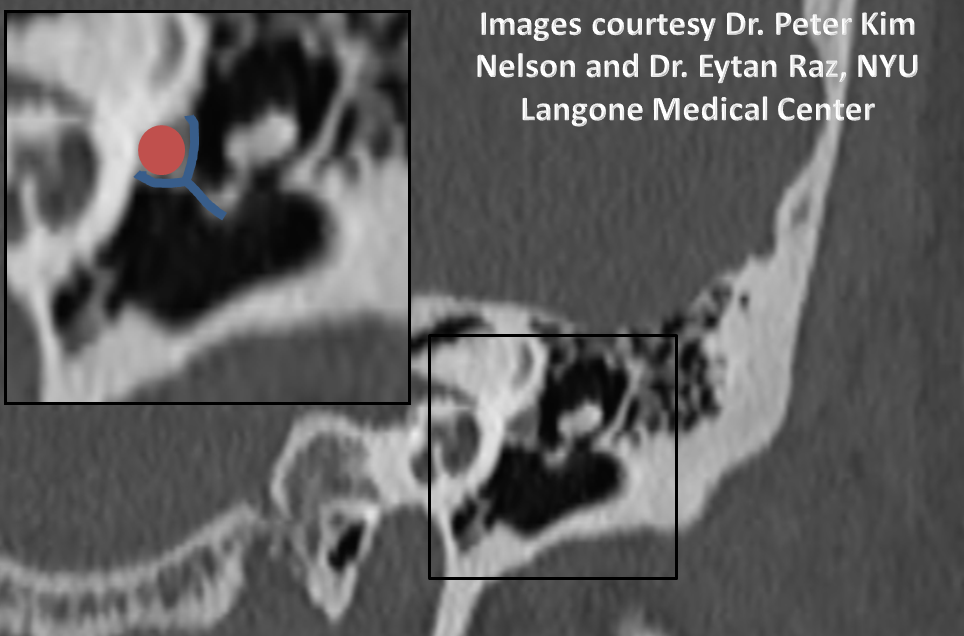
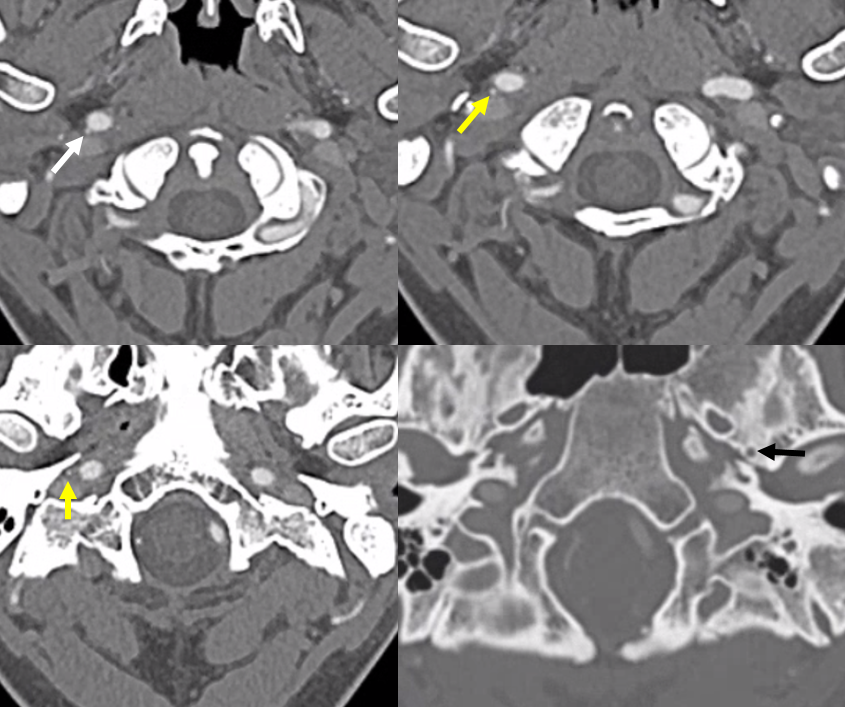
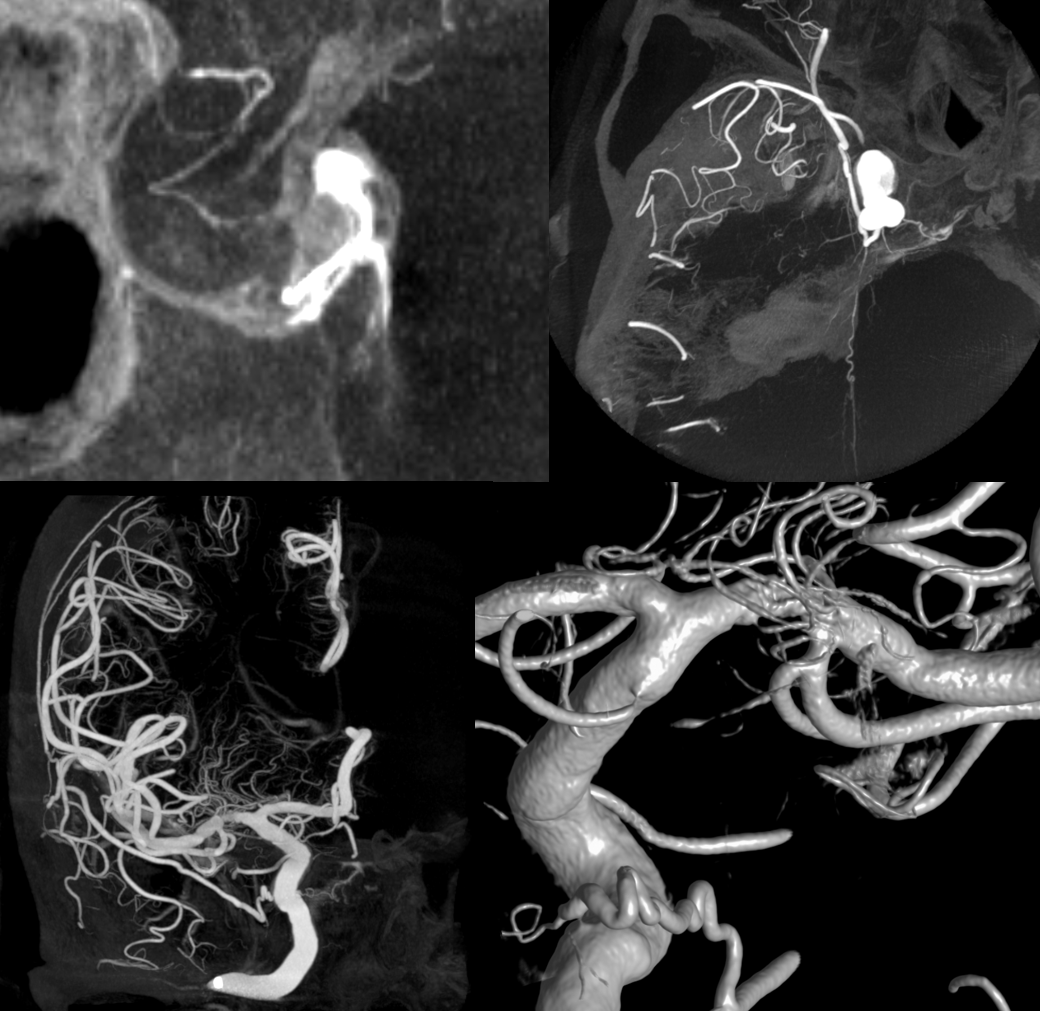
This hyostapedial stage explains several rare variations of MMA origin. One is the so-called “partial hyostapedial persistence“, when the hyostapedial branch remains the source of MMA supply. In this really cool variant, the enlarged hyostapedial artery (ie proximal MMA) goes thru the stapes crura, where it can be readily picked up on a temporal bone CT, and where it has been blamed for pulsatile tinnitus. The foramen spinosum is usually absent. The IMAX is almost always annexed by the ECA and does not stay with the hyostapedial (thus “partial” hyostapedial persistence)
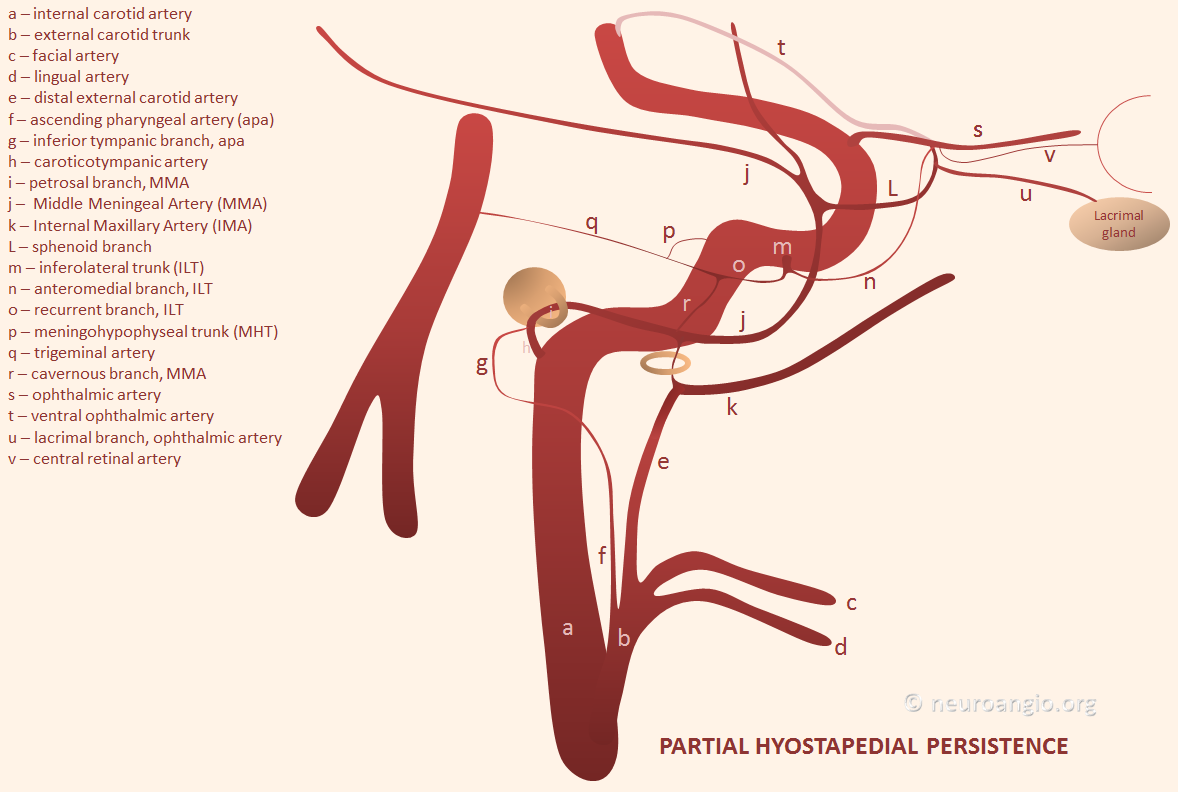
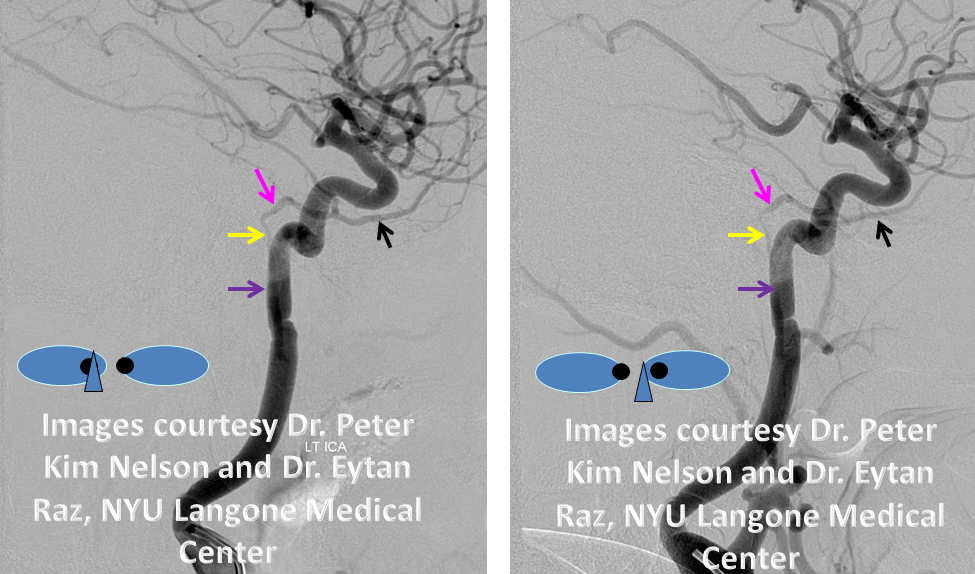
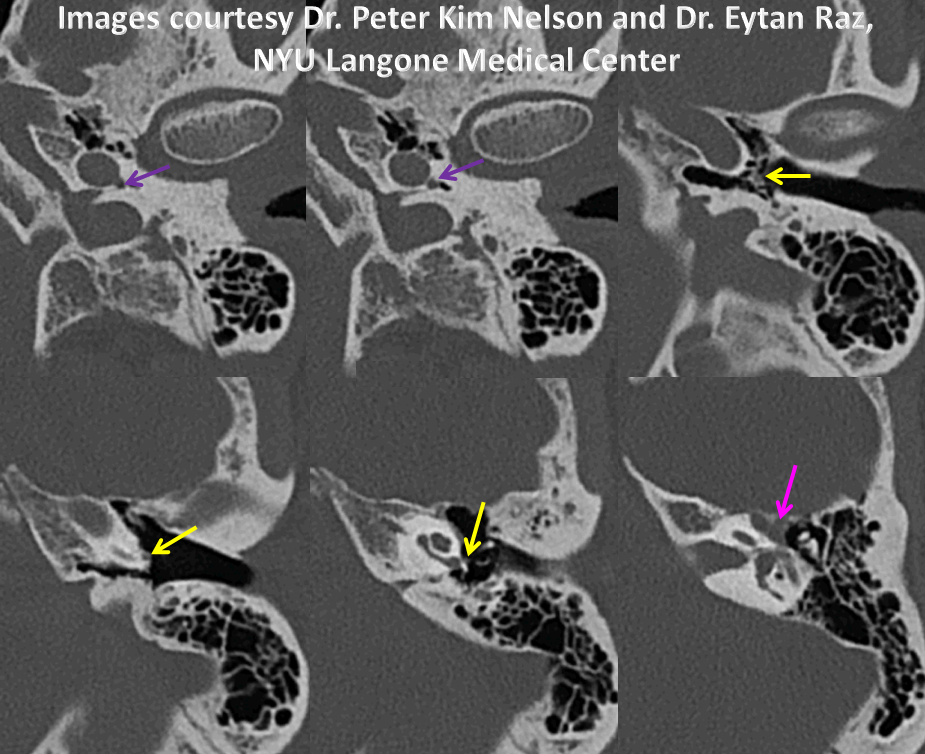
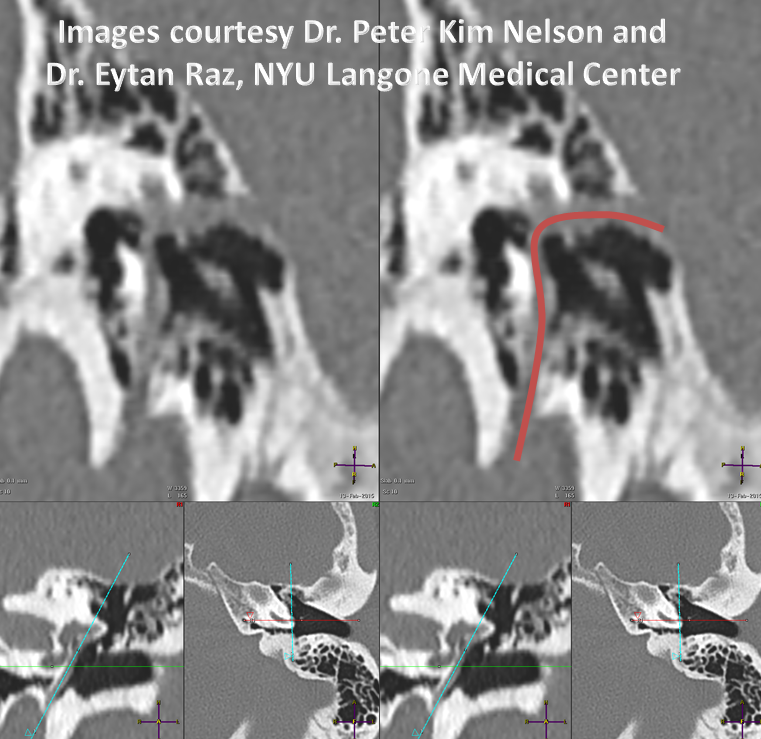
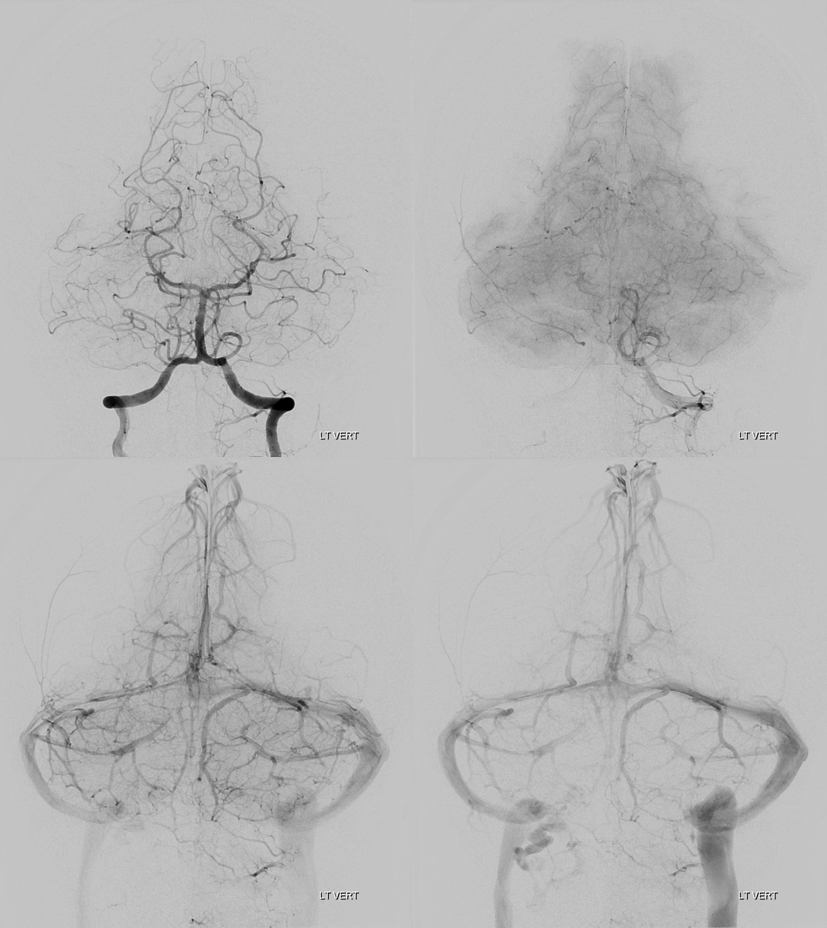
Below are some real-life examples. ICA = purple; hyoid artery = yellow; stapedial artery = pink; MMA = black; images with eyes are cross-eye stereo pairs
Another stereo example, courtesy Dr. Daniel Sahlein
Cervical origin hyostapedial variant
The hyostapedial artery is usually shown as originating in the petrous segment but that is not always the case either. Variants of variants…
Here the MMA originates from the cervical segment of the ICA (anaglyph stereos)
ECA injection shows no MMA
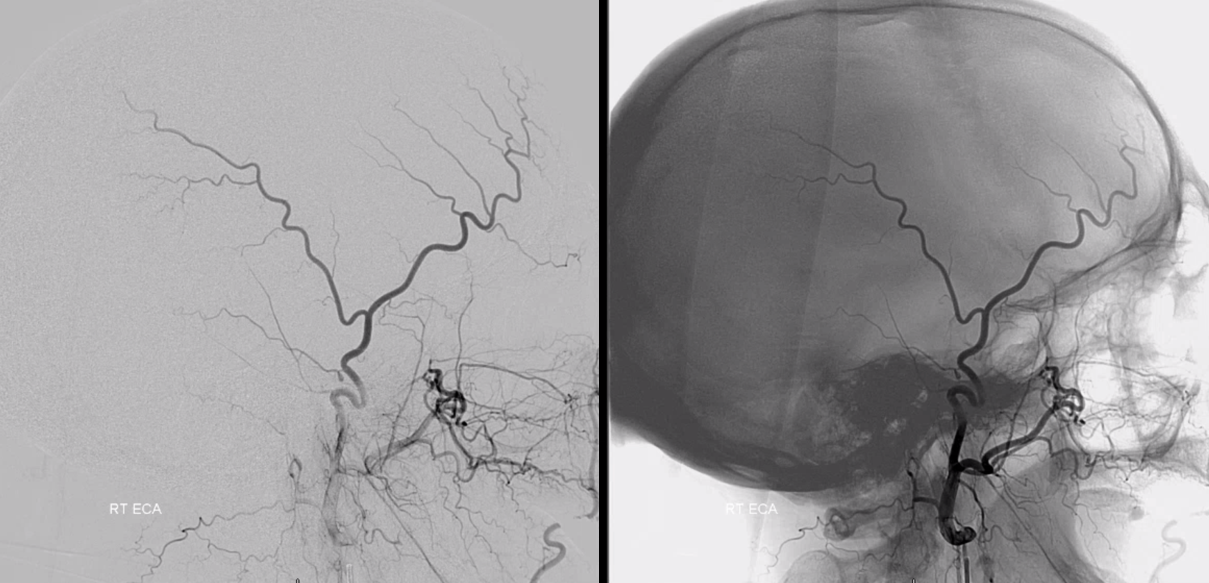
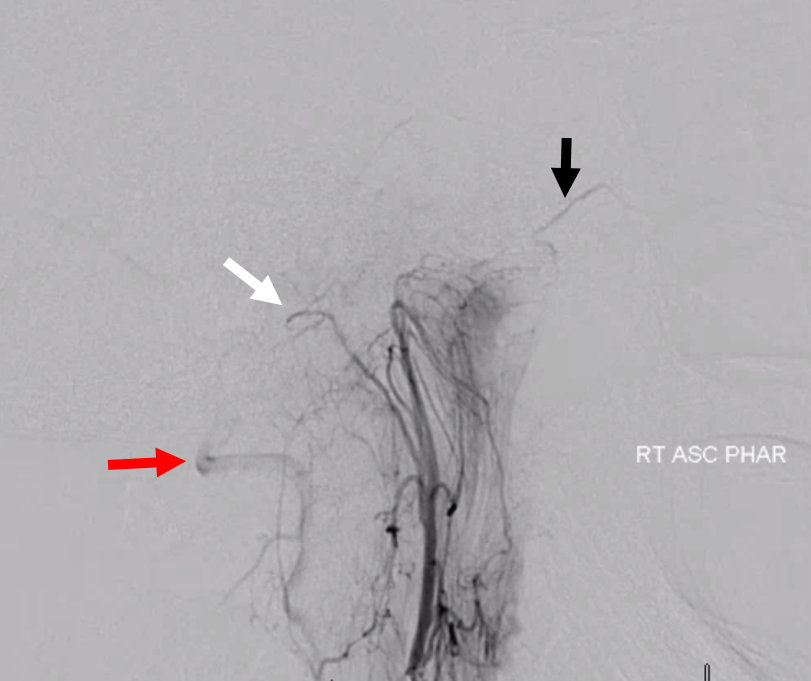
Ascending Phyaryngeal does not contribute to MMA. Notice anastomosis of the superior pharyngeal branch with the vidian system (black), intact neuromeningeal trunk (white) and muscular anastooses with the vert (red)
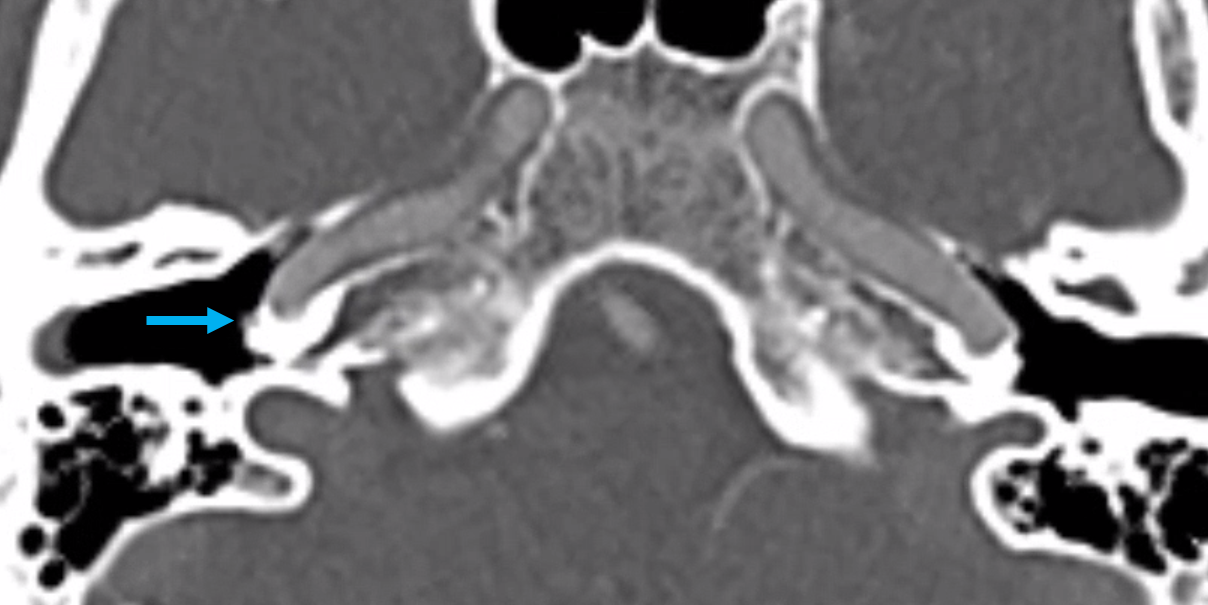
CT cervical origin (white) entering carotid canal where typically the hyoid artery origin would be (yellow). No spinosum on right, yes spinosum on left (black)
CT with stapedial artery over the promontory
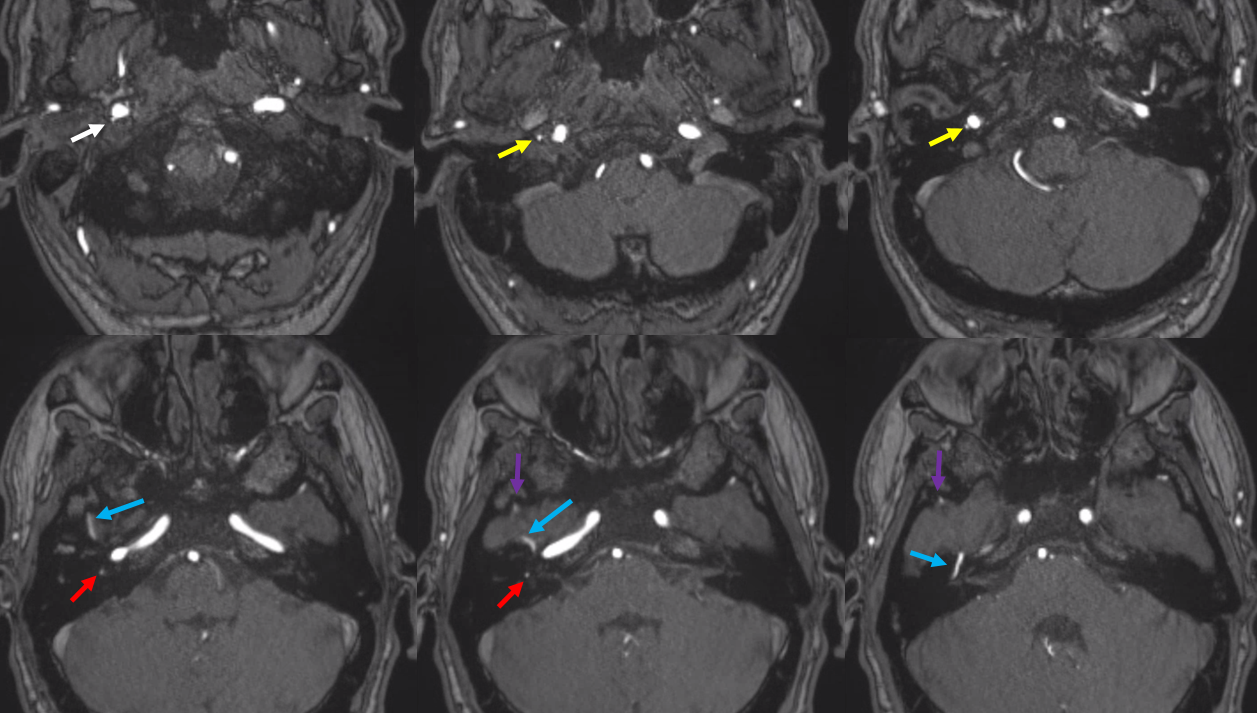
MRA — modern MRA can see it all. Cervical origin MMA (white), coming up into carotid canal (yellow), then posteriorly as the hyoid / caroticotympanic artery (red), then existing middle ear with petrosal nerve (blue) into middle cranial fossa (purple)
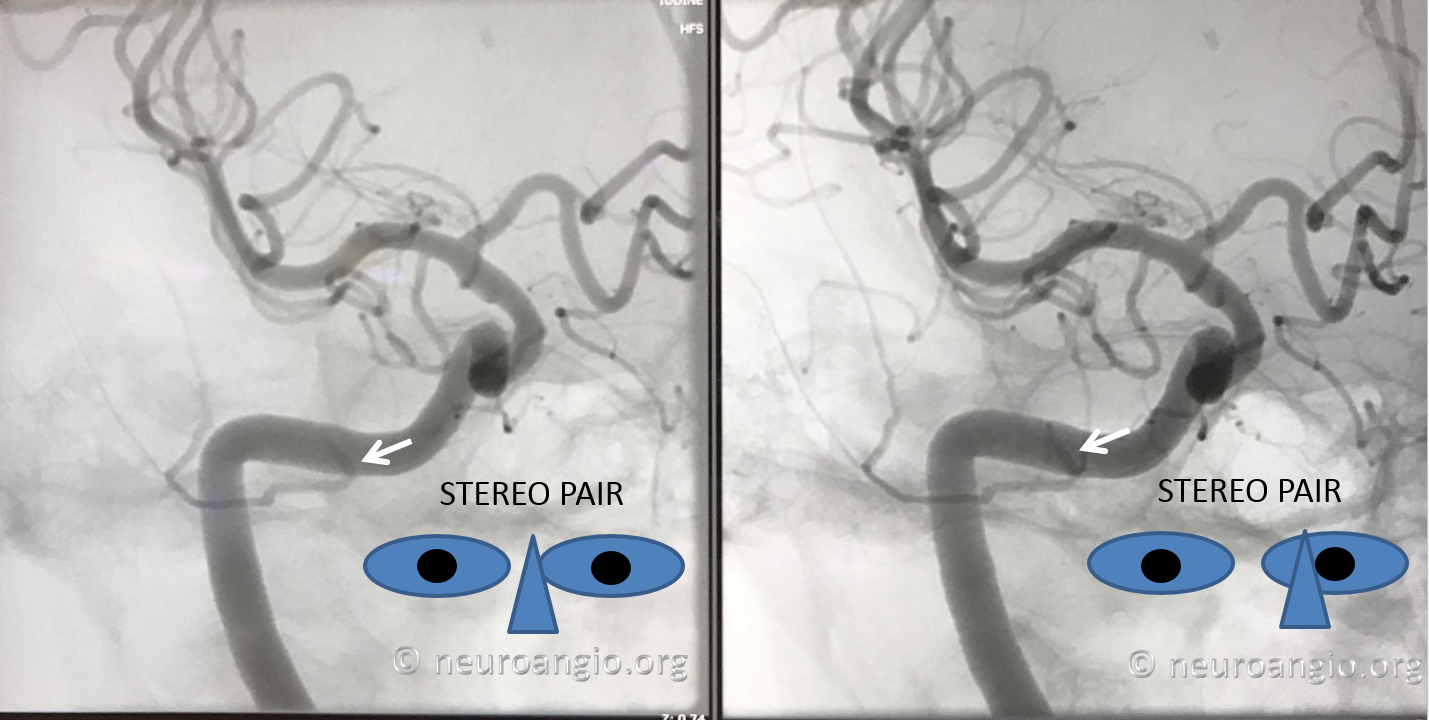
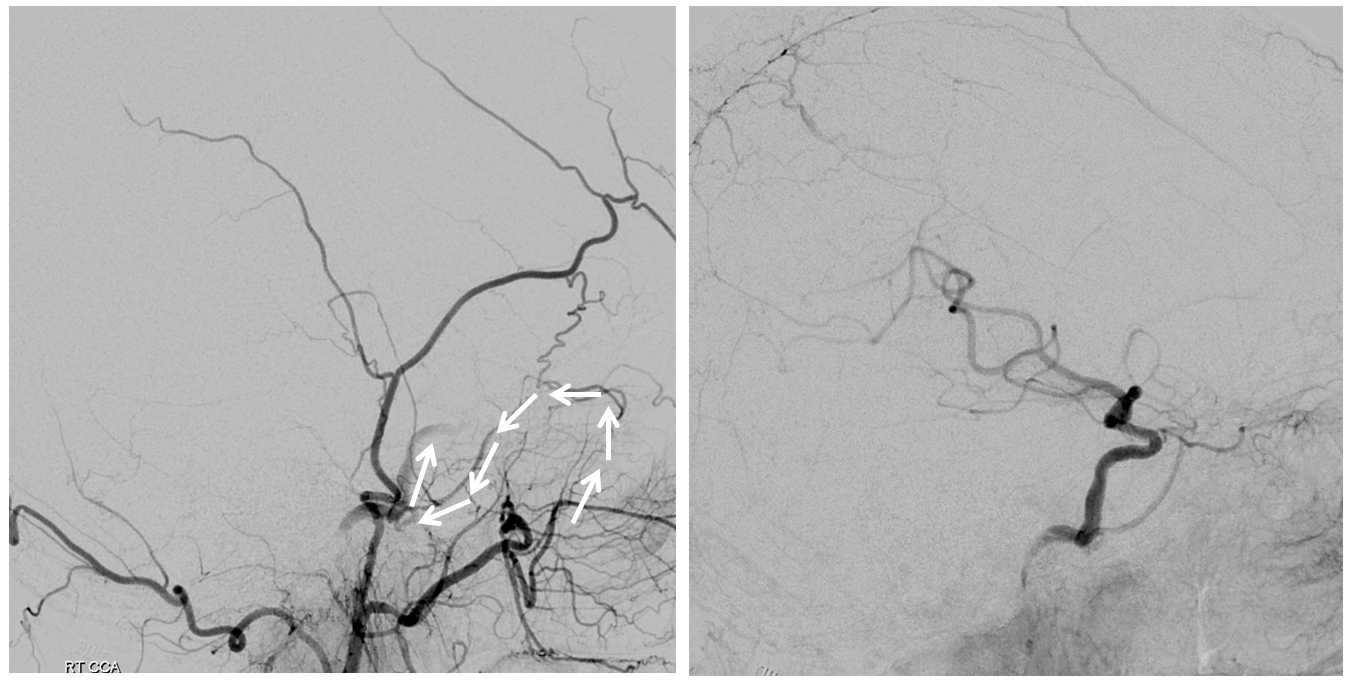
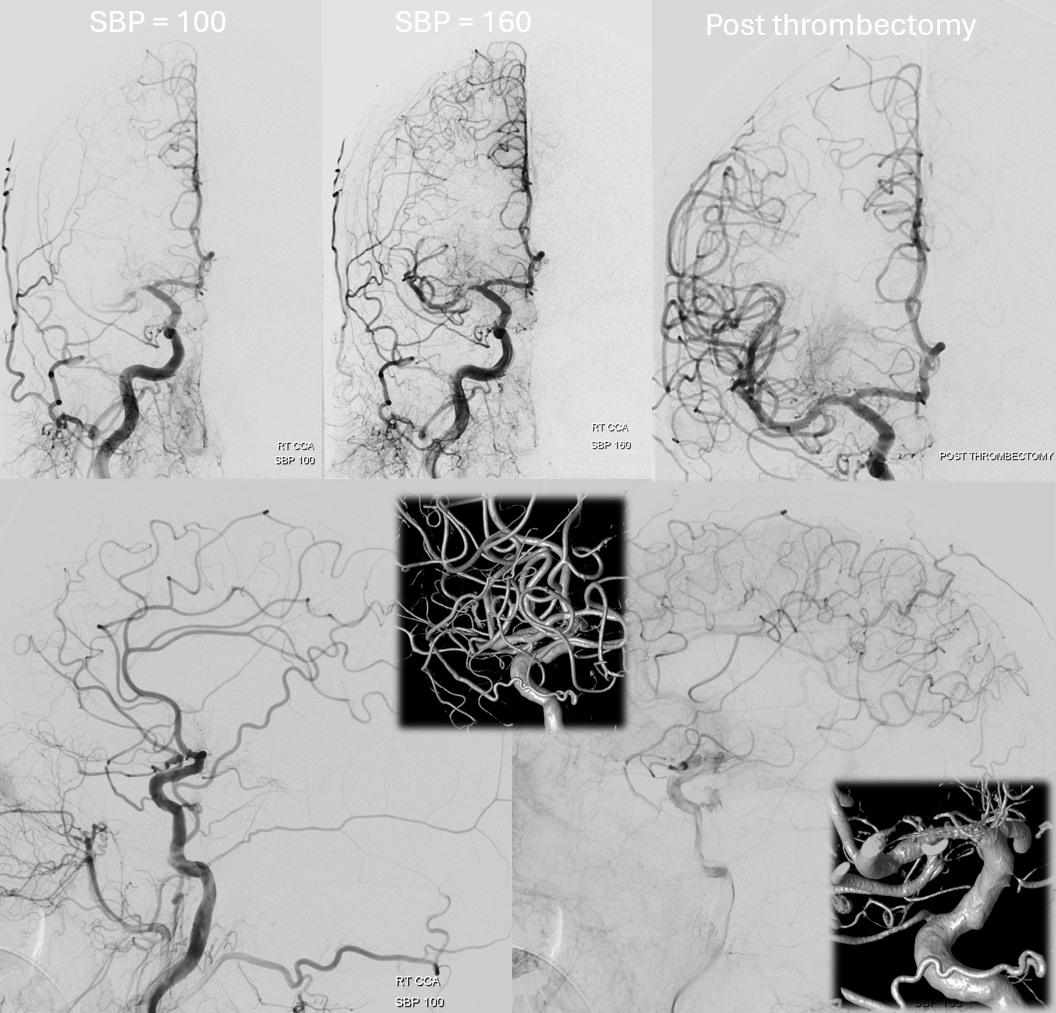
Clinical Significance — check out here this great case of hyostapedial origin MMA reconstituting the ICA in a patient with acute tandem ICA/MCA occlusion, courtesy Dr. Eytan Raz
Pharyngotympanostapedial variant
Fancy name for MMA origin from the Ascending Pharyngeal Artery, via its inferior tympanic branch. This is an important branch, more because it is part of what makes up the “aberrant carotid” artery (see below). As far as MMA is concerned, this is what happens. The anastomosis in this case does not go through the crura of stapes.
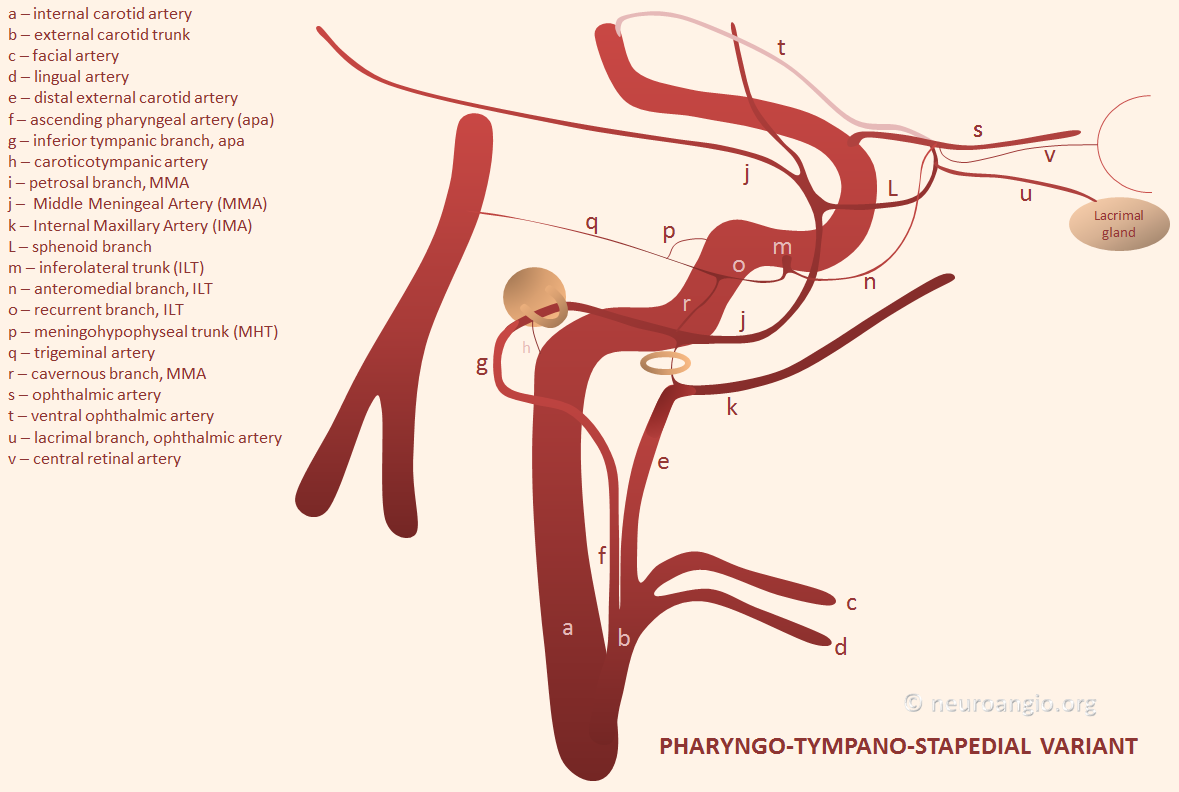
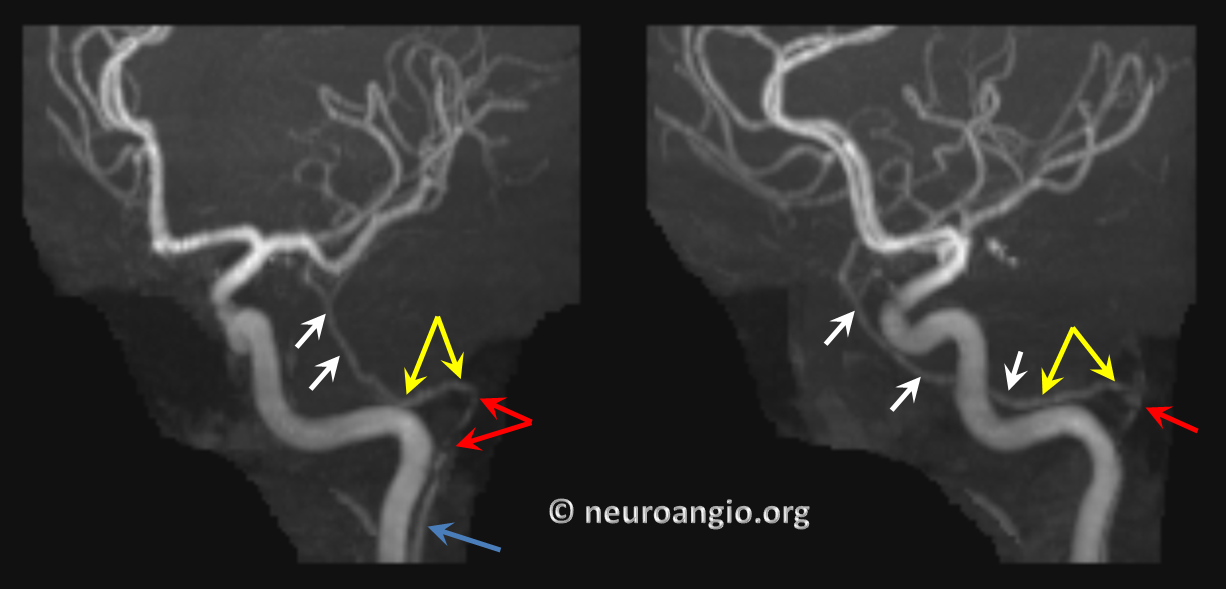
Here is an MRA example, courtesy Dr. Eytan Raz. It looks almost like the hyostapedial variant, except that the MMA can be traced to the Ascending Pharyngeal
Blue – inferior tympanic branch; red = stapedial; yellow – petrous/horizontal facial nerve canal branch; white = MMA
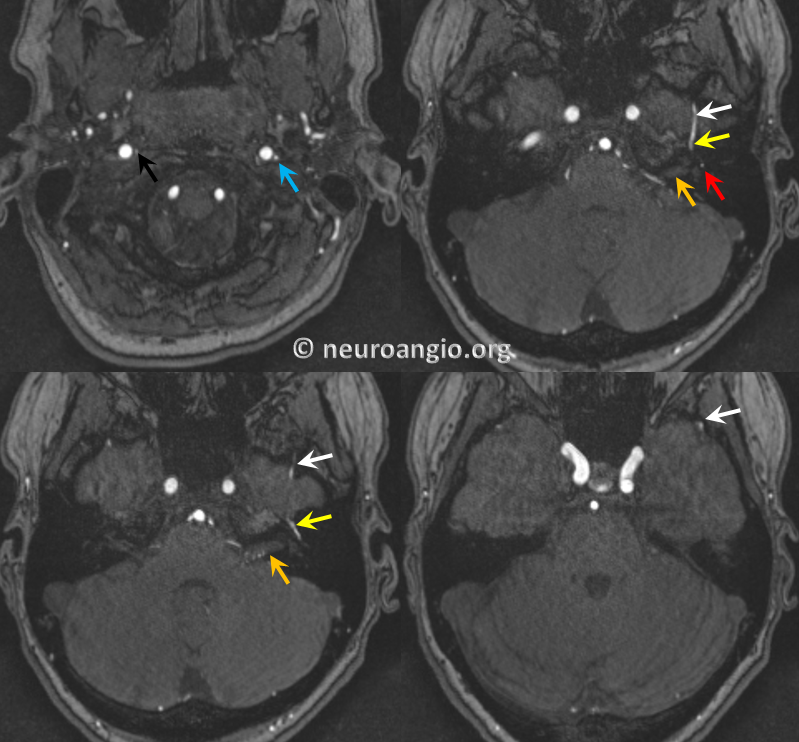
Axial views, with right AP in clandestine black; the IAC is orange
Vidian Branch origin of the Middle Meningeal Artery
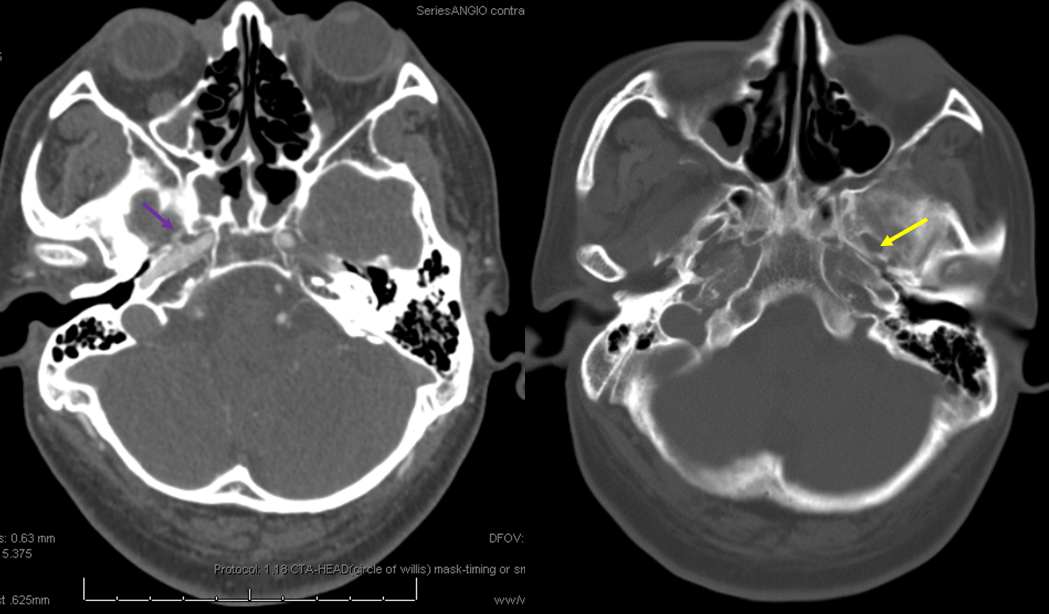
In the following case, the MMA does not originate from the stapedial branch, but more anteriorly, from the mid-section of the horizontal petrous segment, in the expected location of the vidian artery origin. By what mechanism the MMA becomes connected to the vidial segment I do not understand.The following image shows absent of foramen spinosum on on the right (yellow arrow on left), and origin of the right MMA from the vidian segment (purple arrow)
Angiogram of the same patient shows the MMA (red arrows) with its Vidian origin (purple) in frontal (top left) and stereoscopic lateral projections (bottom)
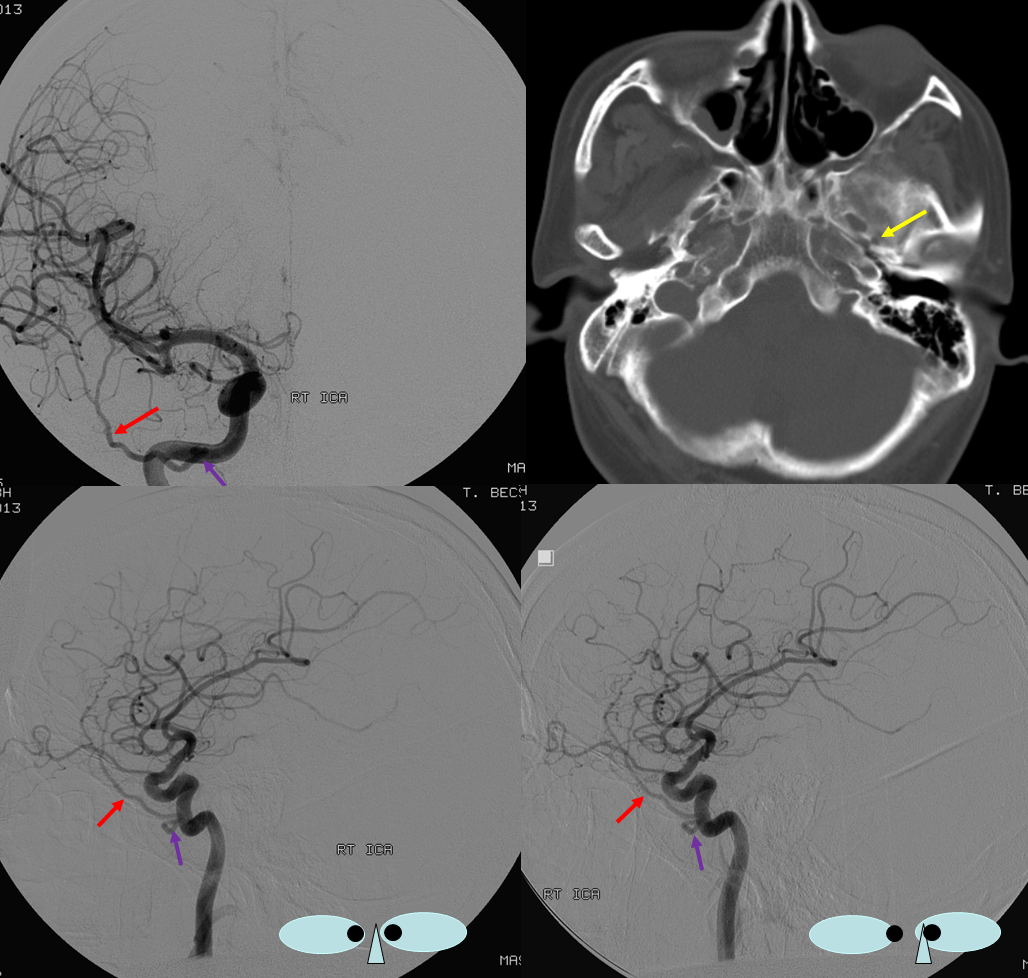
Trigeminal Artery origin of MMA
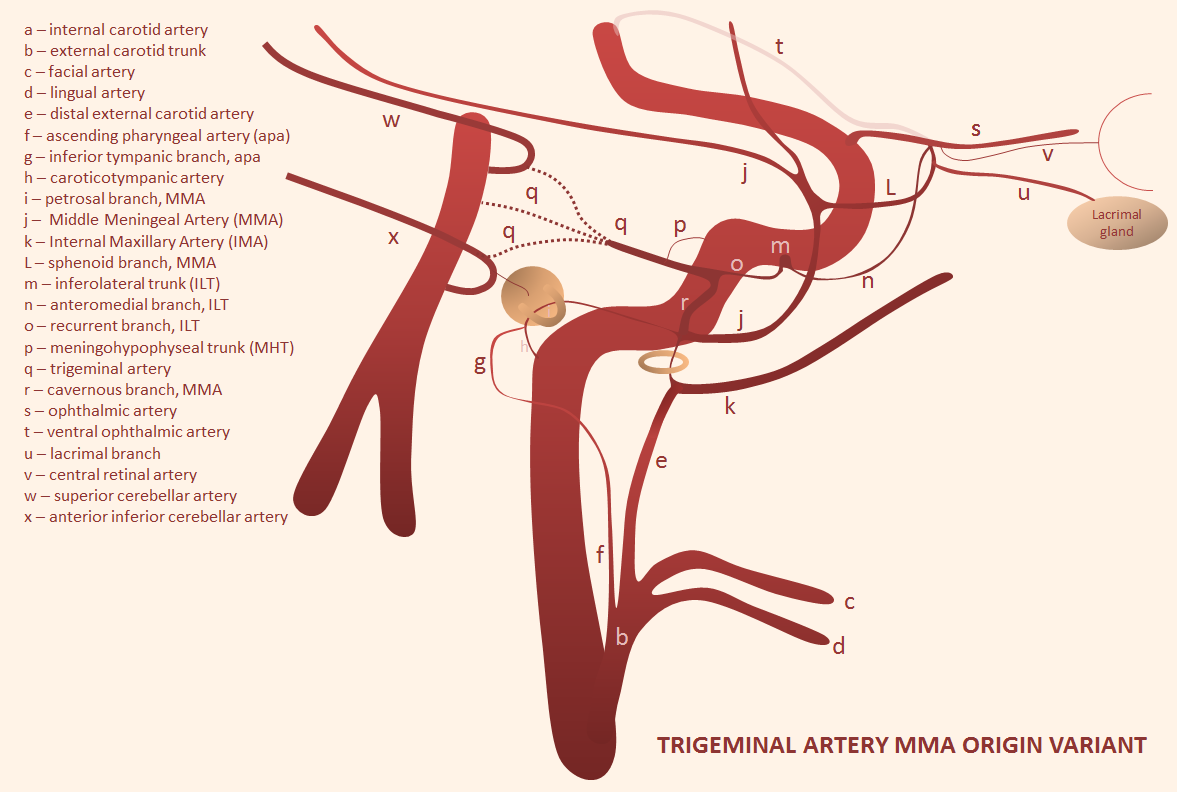
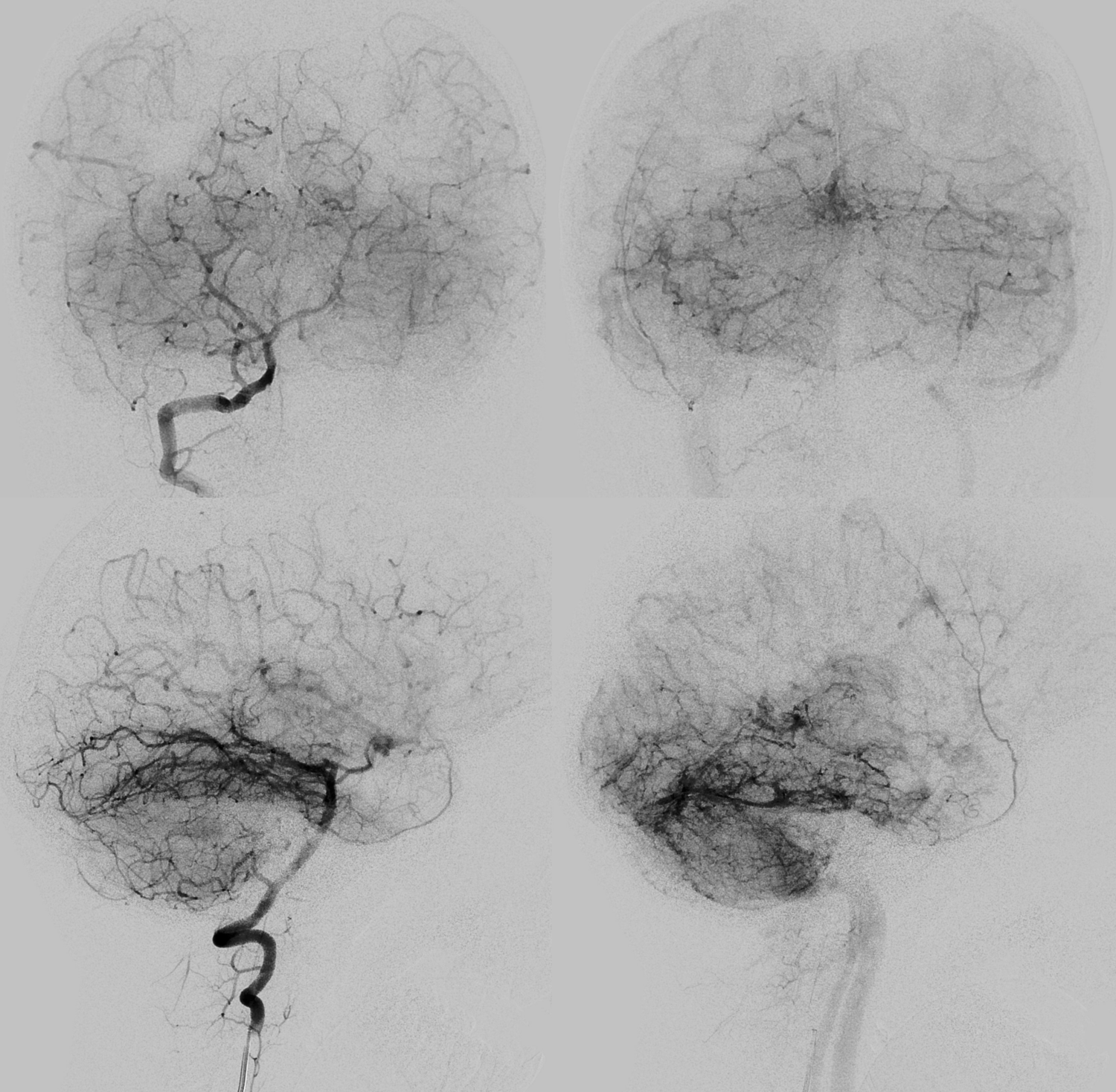
On some level its just probability — any arrangement is possible, including some extremely rare ones. This one, courtesy Dr. Erez Nossek, shows how MMA can arise from Trigeminal Artery (q) via its connection with the cavernous branch (r) of the MMA. Because the Trigeminal Artery (see dedicated page here) can arise from either basilar, SCA, or AICA, the MMA can do the same. In fact, this here is the only known case of SCA-origin MMA we are aware of. We are going to keep it here and spare everyone the pleasure of reading it in some journal of obscure case reports.
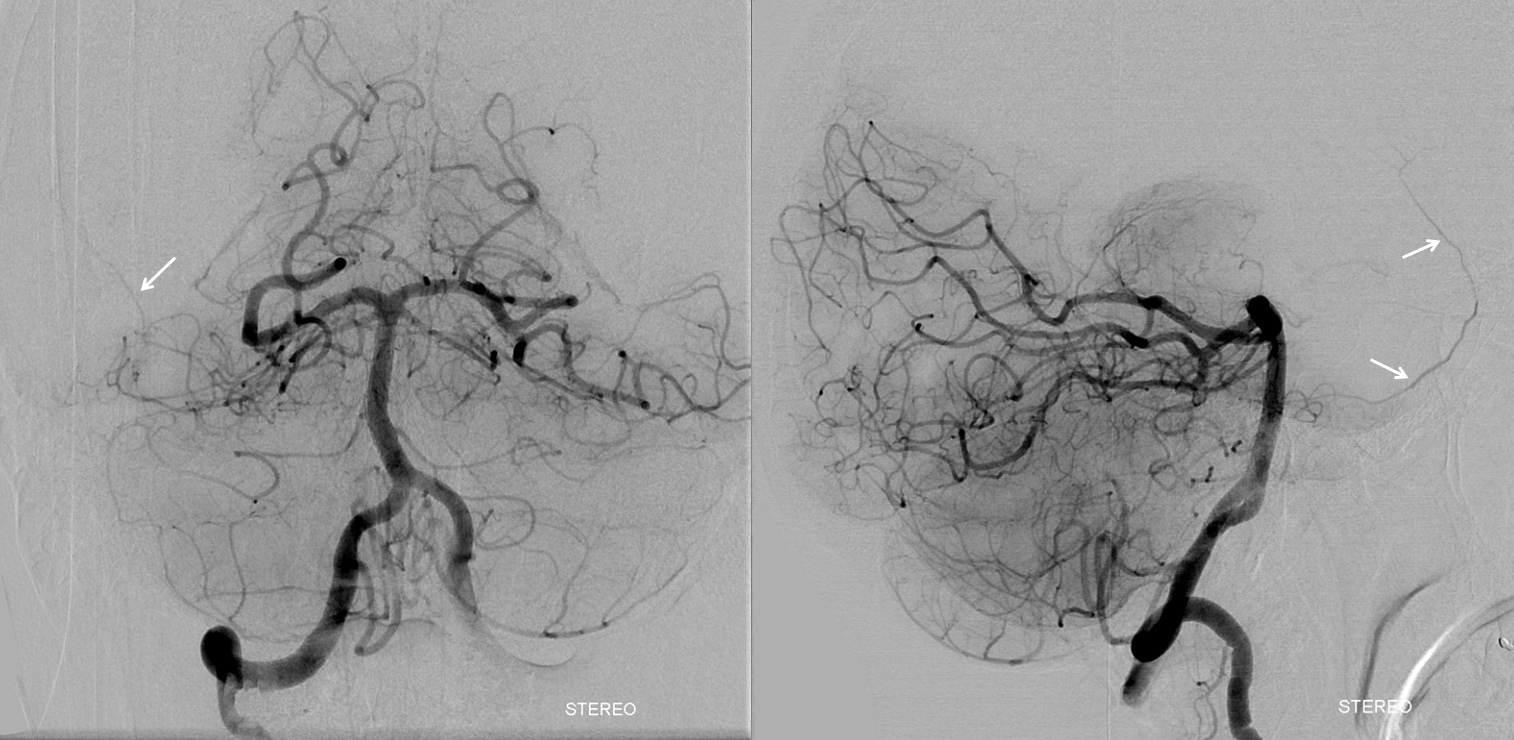
Cross-eye stereos
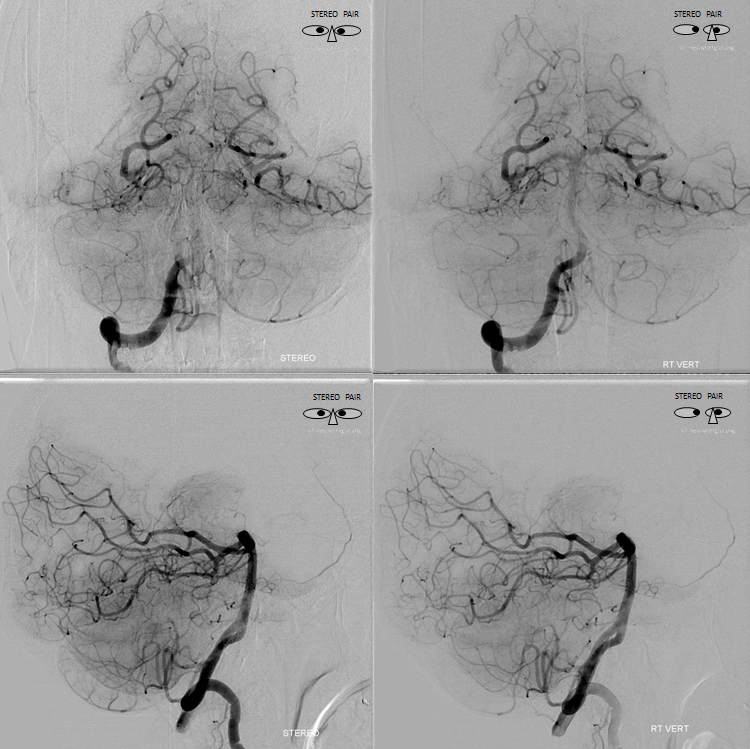
Anaglyph stereo frontal views
Lateral anaglyph
Another example — patient under anesthesia now so images are even more spectacular
ECA with missing frontal division of MMA — petrosquamosal branch is shown by dashed arrows
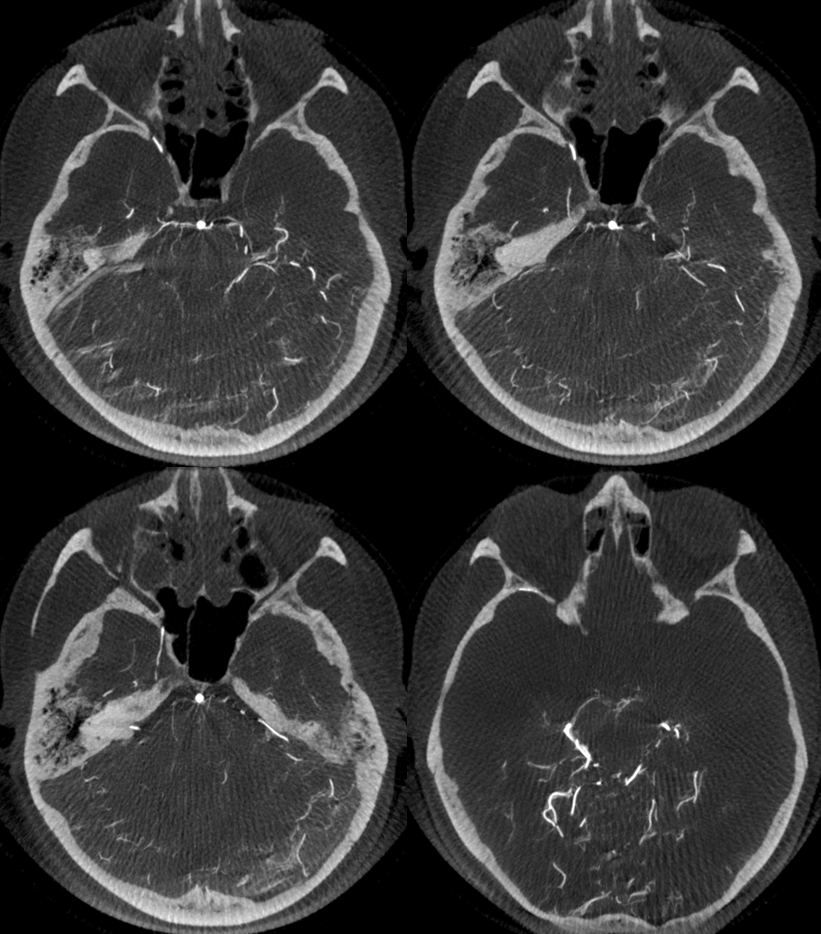
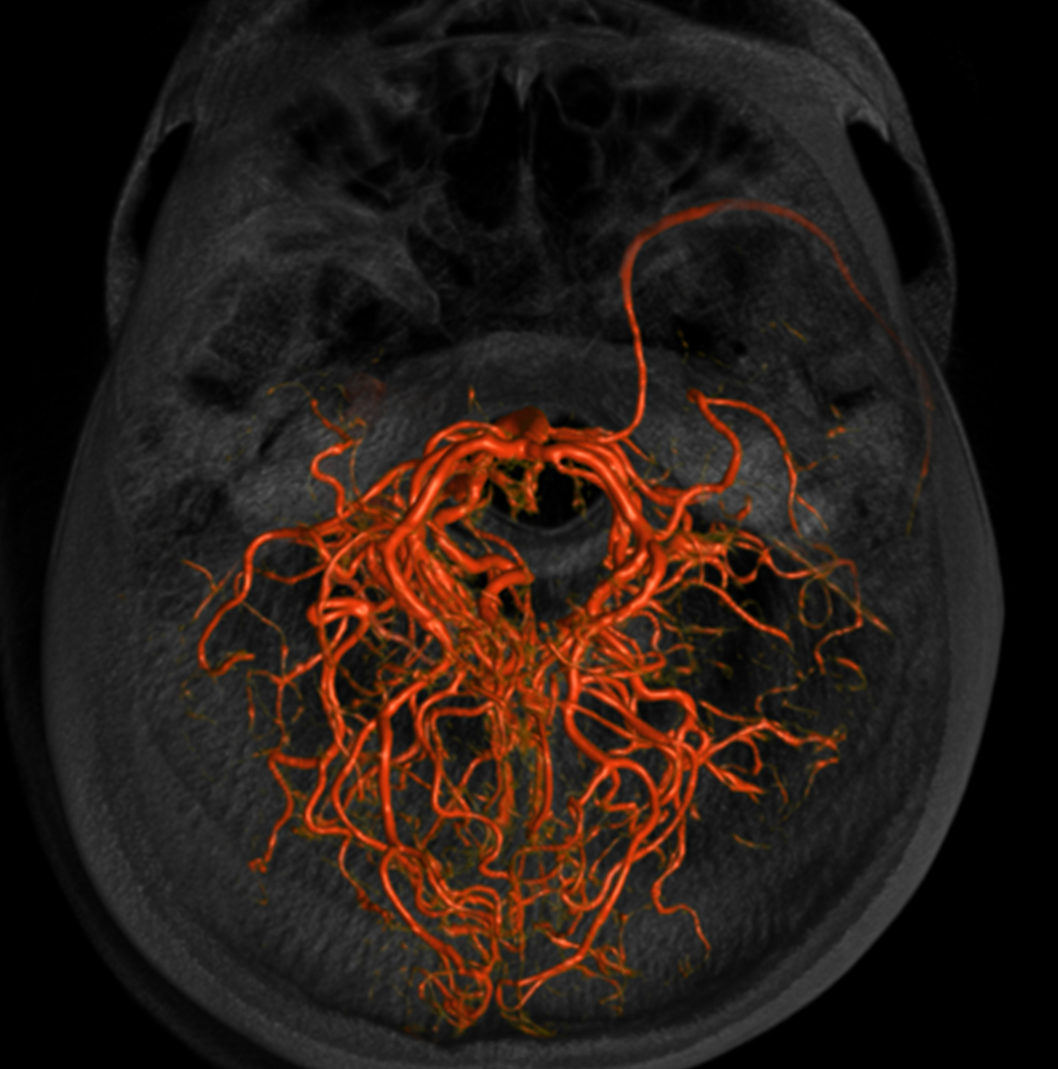
Vert
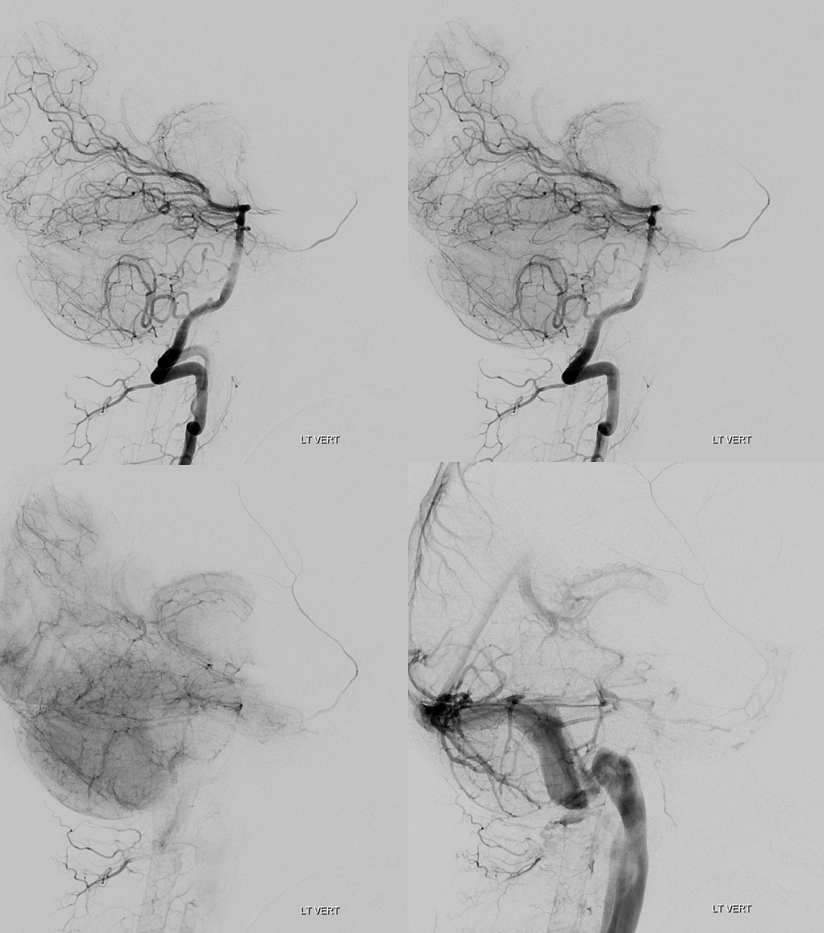
Rotational angio and 3D MIPs
Stereo Volume Rendered images
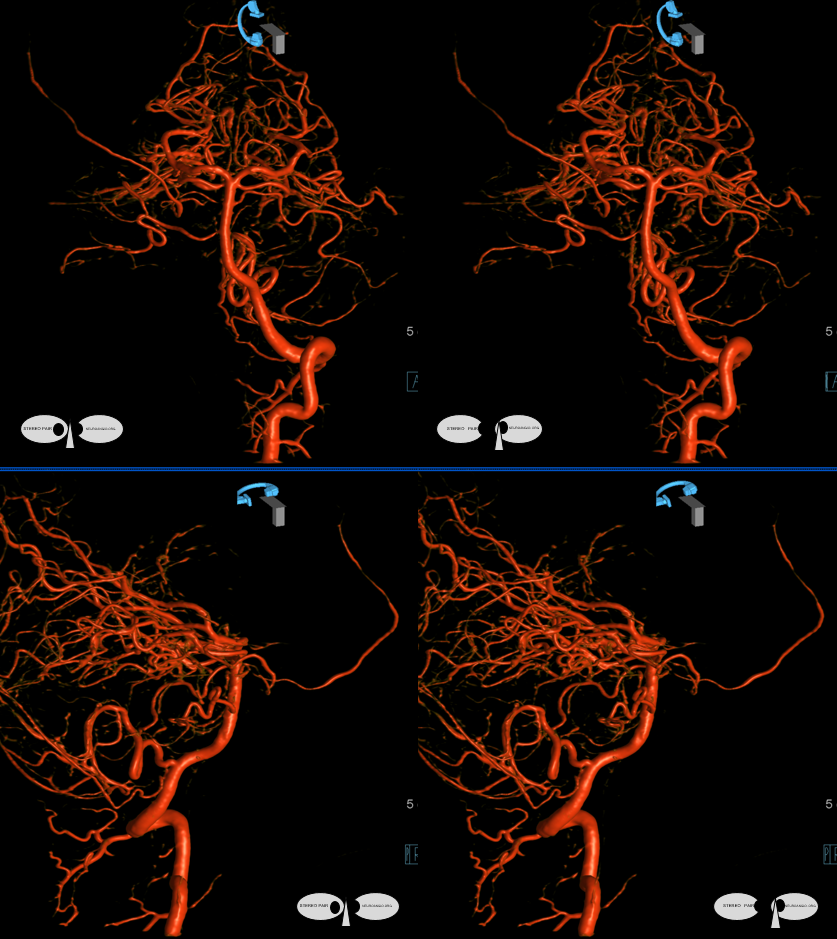
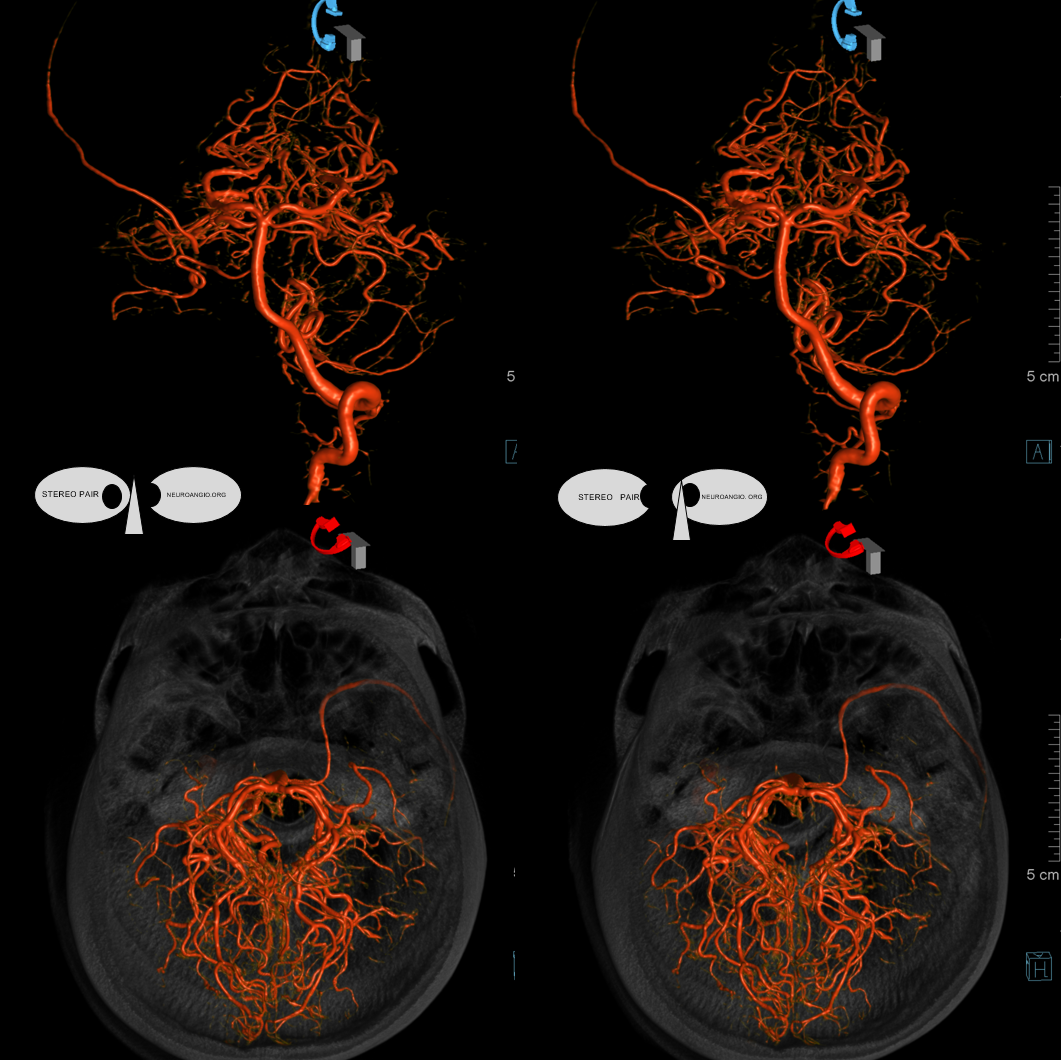
Anaglyph stereos
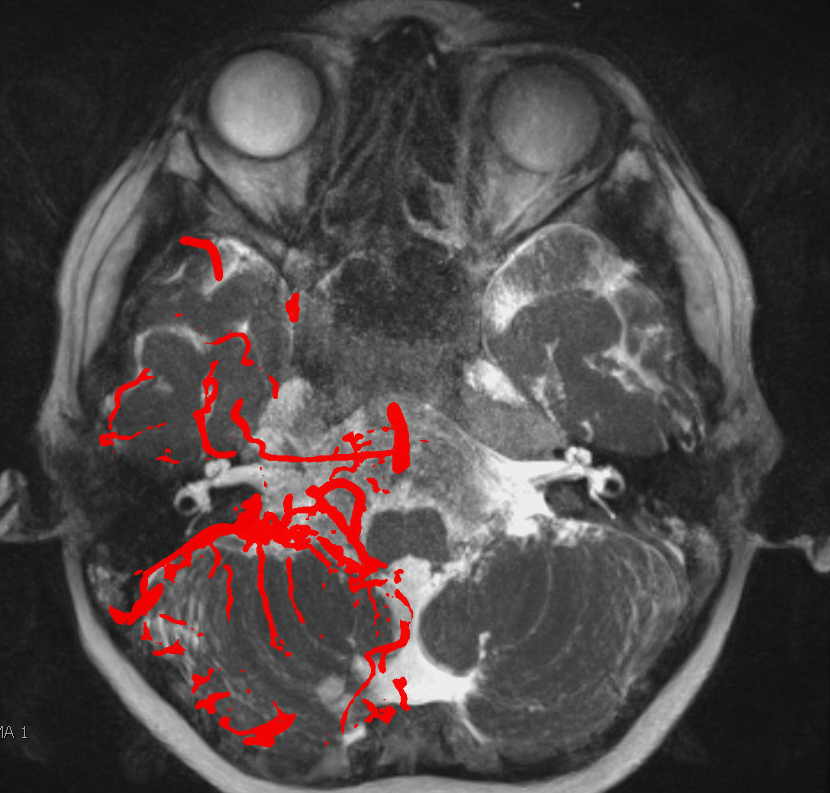
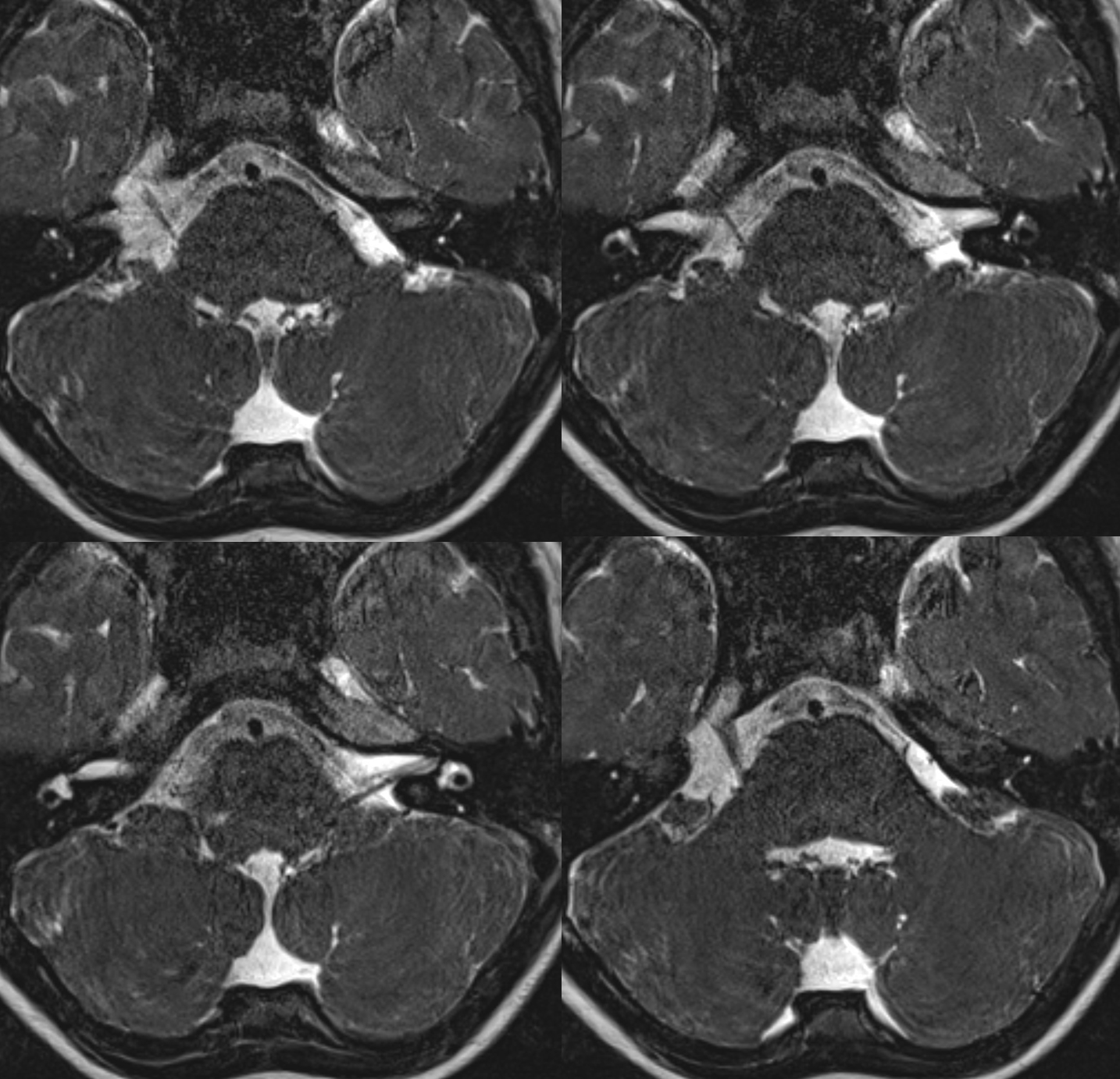
And, in case you really dont believe its the trigeminal artery, check out these co-registration images of angio (axial MIP reconstructions of rotational angio) and finally with superimposed tractography of the Vth nerve! Images courtesy Dr. Nader Delavari
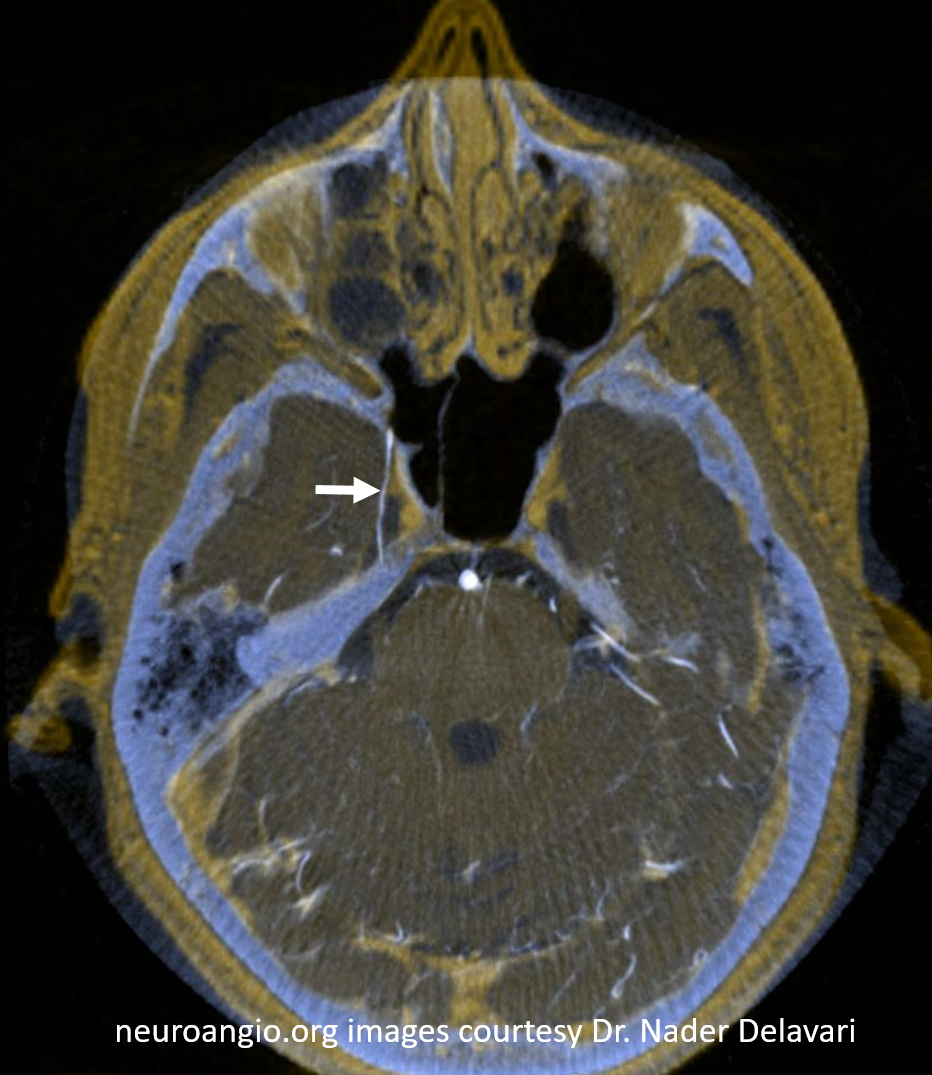
Superimposed MRI and angio axials (our trigeminal artery is shown bi arrow)
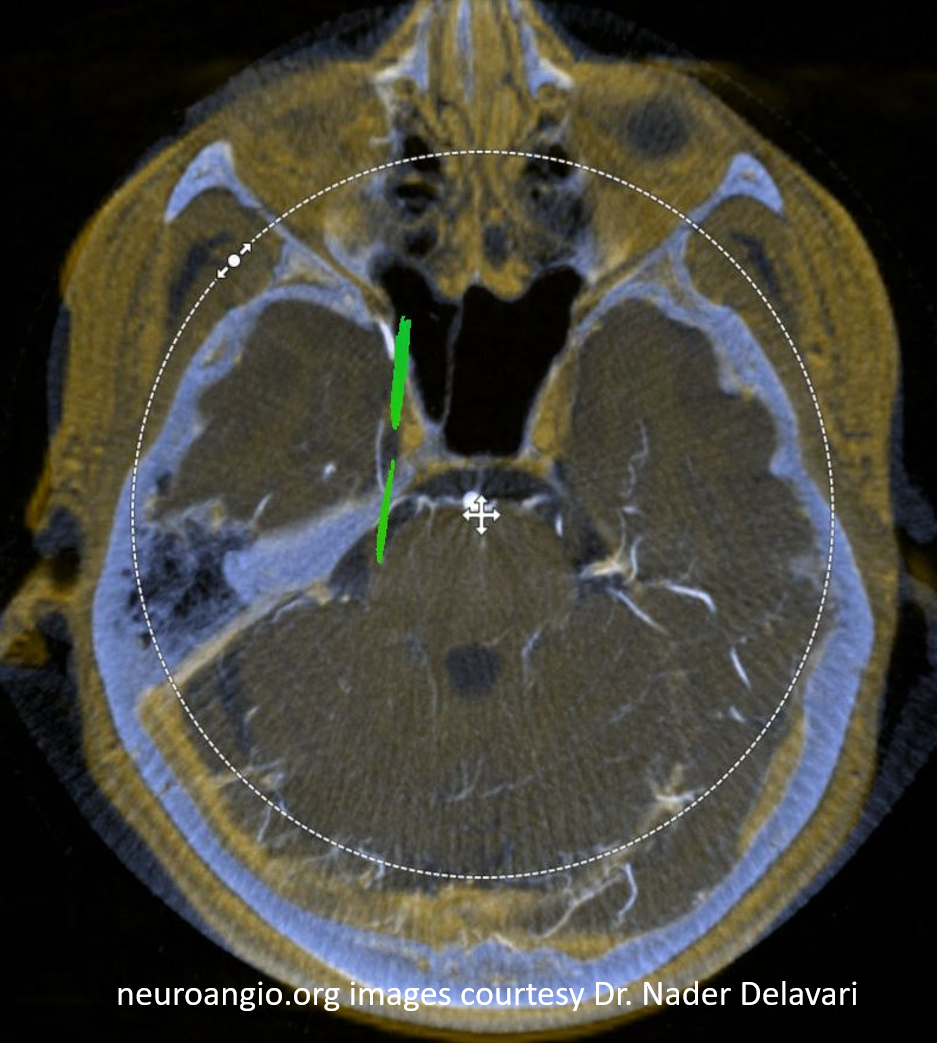
Now with tractography of CN V
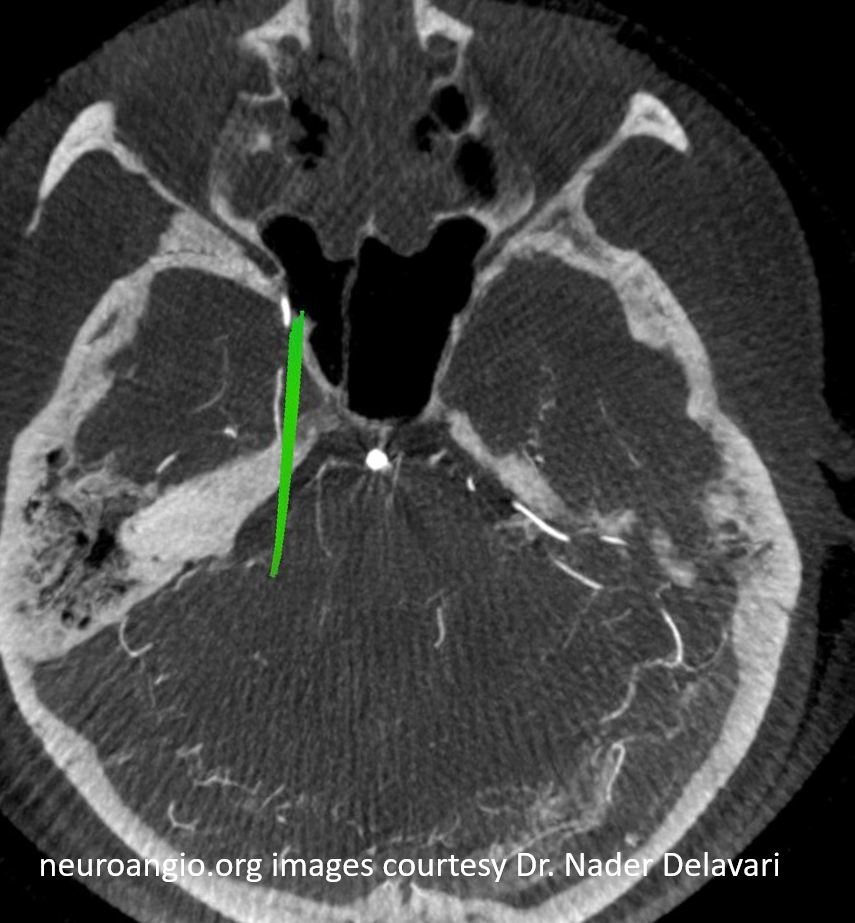
Check out Trigeminal Artery page for more on that vessel
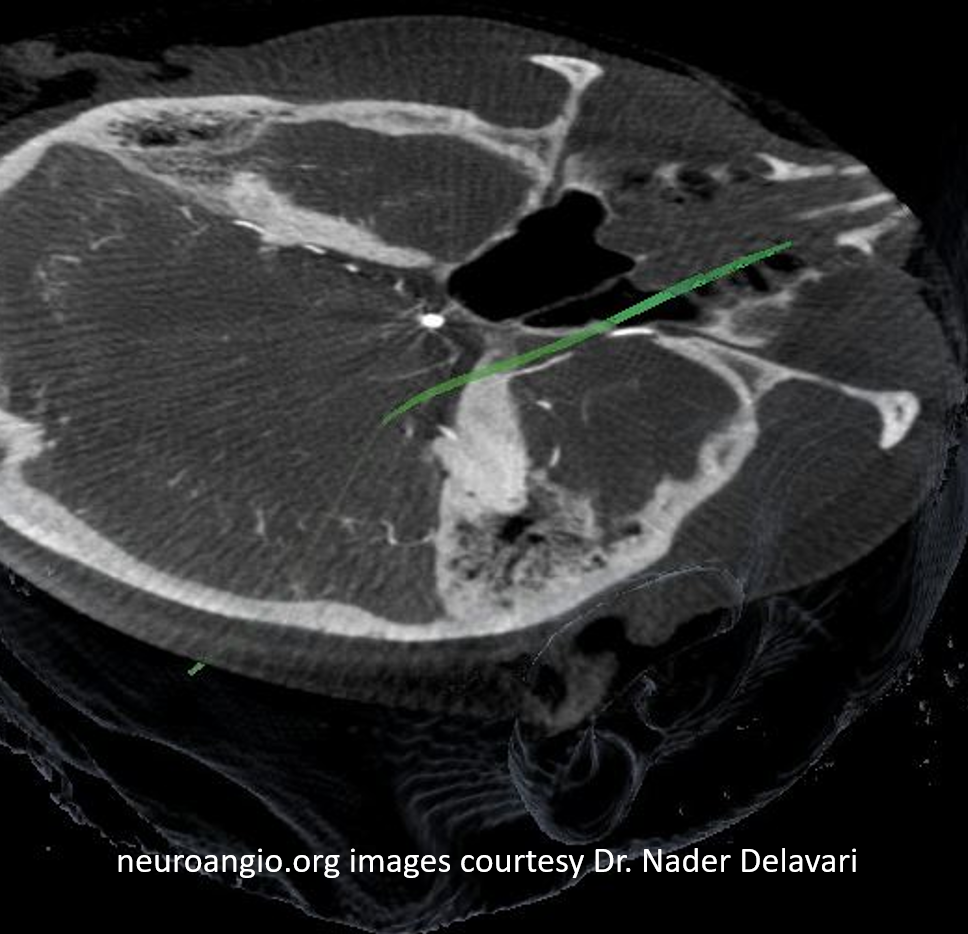
Basilar Origin IAC Course without CN VII or VIII
A unique case of MMA frontal division origin from basilar, with course towards IAC, and congenital absence of CN VII and VIII. Something possibly related to AICA…
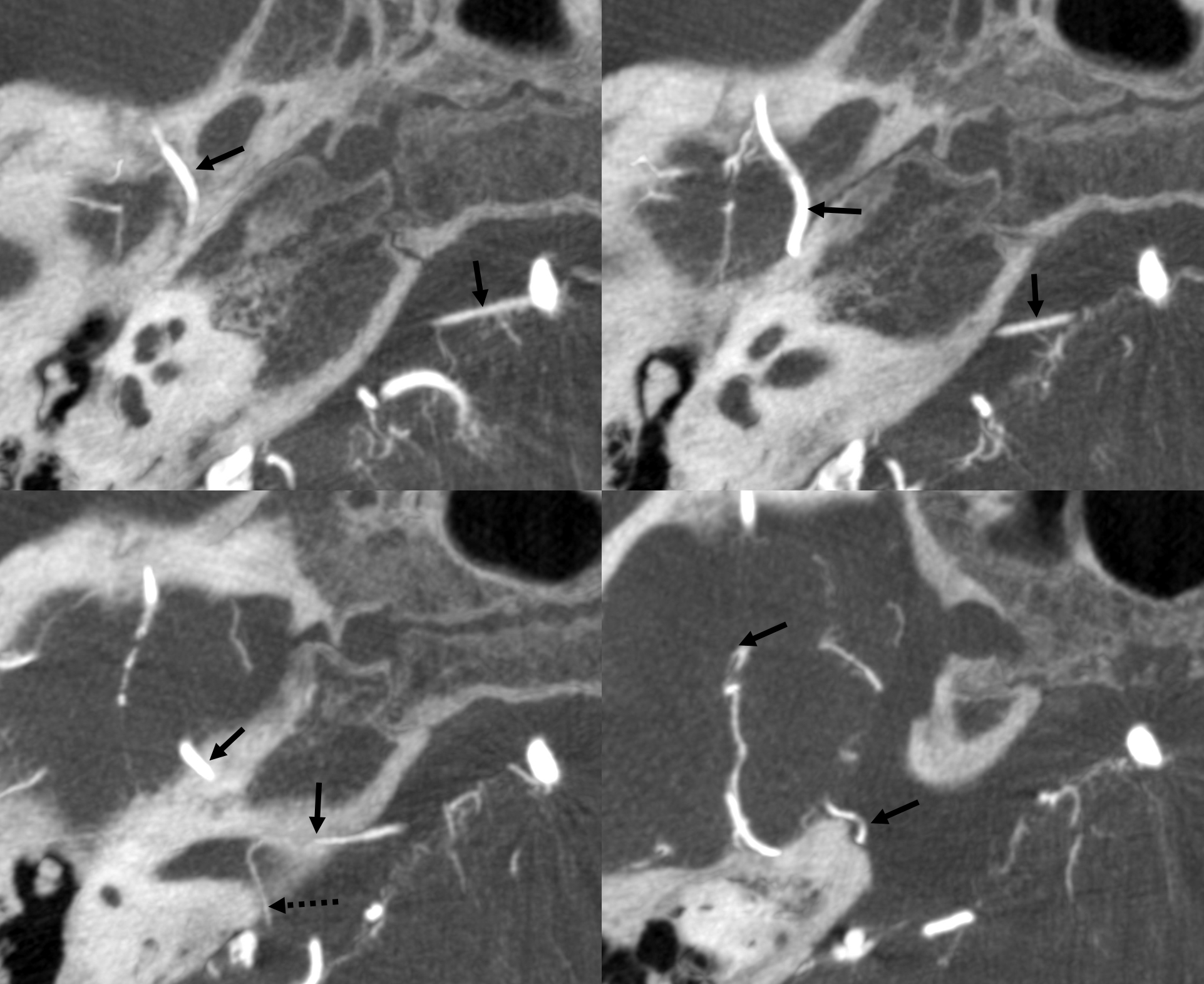
MHT origin of the MMA — via the Sphenoid Ridge Branch
Stroke — the gift that keeps on giving. Note posterior/parietal MMA division usual origin and course. See MMA page for discussion of the spectrum.
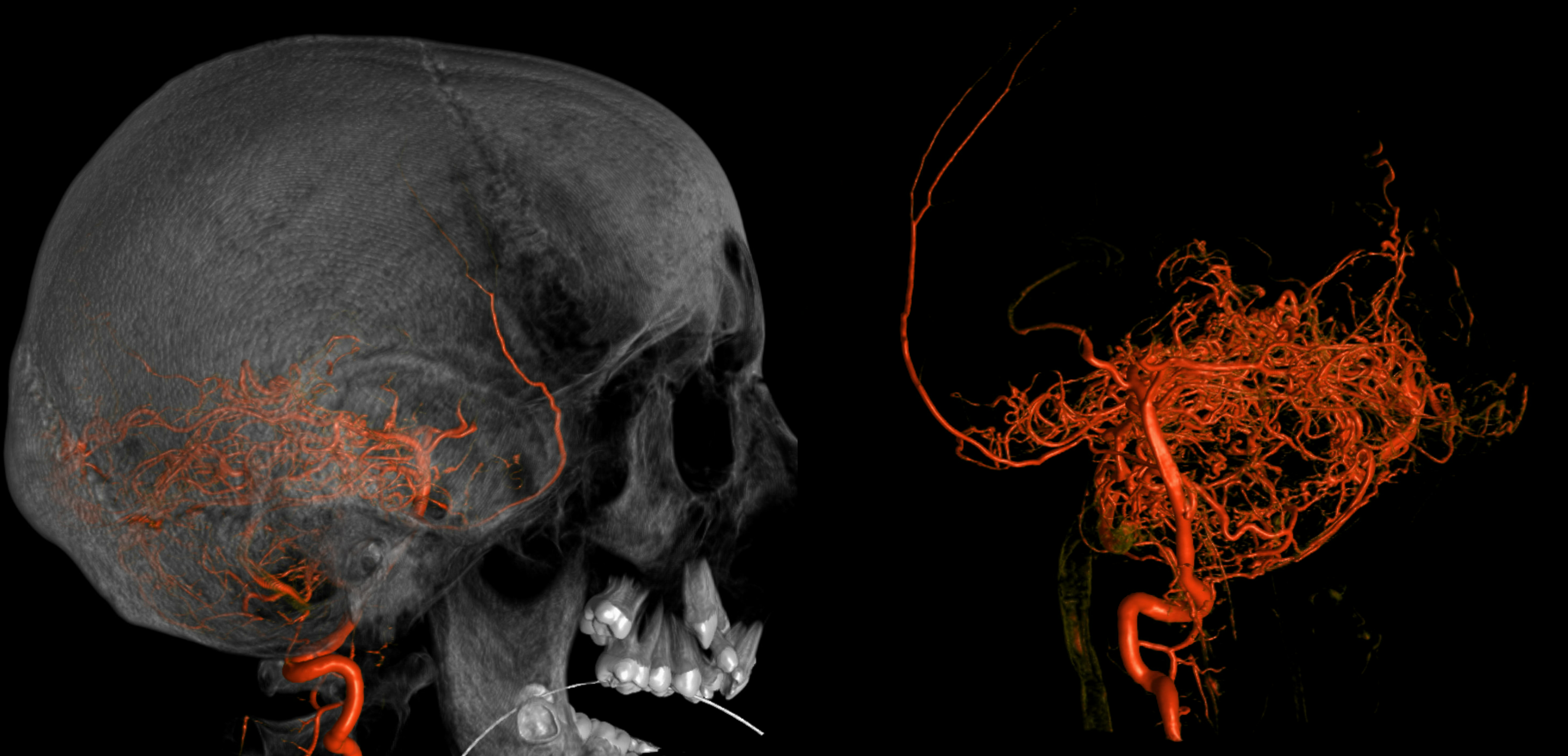
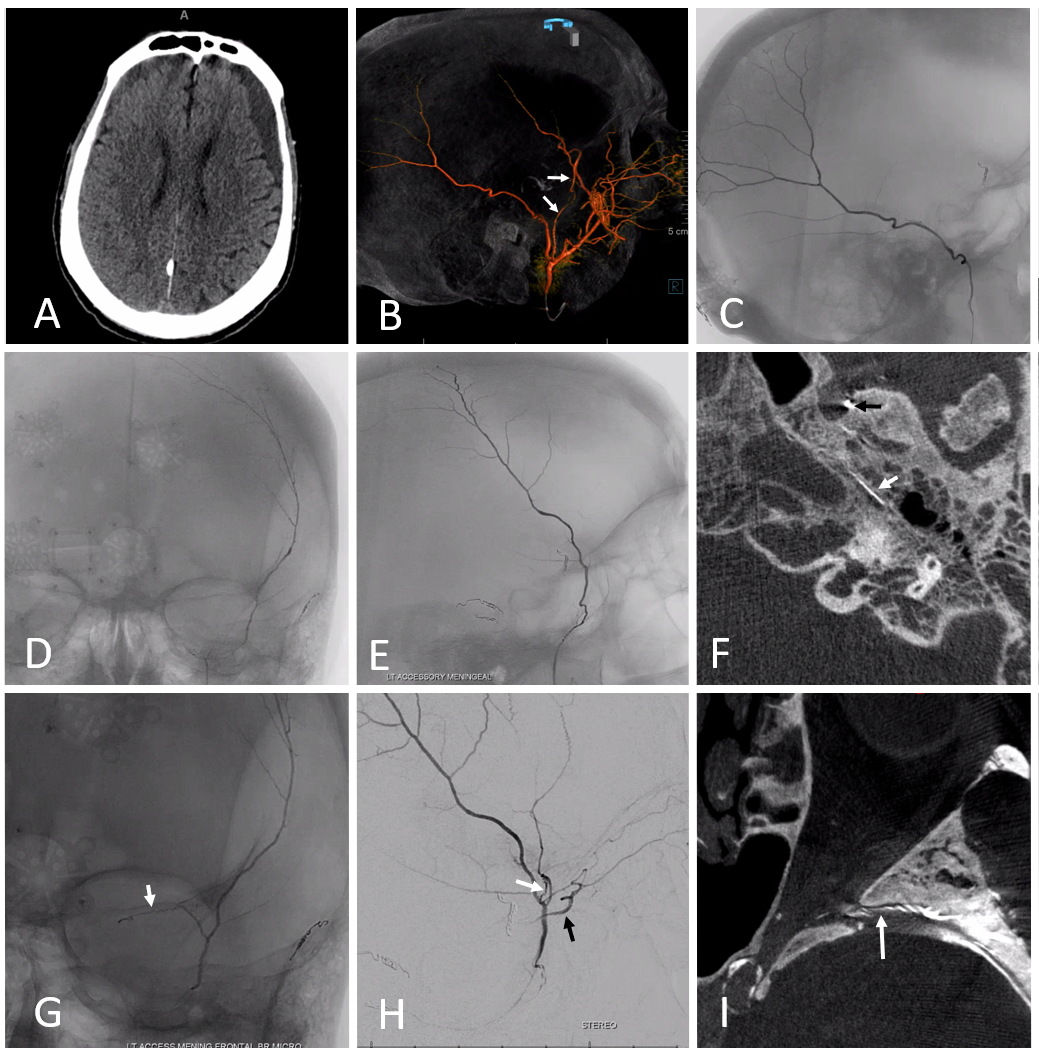
Accessory Meningeal Supply of Frontal Branch and Petrous branches of MMA
The frontal branch variant is rare. The petrous one might not be so rare after all. Just because ovale is more anterior than spinosum doesn’t mean it wont do the job.
In the case below, a variation is present with frontal MMA branch (B, white arrows) supply from the accessory meningeal artery, which also supplies the petrous branch (F, white arrows. Black arrow is on catheter tip in foramen ovale). Images G, H, and I show sphenoid ridge branch (white arrows) opacifying the ophthalmic artery (black arrow) during superselective catheterization of the frontal branch (in this case of AMA origin) — this connection was not visible on a nonselective injection (B) So, be careful…
Complete Middle Meningeal Artery Supply by the Accessory Meningeal Artery — Extremely Rare
Again, we return to the idea of the spectrum — central notion of the neuroangio approach to anatomy. Basically, the most “balanced” arrangement between two or more territories is most common and by definition “classic”. Progressively greater takeover of one territory by another is correspondingly rarer. In other words, the more completely one territory supplies the classic distribution of another, the rarer that is seen. This extremely rare variant of complete MMA supply by the accessory meningeal was seen by Dr. Raz in context of MMA embolization.
Distal ECA — see the unusual origin and course of vessel supplying the MMA territory — more distal origin along IMAX and a posterior course along the floor of middle cranial fossa?
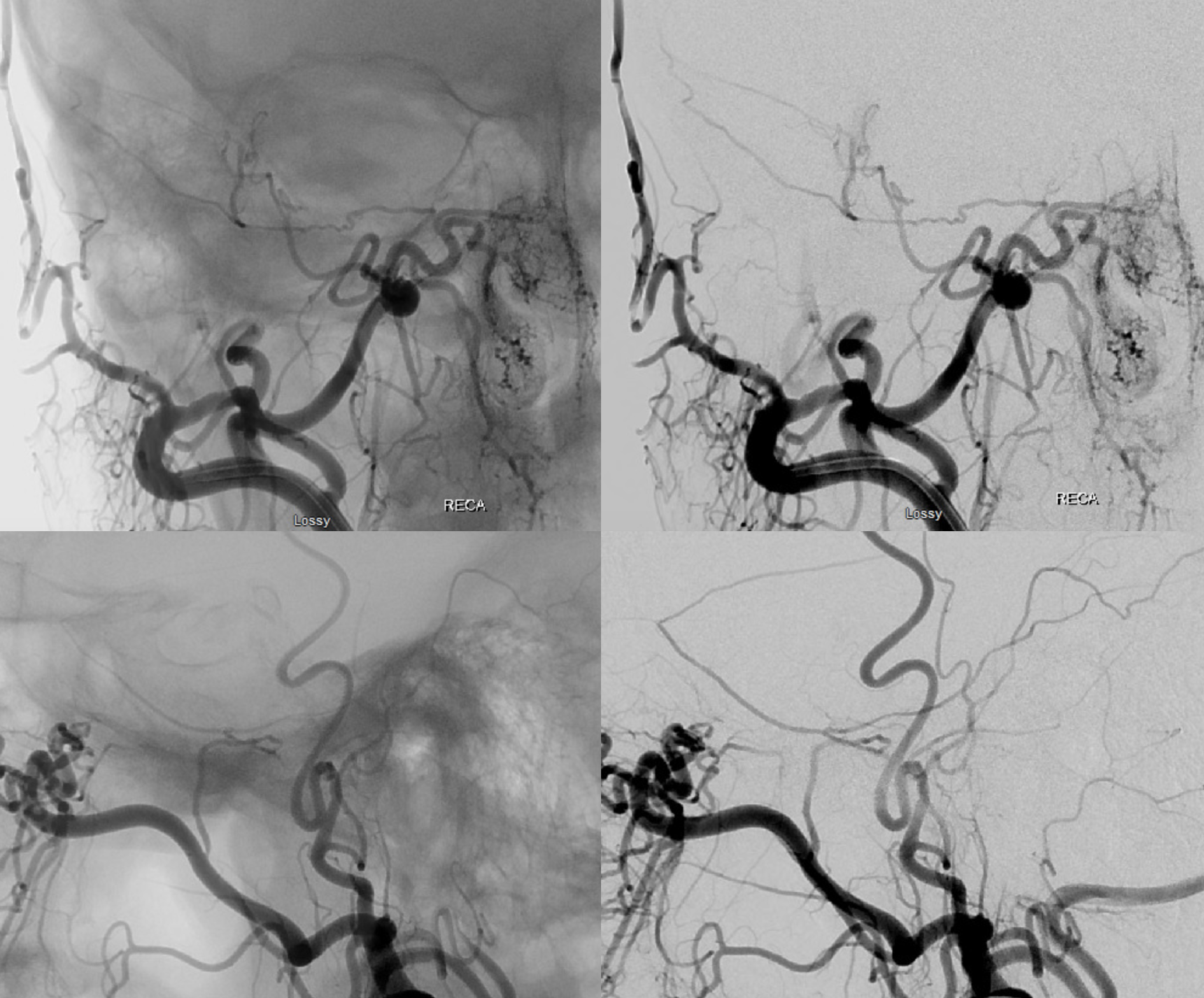
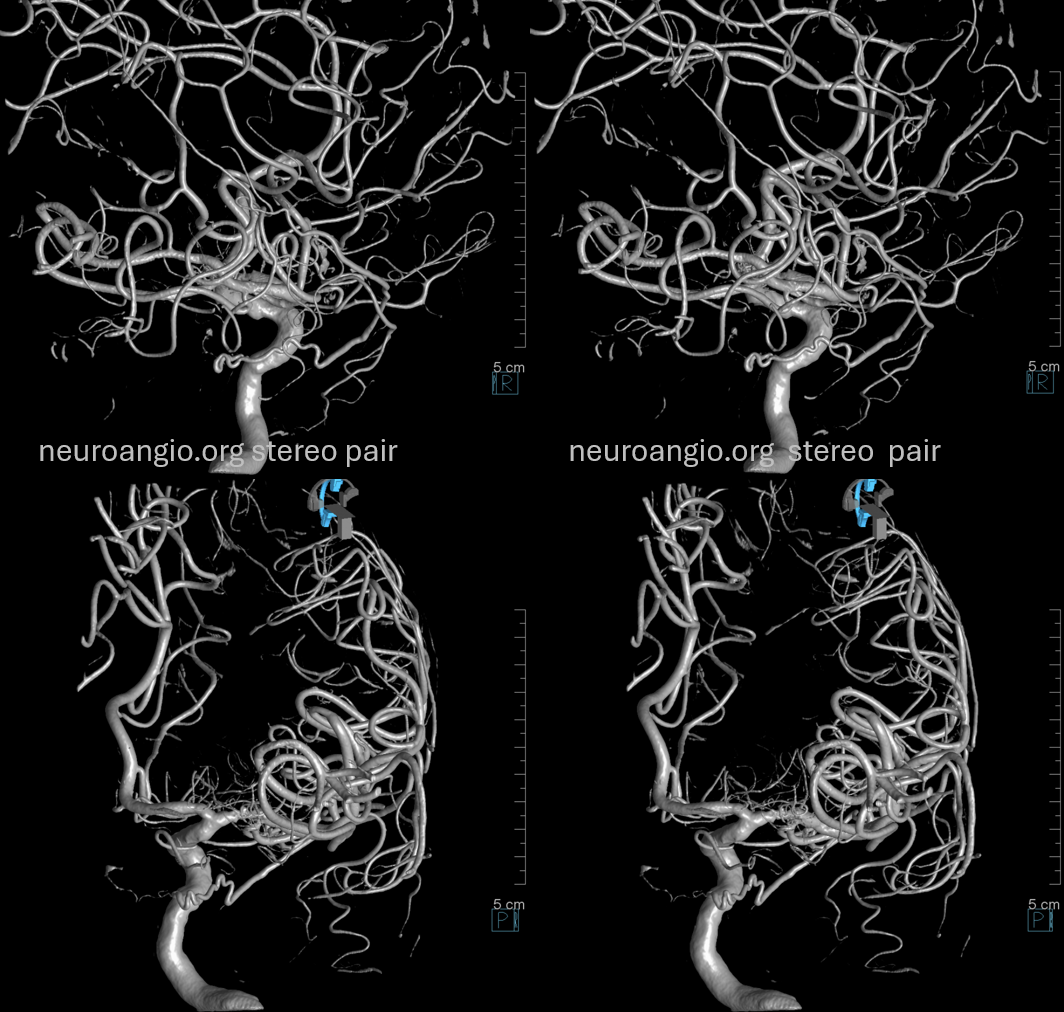
Stereos!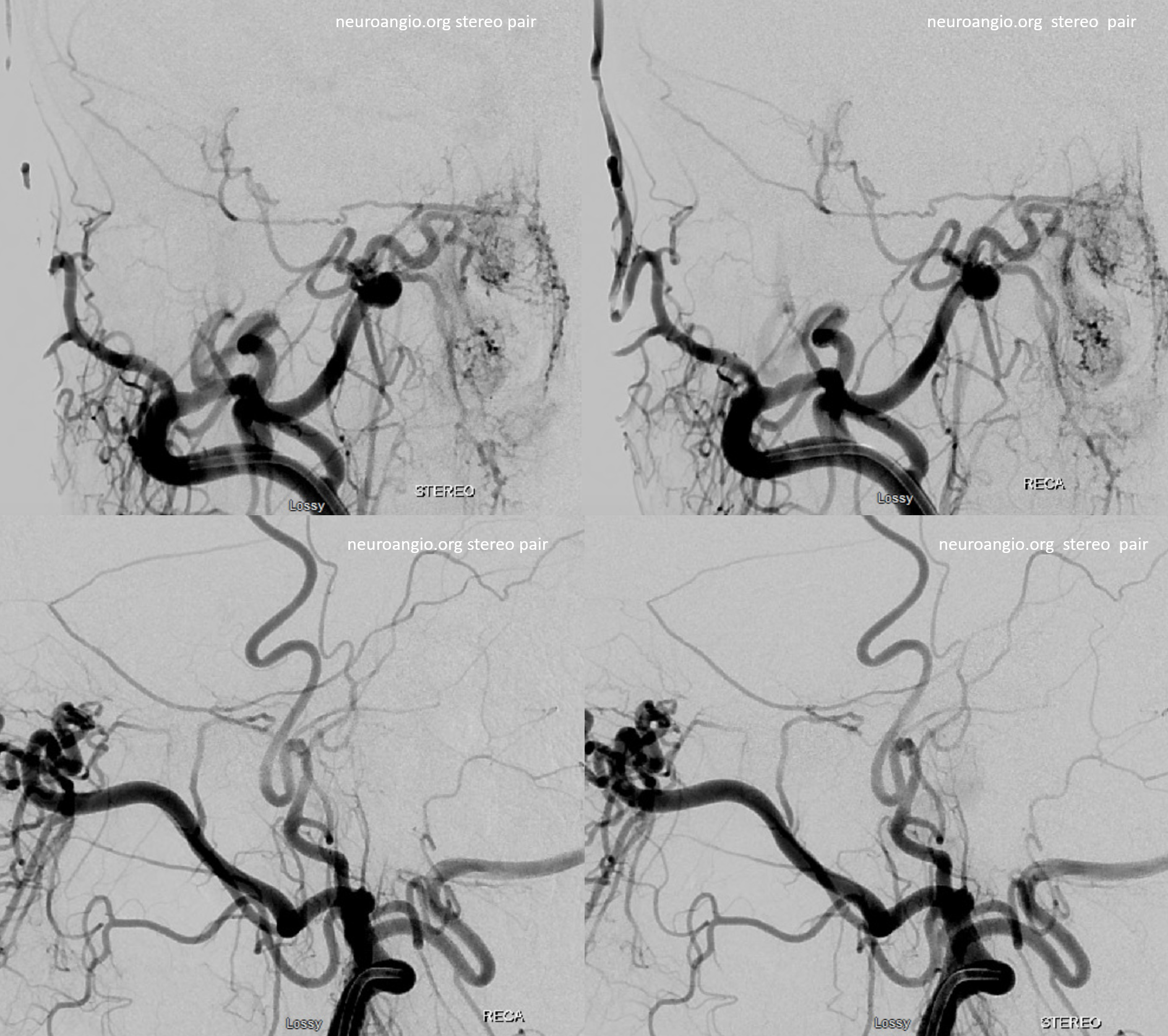
Extremely important technique point. Images on the left show proximal accessory meningeal injections — nothing to worry about right? Images on the right are “wedged” or “flow control” position — see the ophthalmic artery now? Forewarned is forearmed… Particle embo from proximal accessory meningeal position would be a bad idea — but not half as bad as any embolization from the wedge position.
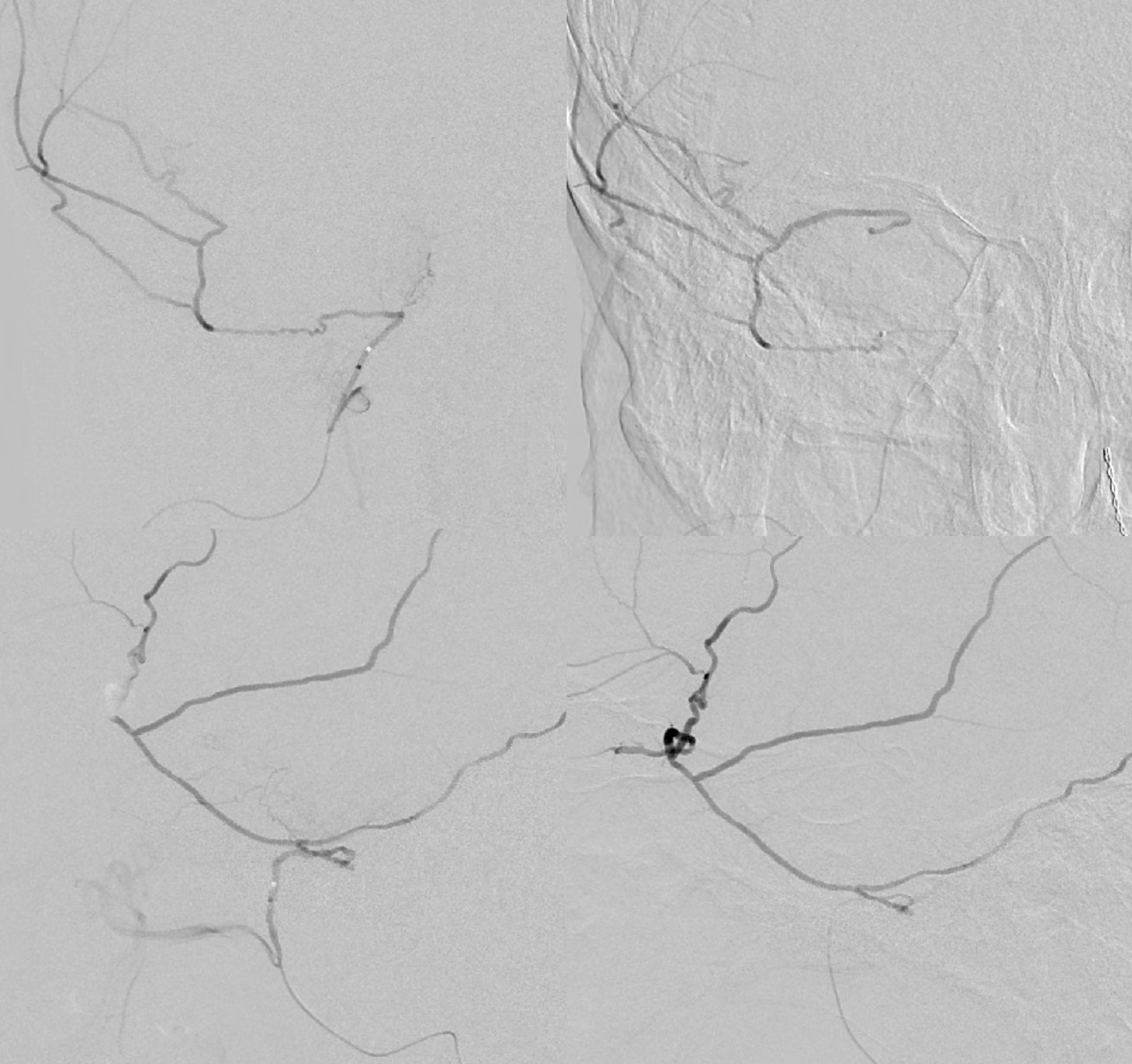
One has to be very careful — see the ICA images below — looks like no problems huh? The connections are always there — its just a matter of how big and how to visualize them.
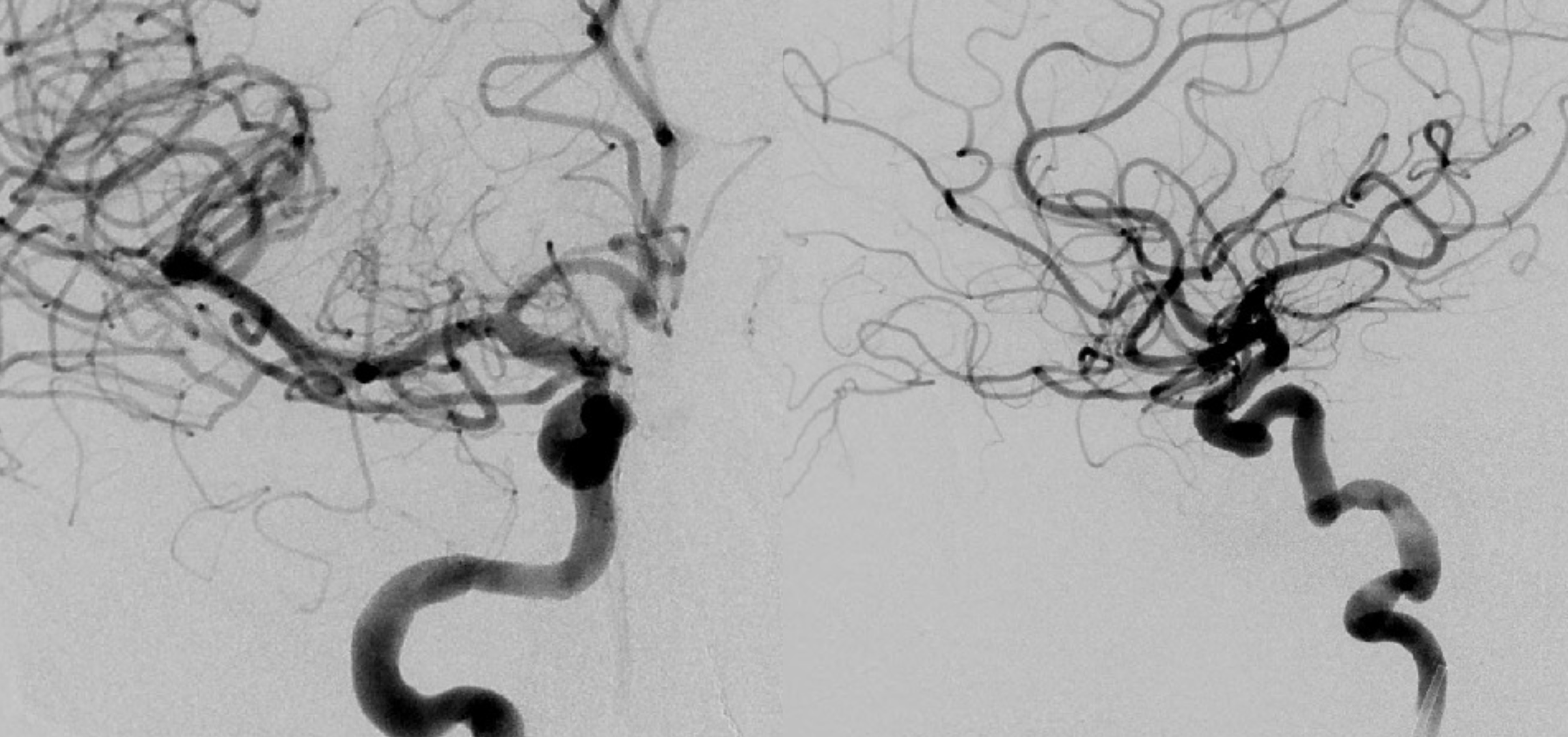
DYNAS — top left is coronal MIP through ovale. Top right is axial MIP through same — the accessory meningeal courses backwards — utilizing the “cavernous” branch of the MMA or the meningeal branch of the ILT — same thing — to reconstitute the MMA. Bottom left is view of ophthalmic artery connection with the MMA via the sphenoid ridge branch. Bottom right is thick MIP showing absence of foramen spinosum.
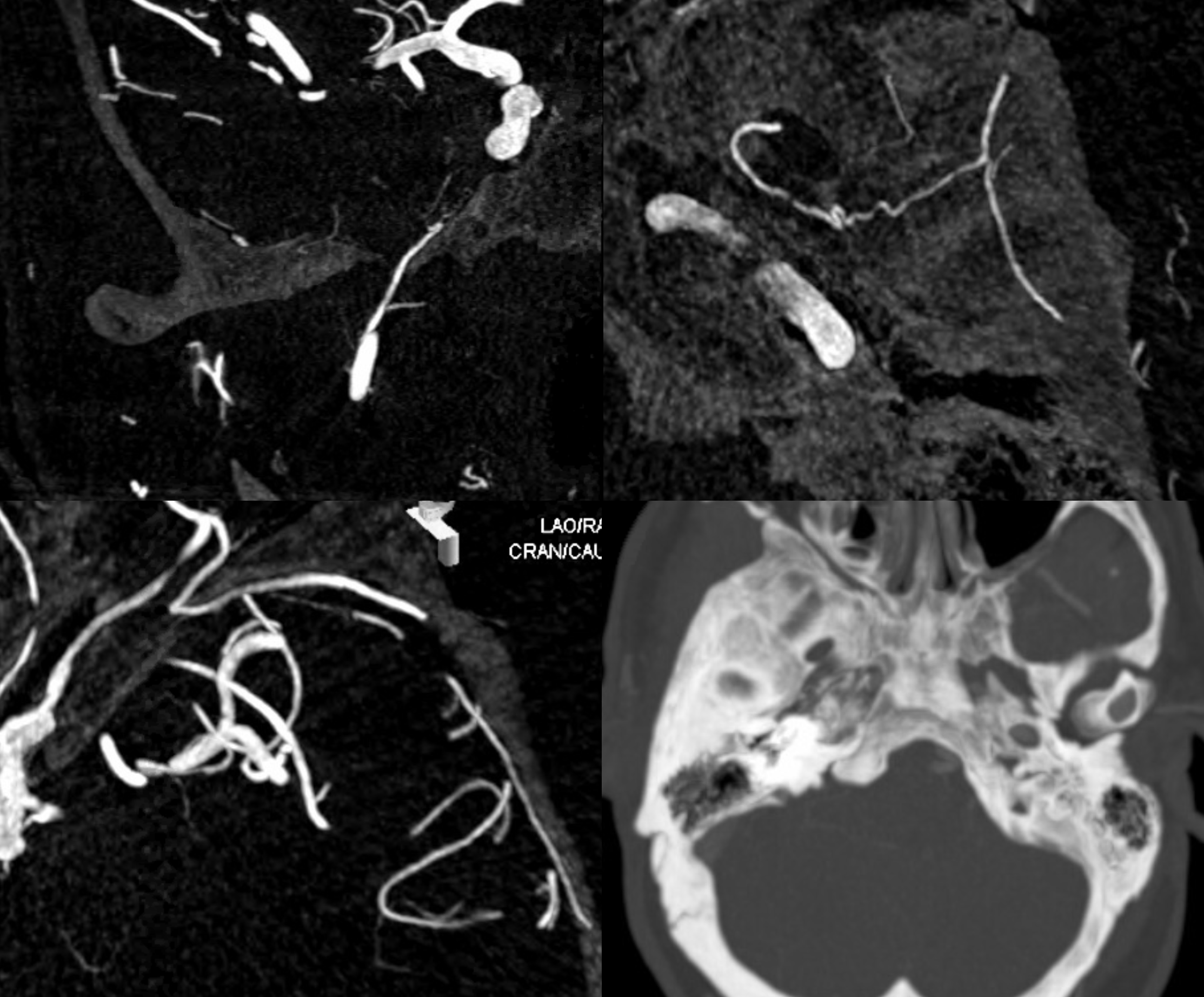
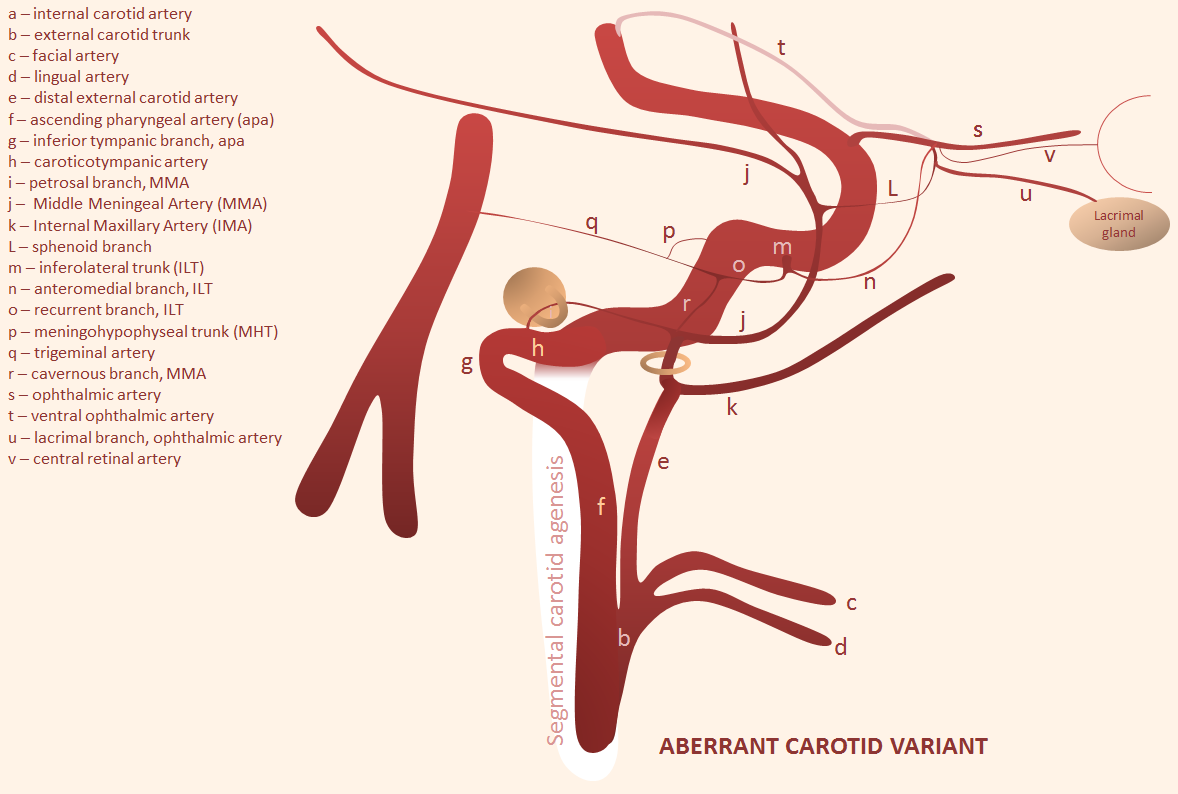
Finally, as an important aside, we illustrate the embryonic origin of the “aberrant carotid artery”. There is agenesis of the cervical ICA segment, which leads to reconstitution of the petrous segment by the inferior tympanic-carotidotympanic anastomosis, like this:
Wanna see some imaging examples? There are plenty on the “Ascending Pharyngeal Artery” Page. Now back to the MMA.
Petrous Branch — facial and petrosal nerve supply. Be careful.
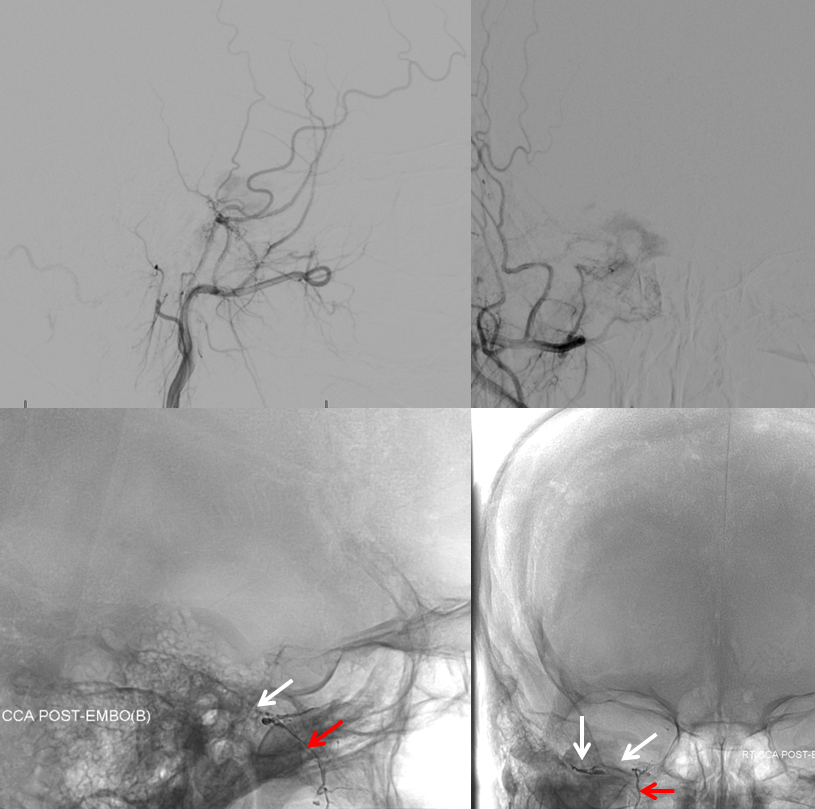
The first intracranial branch, takes off right after MMA exits foramen spinosum, runs posterior. It is simple — proximal MMA embo, especially with liquid embolics, risks facial/greater petrosal nerve injury. Embryology tells you that there is always going to be a petrous branch of the MMA. It is a remnant of the ultra-early hyostapedial system. Petrosal branch is part of the “facial nerve arcade” (together with the occipital or posterior auricular artery), and supplies not only facial but also (more proximally) the greater superficial petrosal nerve. Don’t mess with them. Because of petrosal branch, embolization from proximal middle meningeal position carries risk of aforementioned nerve injury. Particles are unlikely to penetrate and disable the nerves if petrosal branch is very small (and usually it has anastomoses). But liquid embolics will penetrate… Like this:
Patient presents with posterior cavernous dural fistula. Supply is from left ascending pharyngeal and left MHT
For some reason, embolization from right proximal MMA is attempted using Onyx. Top row is pre-embo. Bottom row shows Onyx in the proximal MMA, refluxing into the accessory meningeal artery (red) and petrosal branch (white). The patient developed an isolated greater petrosal nerve syndrome (taste aberration, lack of tears, and lack of nasal secretion production on the right. CNVII remained intact likely because of Onyx did not go posterior enough. All symptoms resolved in 3 months)
Another example — cavernous dural fistula (purple) presenting with vision loss in left eye. Even though fistula is small, both superior and inferior ophthalmic veins have thrombosed, leading to marked occular venous hypertension. Full case is here. Supply is primarily via right MHT
There is suggestion of supply from right ECA (below)

Catheter in proximal MMA position shows supply to fistula via small cavernous sinus branch of the MMA (black arrow). The Petrosal Branch is marked with red arrows. Images on right show nBCA in the petrosal branch. The patient awoke with complete facial nerve paralysis, and made only partial recovery. The fistula was closed by nBCA embolization via the right MHT. See full case here
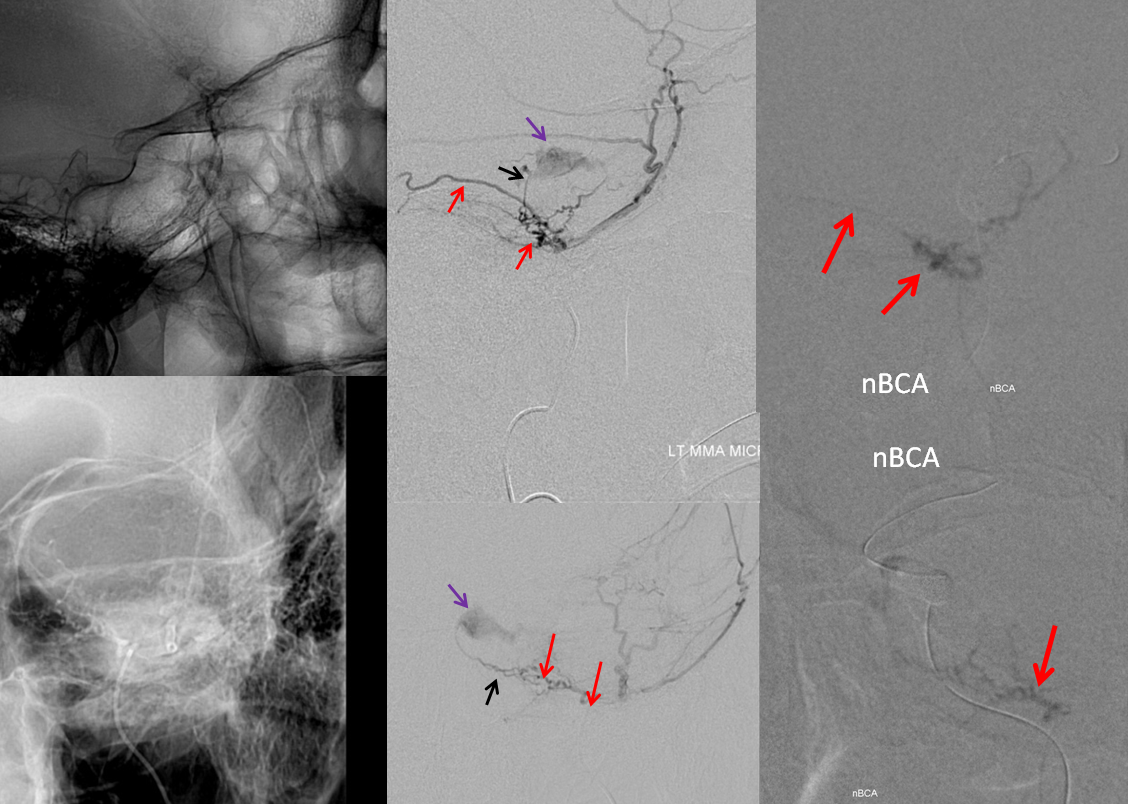
Conclusion — proximal MMA embolization, especially with liquids, is a treacherous undertaking
Meningioma Petrous Branch Supply
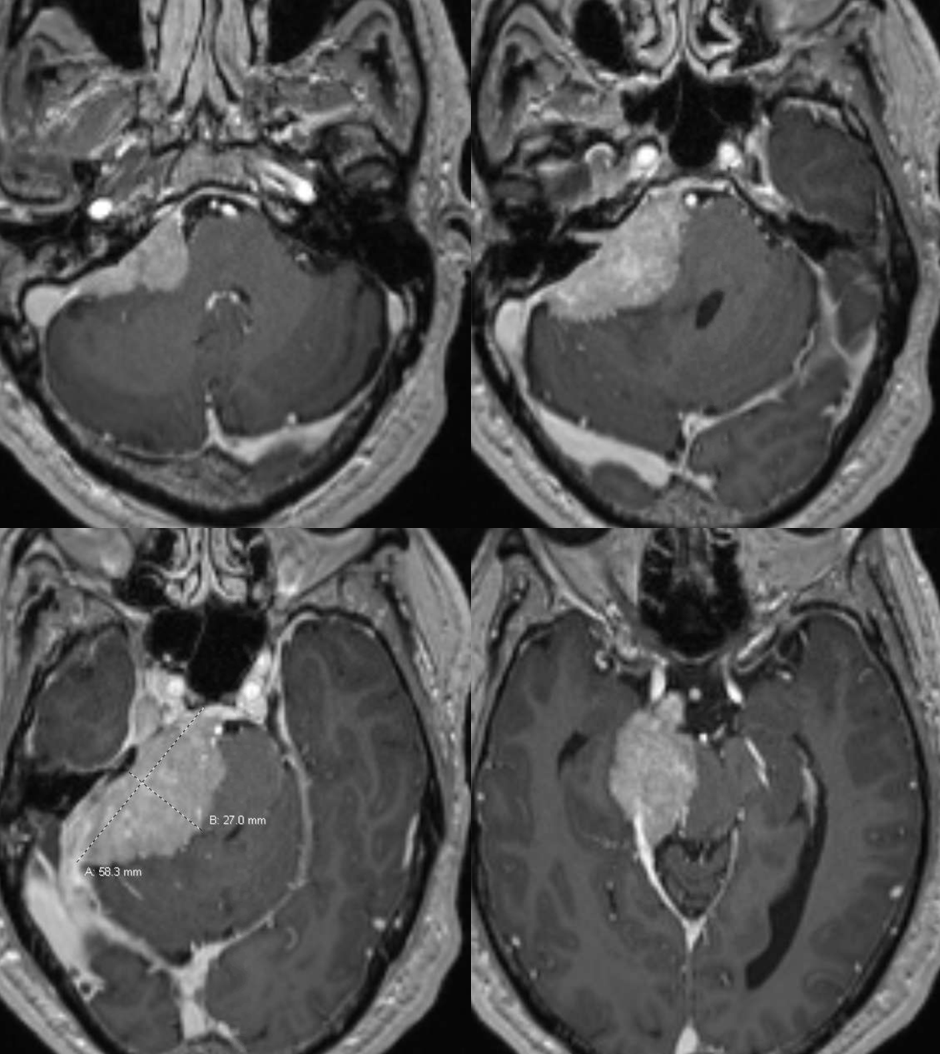
Petrous branch (33) supply – the branch is enlarged because of additional mening supply. Typical course.
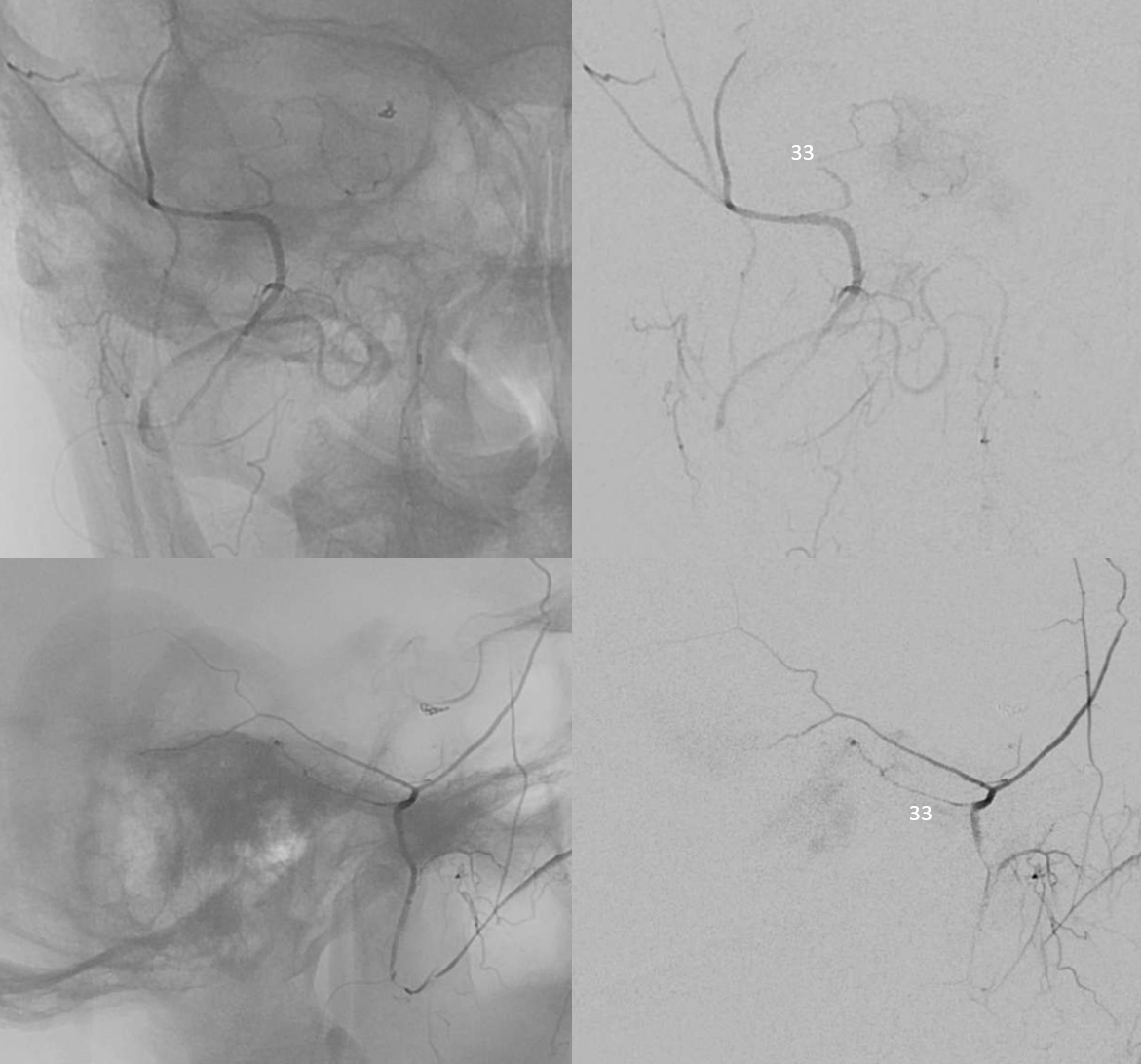
Additional supply from MHT, ILT, and jugular division arising from occipital artery

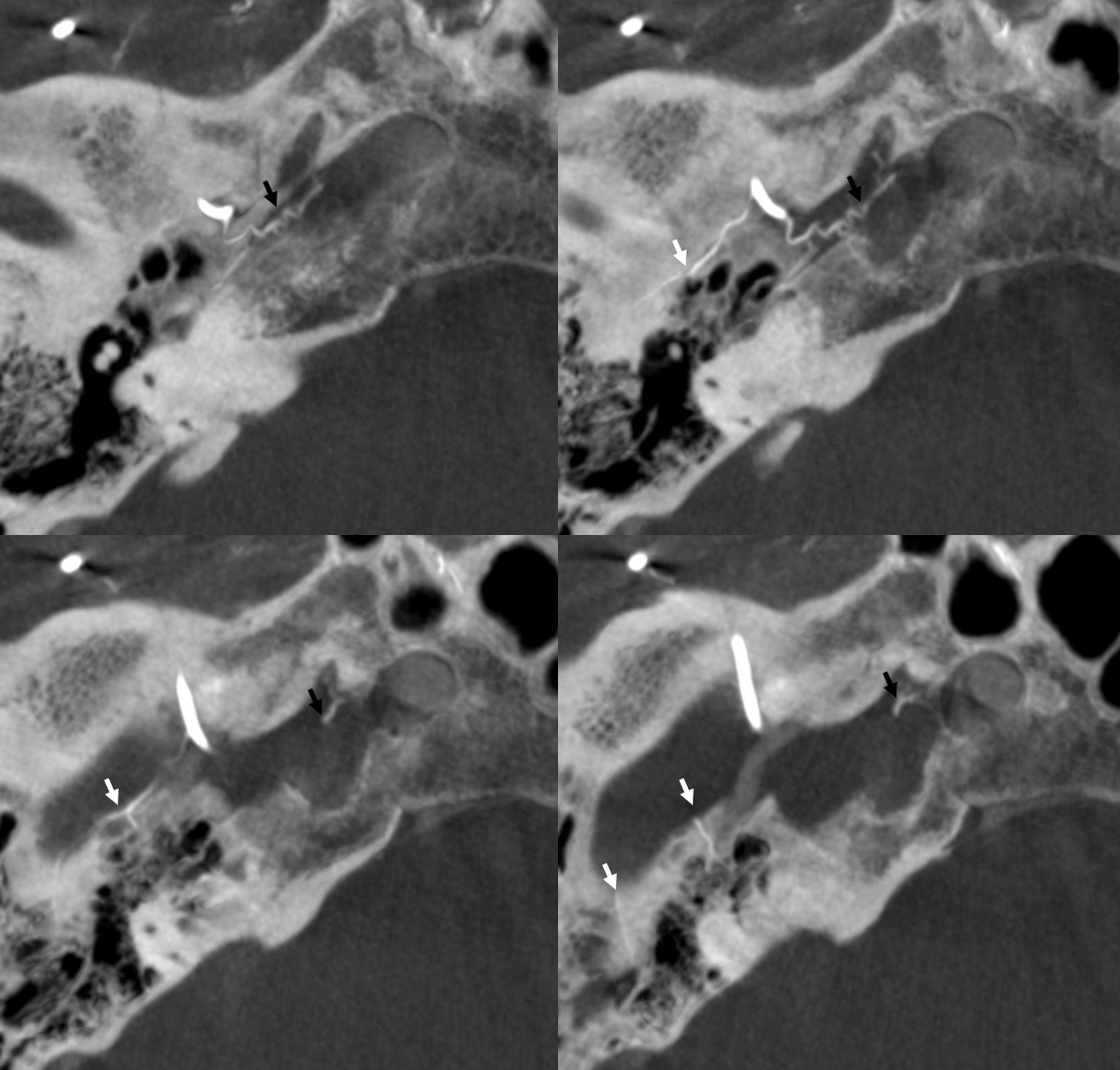
Cavernous Branch
Much less often discussed than petrous branch. Connects MMA with ILT — it is the same branch as the posterolateral branch of ILT. Can supply nerves of the Meckel cave and cavenous sinus. Another reason not to embolize from a proximal MMA position. Here both cavernous (black arrows) and petrous (white arrow) branches are shown well
Cavernous branch reconstituting ILT territory in a case of occluded ICA — including continuation into the orbit as the anteromedial ILT branch to reconstitute the ophthalmic artery (arrows)
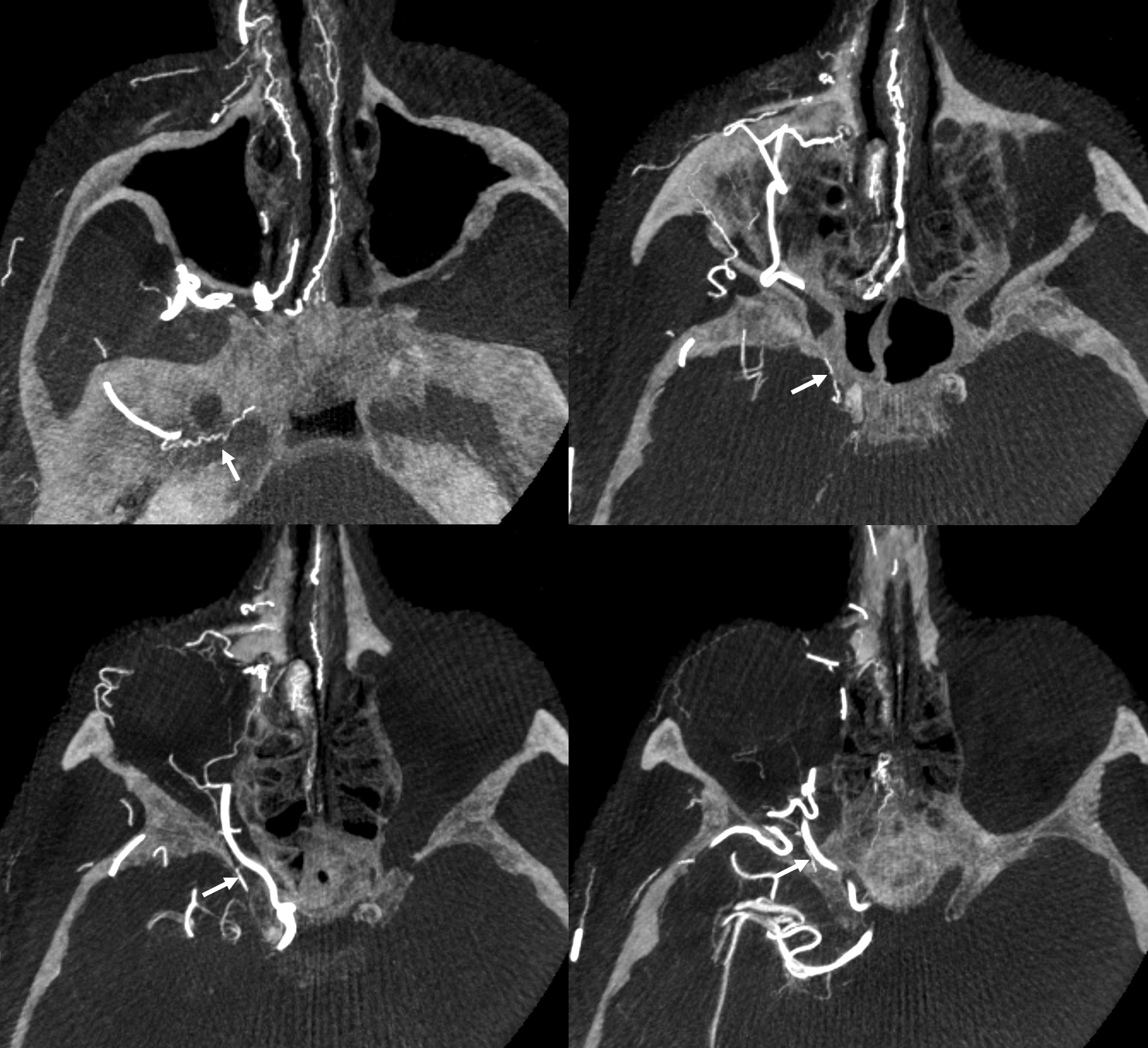
Facial Nerve Supply — Beautiful DYNA CT demonstration
Very prominent facial arcade — so much so there are two branches in the facial canal…
Axials
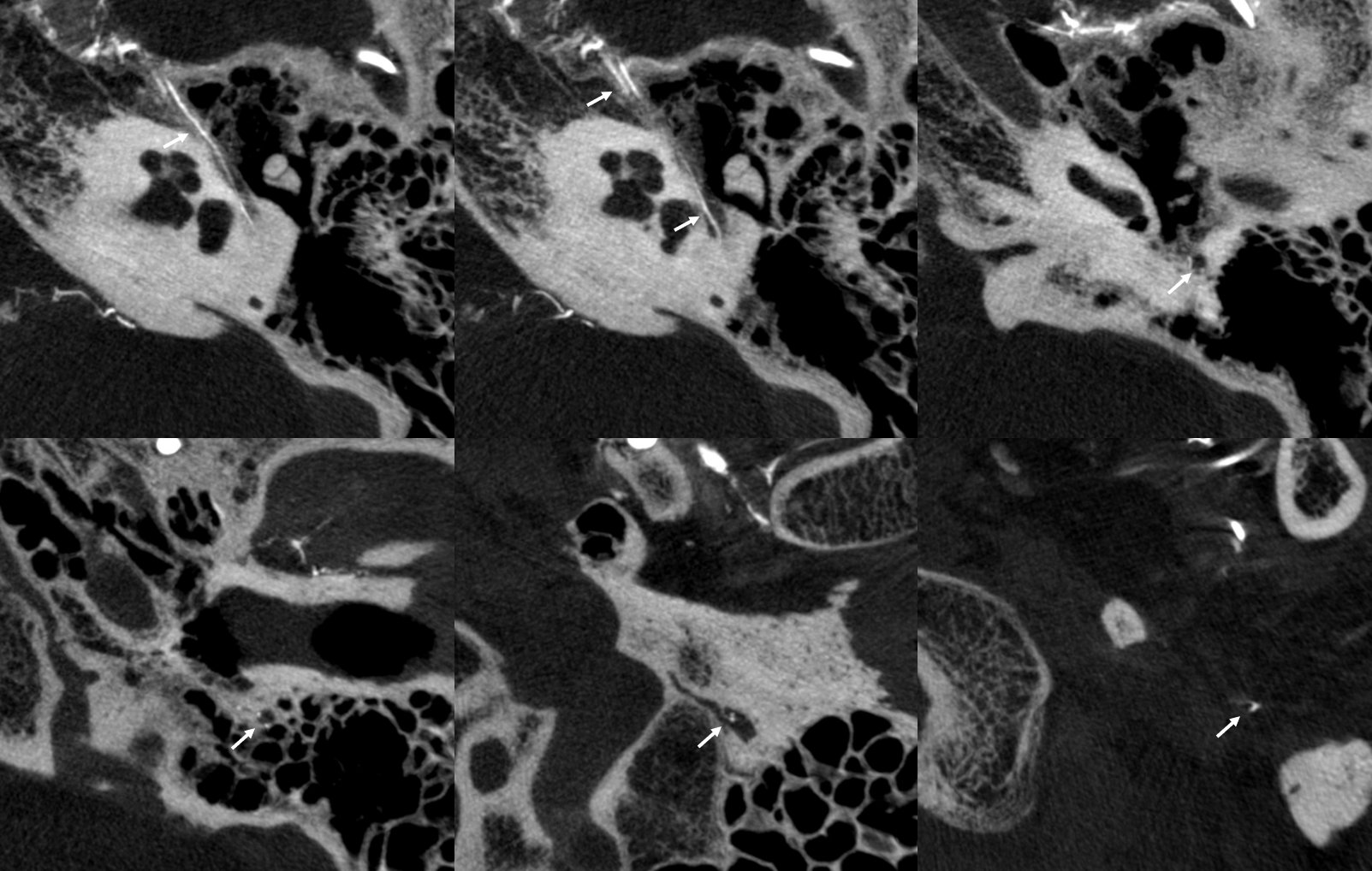
Sagittals
Coronals
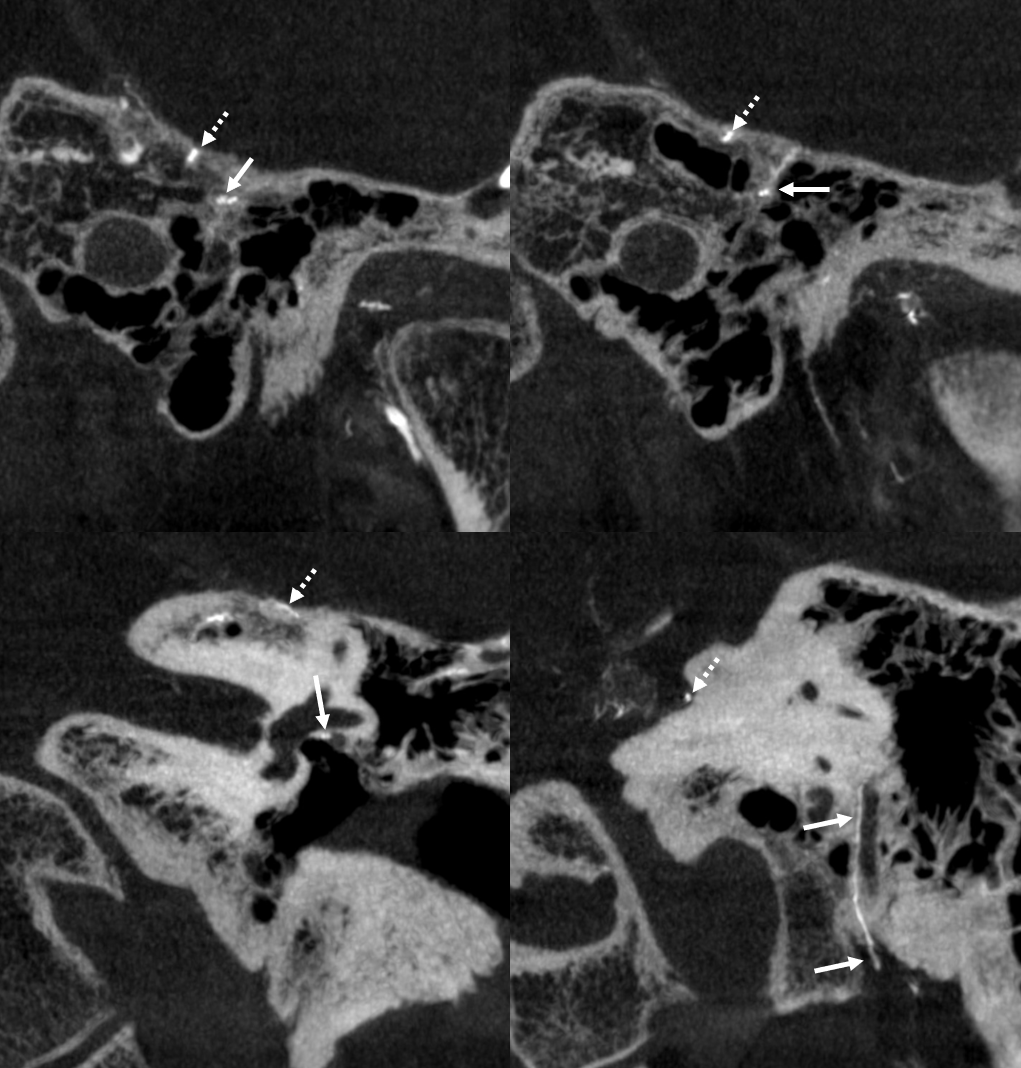
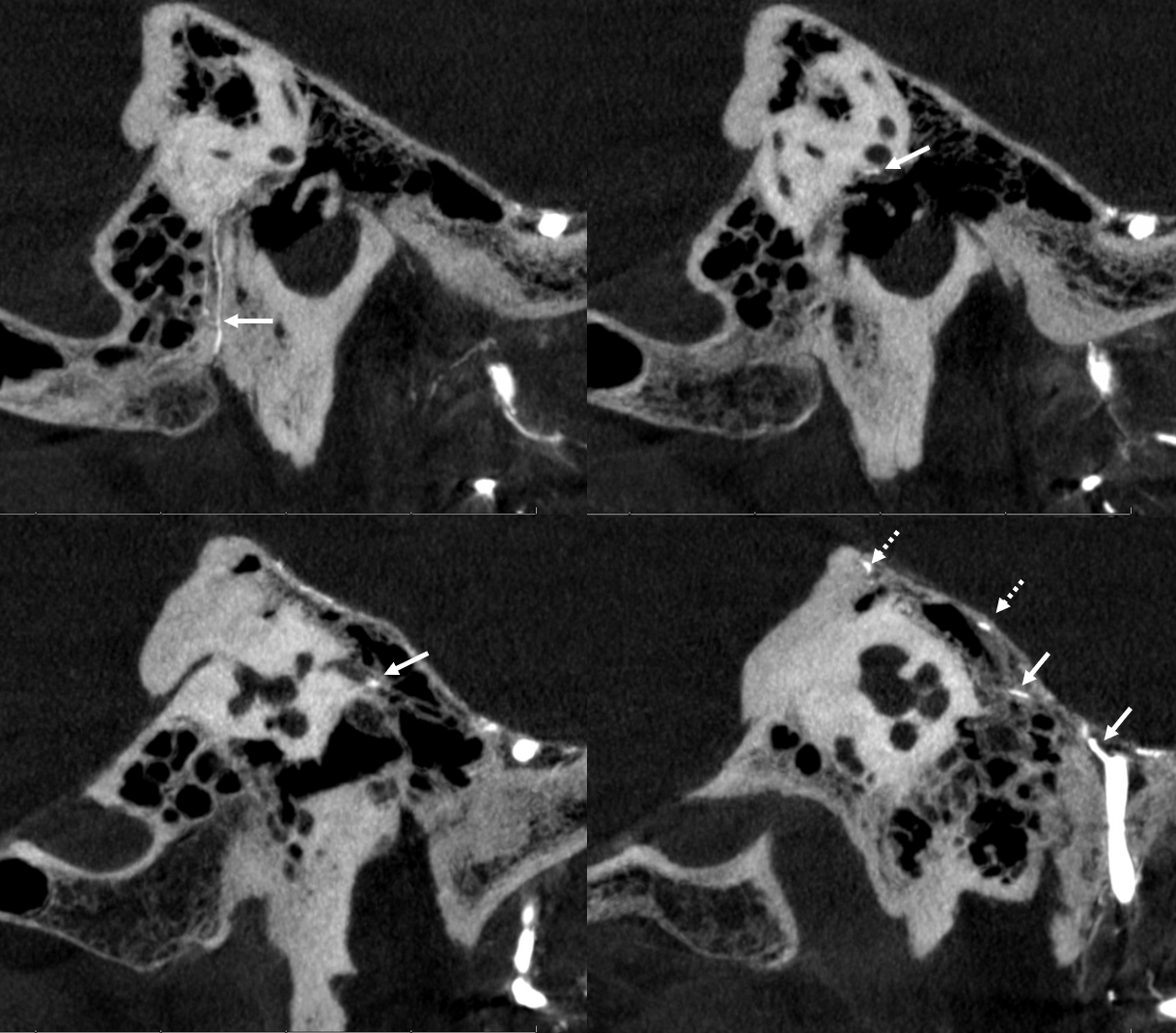
Another example — here the cavernous branch (arrow) is prominent. The petrous one (dashed arrow) is tiny as it often is. Better seen in DYNA movie below
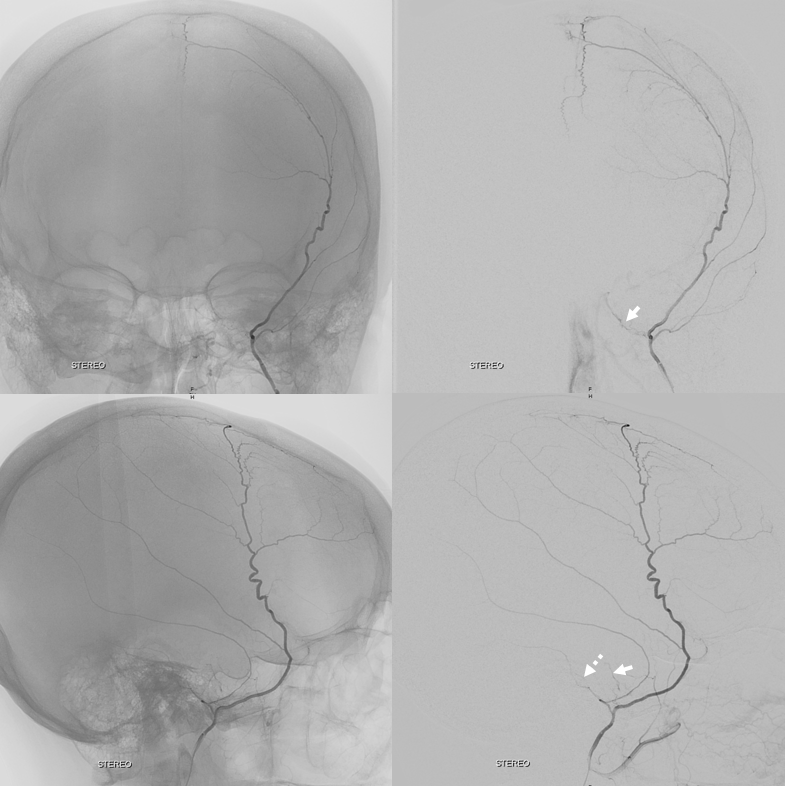
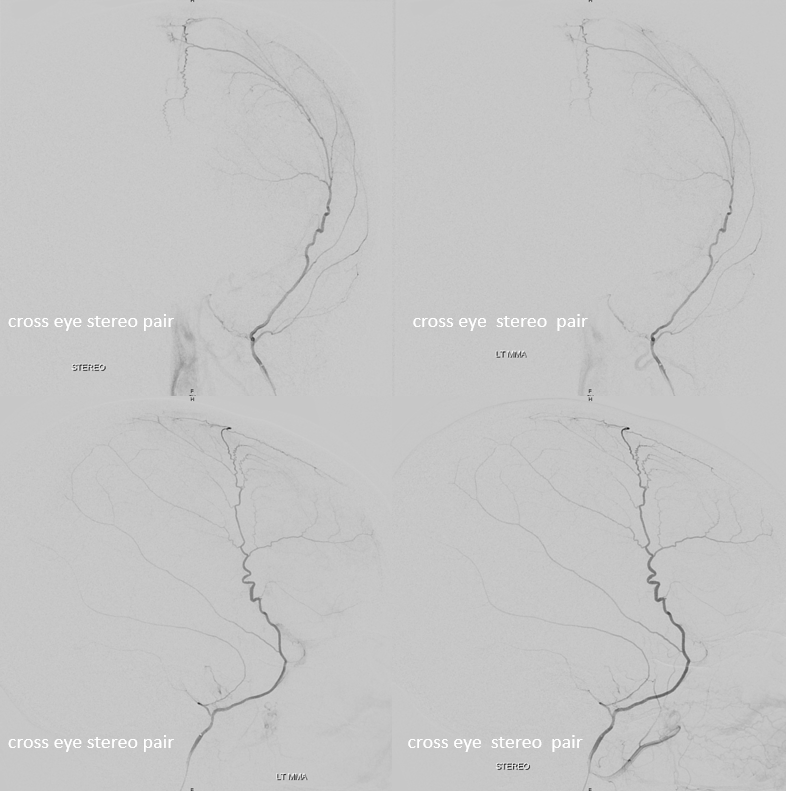
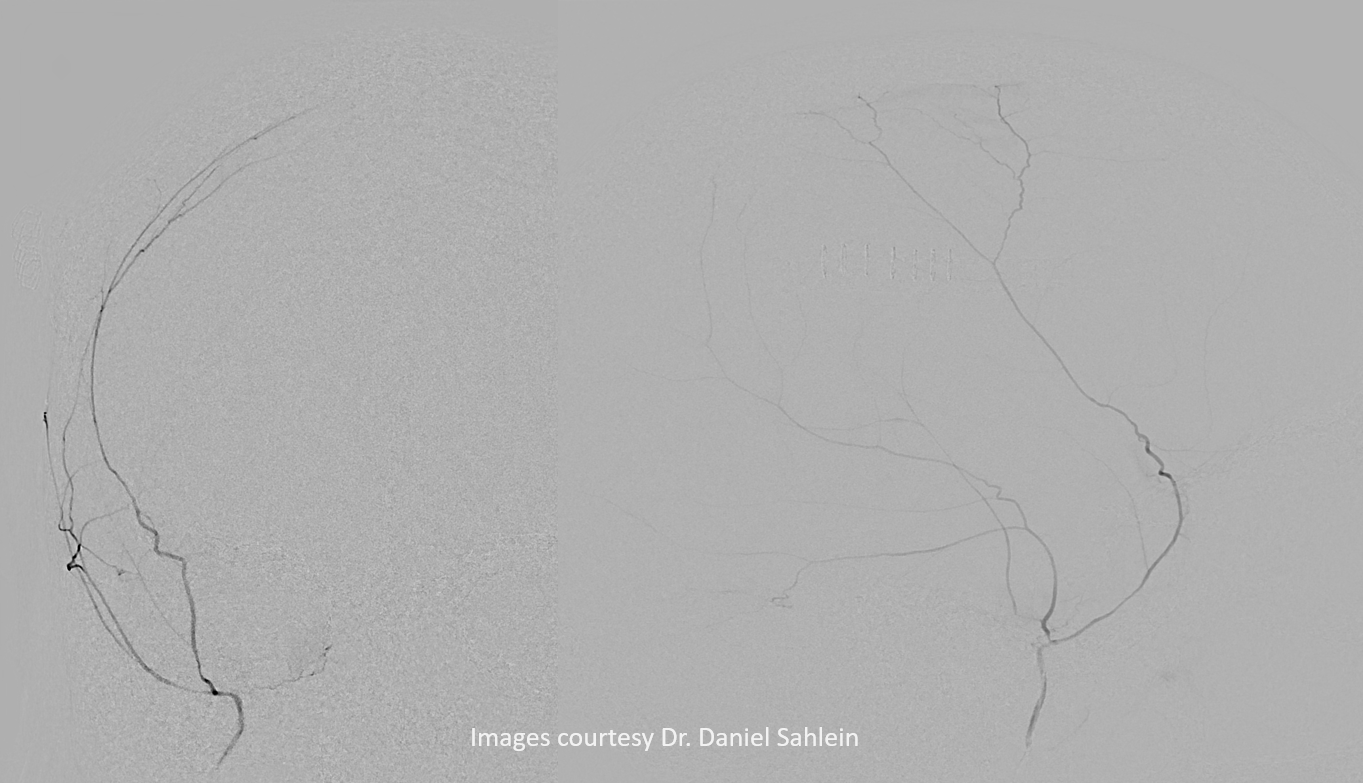
Cavernous and Petrous Branches — especially beautiful DYNAs, courtesy Dr. Daniel Sahlein
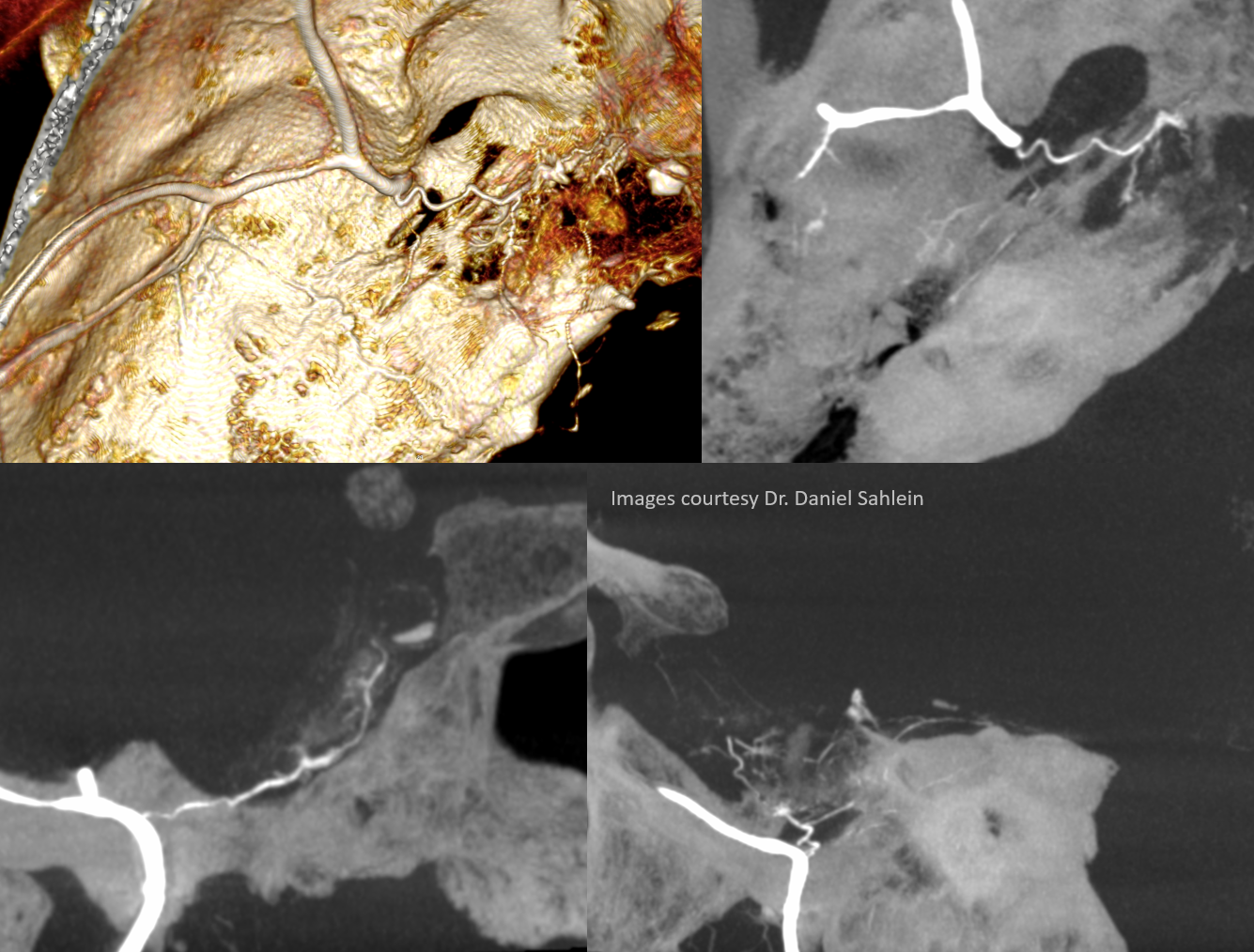
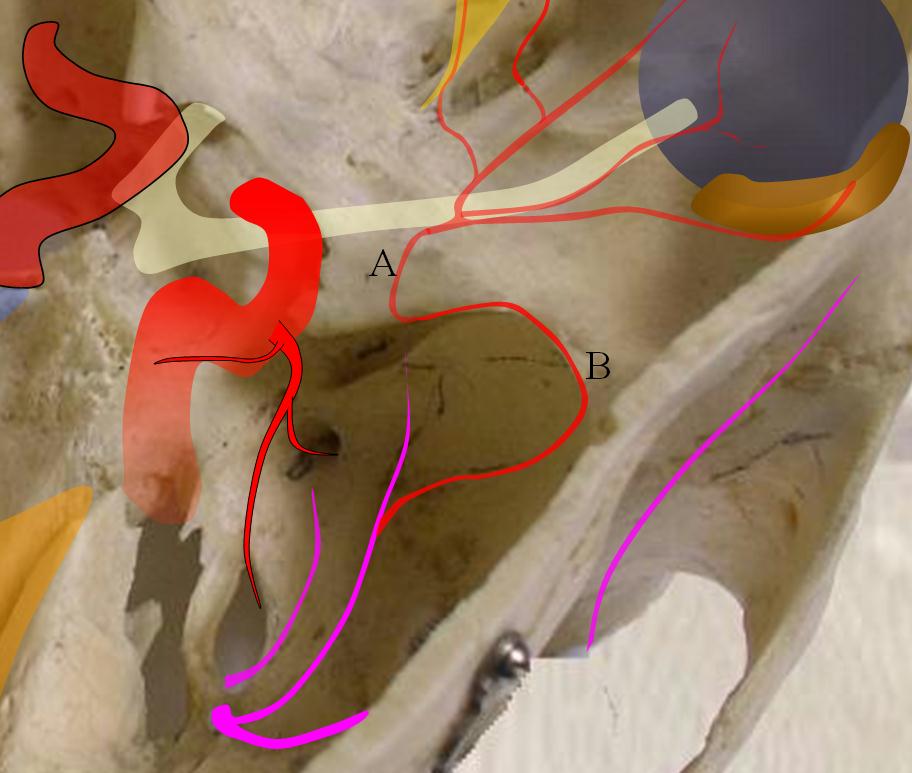
Orbital Anastomoses and Variants
The most famous of “dangerous anastomoses” MMA has embryonic connections in the orbit. Like anything, there is a spectrum. Sometimes, the connections are tiny, sometimes large in a way that makes MMA the primary supply of orbit, and anything in-between. And vise versa — the “recurrent meningeal” is when the ophthalmic artery supplies the MMA — part or all of it.
Meningophthalmic and Meningolacrimal arteries
When MMA arises “normally” from IMAX, connections with orbital vessels come from the sphenoid branch of the MMA — running forward and up from the spinosum to get to the sphenoid ridge. From there, connections enter orbit via the superior orbital fissure (SOF) and/or via its own foramen, known as foramen of Hyrtl. Two broad patterns are present — complete MMA supply of the orbit — meningophtalmic (or meningo-ophthalmic) artery — in which case it enters via the SOF, and partial supply restricted to the lateral orbit and specifically the lacrimal gland — meningolacrimal artery — in which case it enters via the Hyrtl foramen. Simple.
IMPORTANT: Like everything else, there is a continuum. One can have a “full” meningo-ophthalmic or meningolacrimal or co-dominance — just like one can have a fetal PCOM, no PCOM, co-dominant PCOM and P1, etc. See below.
Meningophthalmic Artery — complete MMA supply of orbit, including central retinal artery
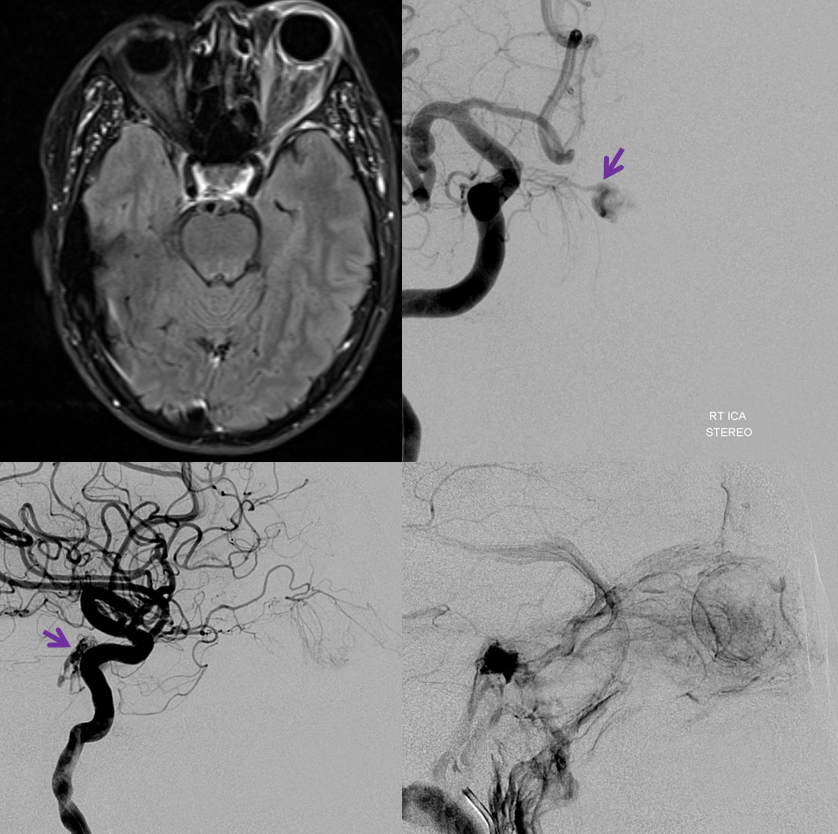
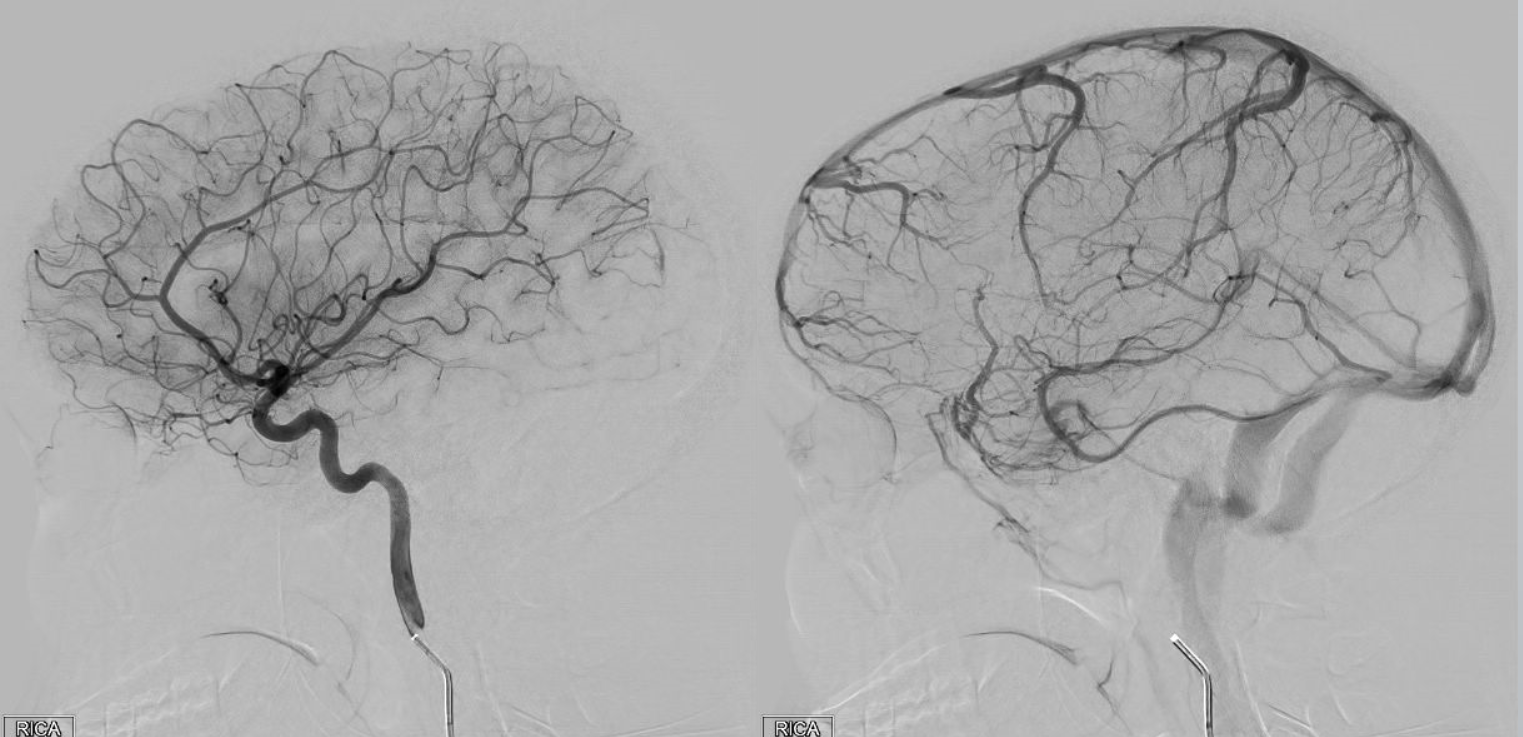
The classic dangerous anastomosis. Particles are the most dangerous embolic in this case, because they will be taken by flow into distal circulation. The central retinal artery is about 160 micrometers in diameter, a true end-artery with no collaterals, usually not visible on angiography. A single small particle will either completely blind the patient or at least give a large field cut. Liquid embolics are less dangerous because they may not penetrate the retinal artery and allow for possibility of collaterals — not that its OK to use them. Here is an example, with no ophthalmic artery on ICA views
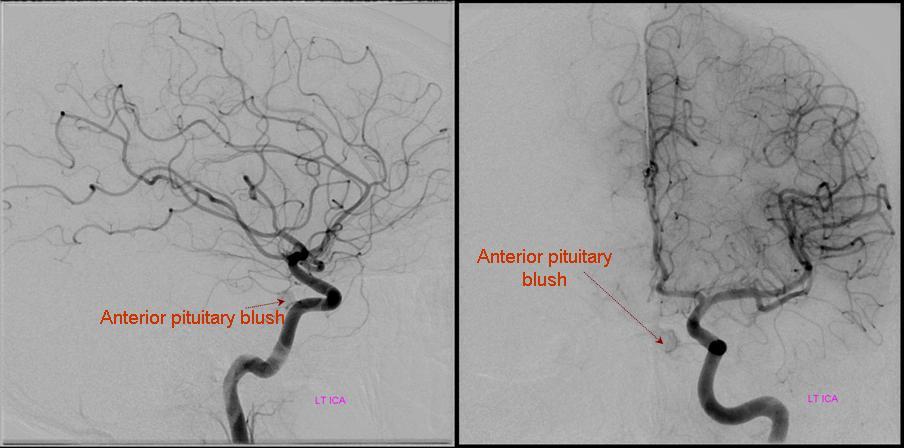
The entire orbit is supplied by the MMA
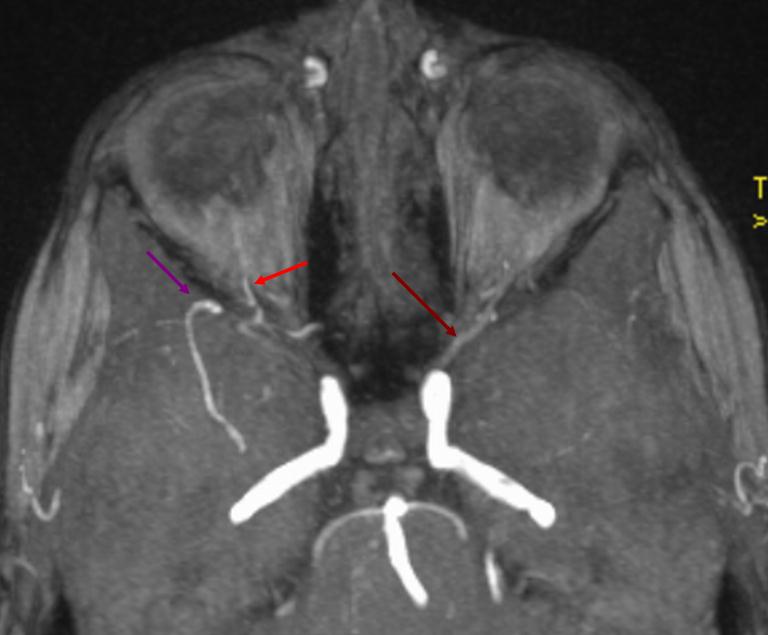
In many cases this variant can be seen on modern MRA (MIP image of “normal” on left (brown arrow) and MA origin ophthalmic on right (red)
Over the orbit may be in the orbit
Cant be over-emphasized. Any ECA injection frontal view artery projecting over the orbit can be in the orbit. makes sense. What is it — occipital or meningolacrimal/meningophthalmic as a rule. Here, the catheter is below occipital origin — unlike in the above example. Occipital is dashed arrows, MMA solid, ophthalmic ball arrow, and choroid blush arrowheads. Boxes are corresponding orthogonal views
Had enough? Of course not. We are not even halfway there yet…
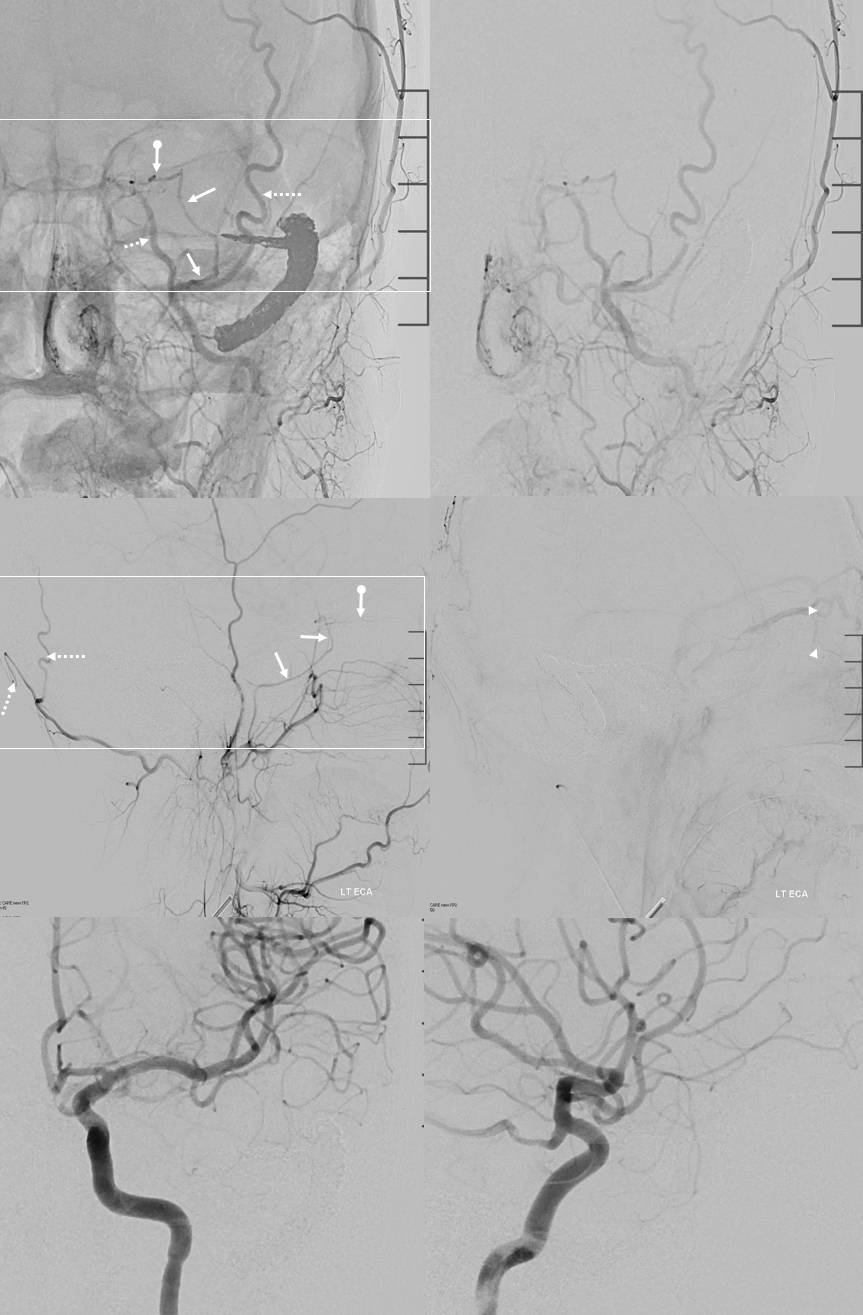
Clinical Significance — patient with left cervical ICA dissection
Collateral reconstitution of the left ICA is provided by right MMA (white arrows) to left MMA (meningo-ophthalmic, black arrows; sphenoidal branch -purple arrow) to ophthalmic artery (red arrow)! Notice also anterior meningeal branch (yellow) reconstituting ophthalmic via the ethmoid artery (pink arrow). Case courtesy Dr. Eytan Raz
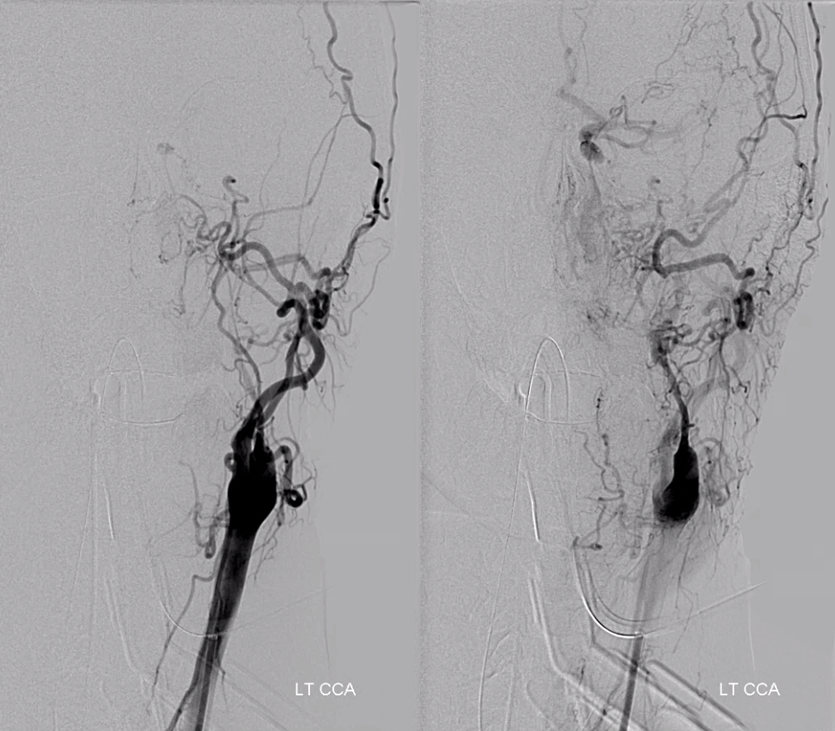
Stereos
Anaglyph stereos
Meningolacrimal artery
MMA supply to lacrimal gland and lateral orbit. Enters via its own foramen of Hyrtl, lateral to the SOF. Intra-orbital connections with ophthalmic artery are usually distal to origin of the central retinal artery, however extreme caution must be exercised nevertheless.
Angio example, with typical origin ophthalmic including choroid blush of the globe
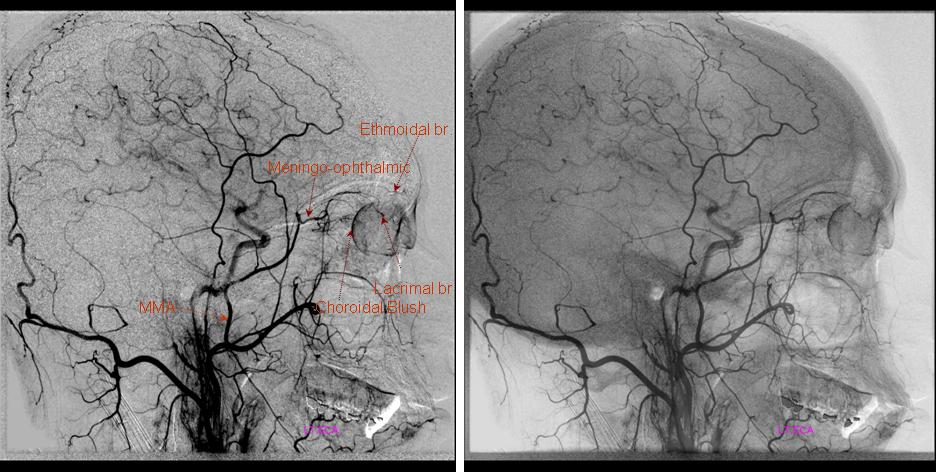
ECA injection shows MMA (purple) and sphenoid branch (white) supplying via the lacrimal branch (brown) the lacrimal gland (peach). Note lateral entry of lacrimal branch into orbit (foramen of Hyrtl). A more medial branch (red) likely connects the lacrimal branch to the ophthalmic artery.
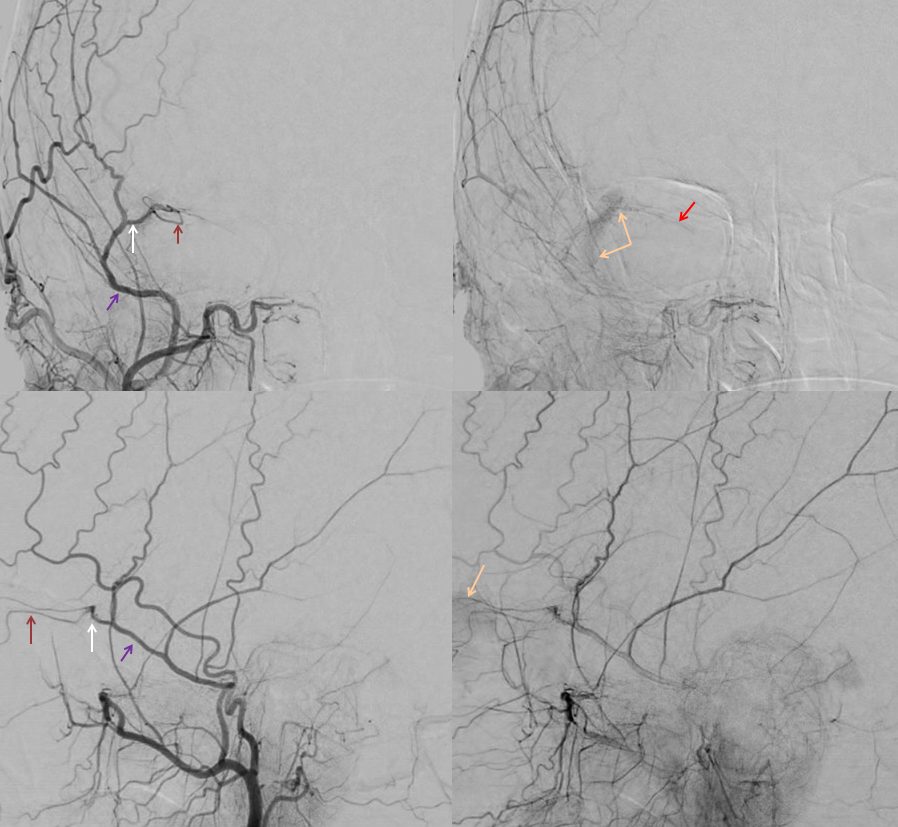
Again, there is a continuum. Bodies don’t read books. In example below, the meningeal contribution to the orbit includes both classic “meningolacrimal” artery via foramen of Hyrtl (arrowheads), and a superior orbital fissure contribution (dashed arrow) that mainly goes to the posterior ethmoidal foramen (ball arrow). Neither directly supplies the orbit. Also present is a rare but important connection to the tentorial arcade (arrow) going backwards — usually supplied by ILT.
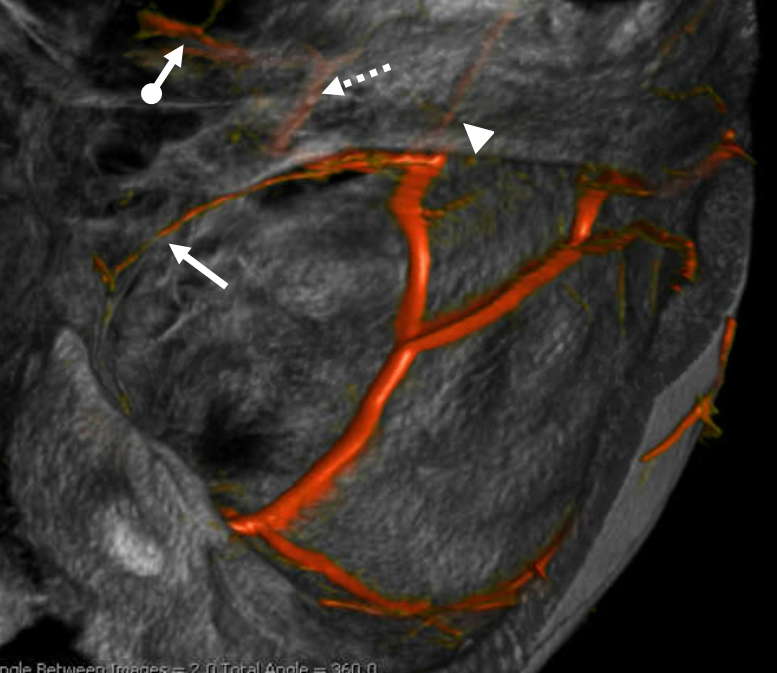
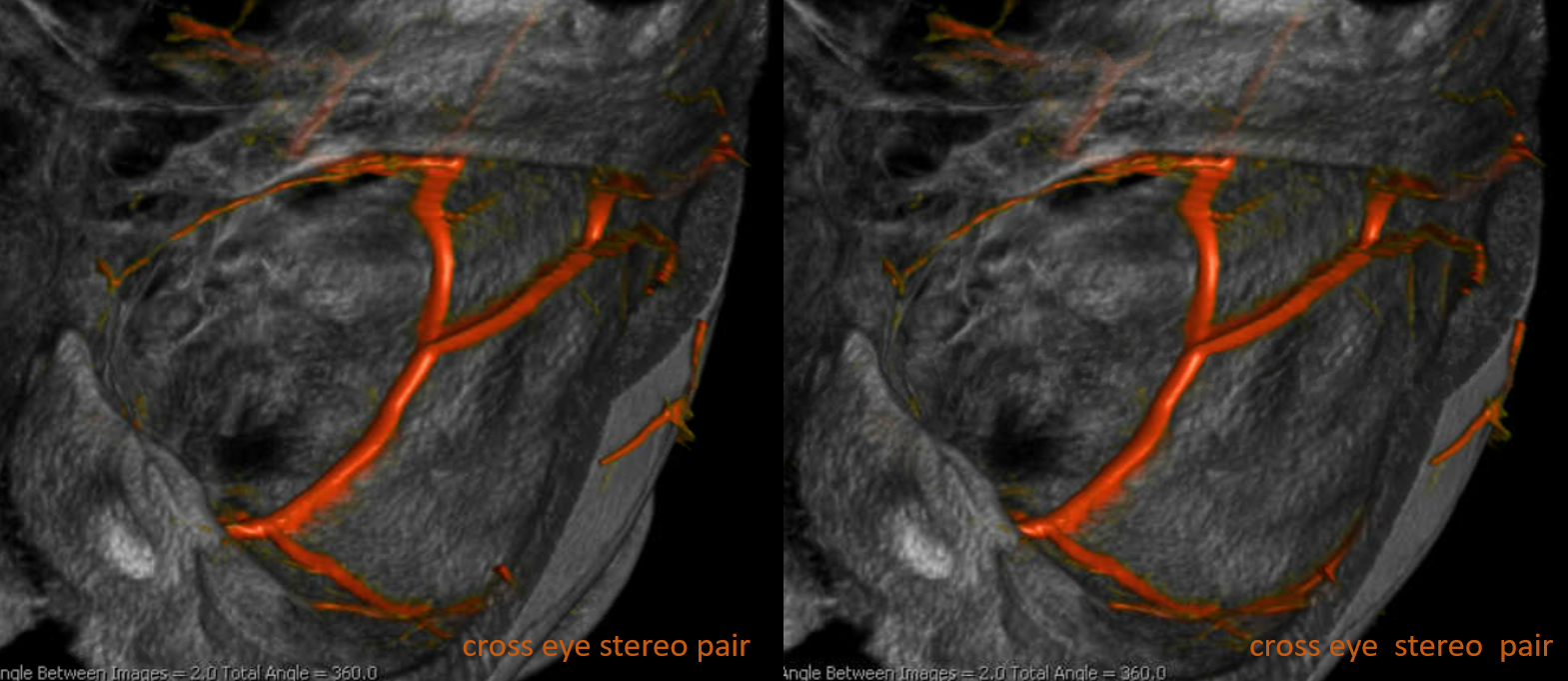
The recurrent tentorial branch (arrows) heads far posterior to become the free margin artery (Bernasconi-Cassinari)
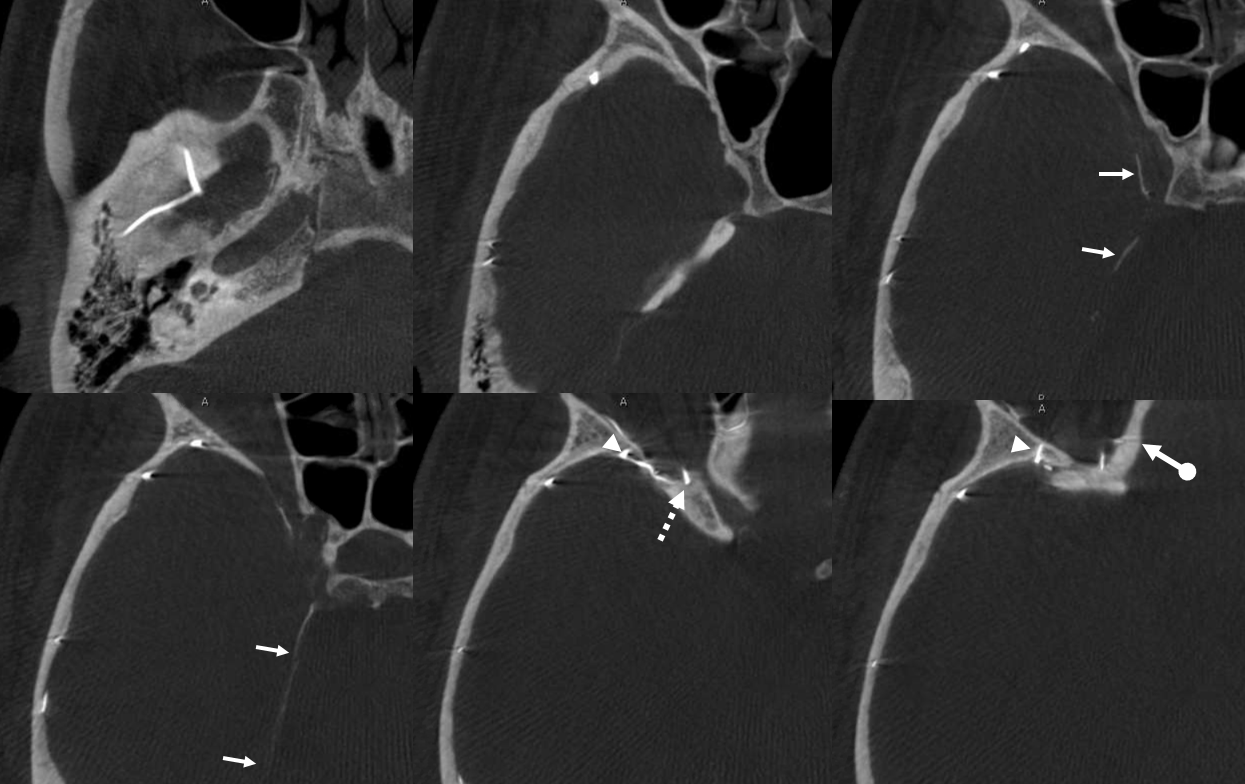
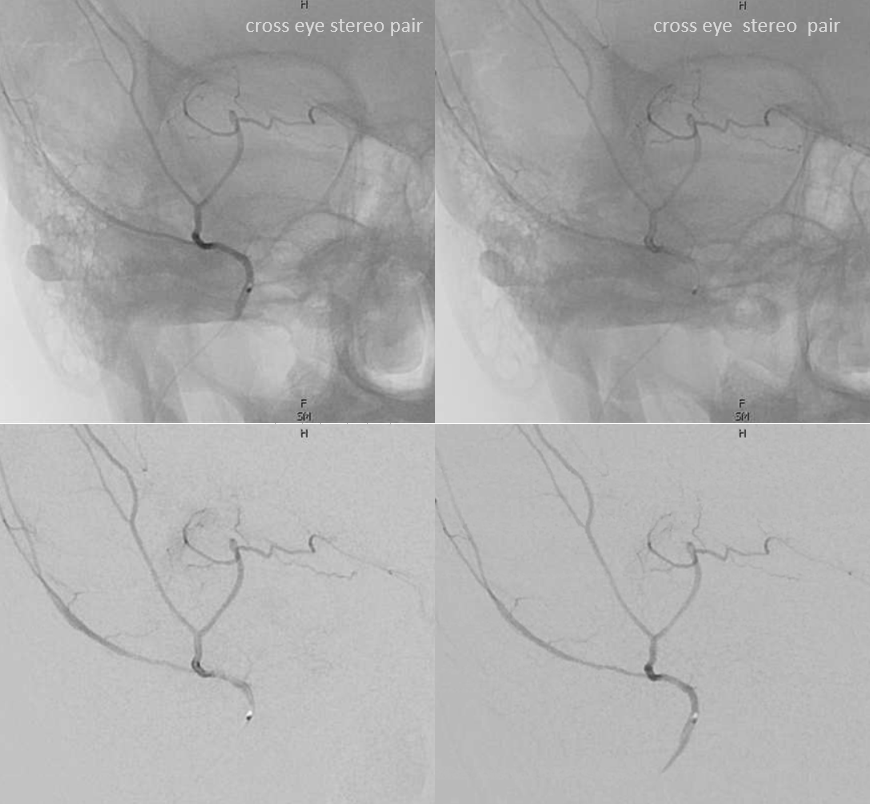
Movie — pause and scroll thru images
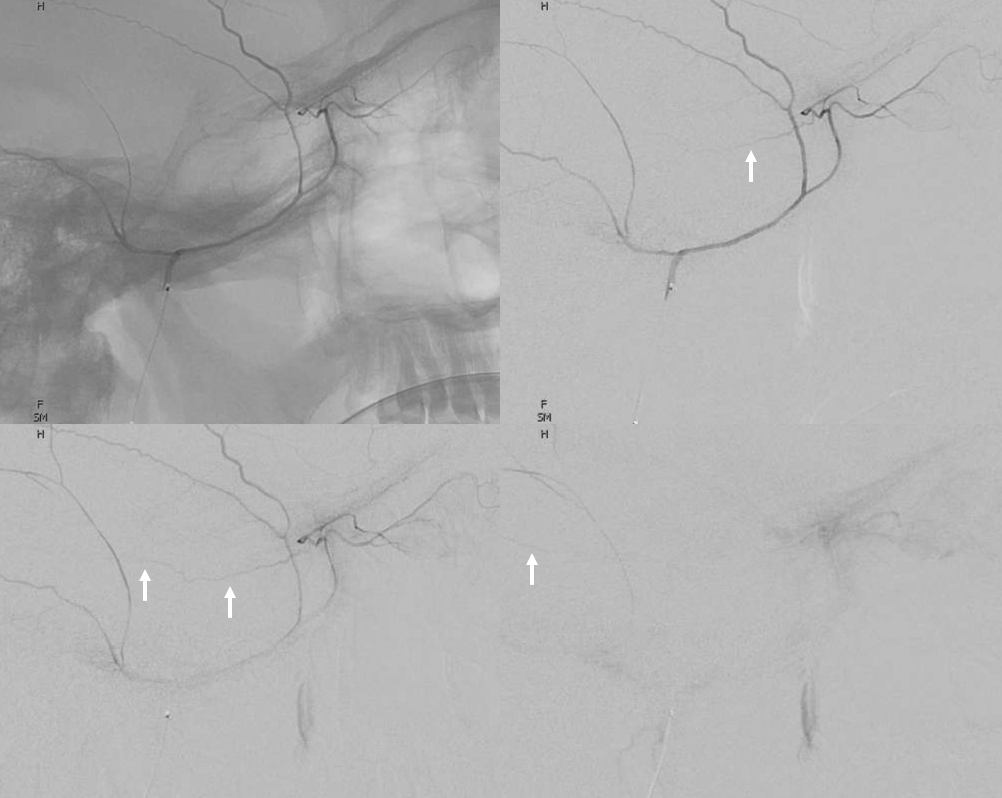
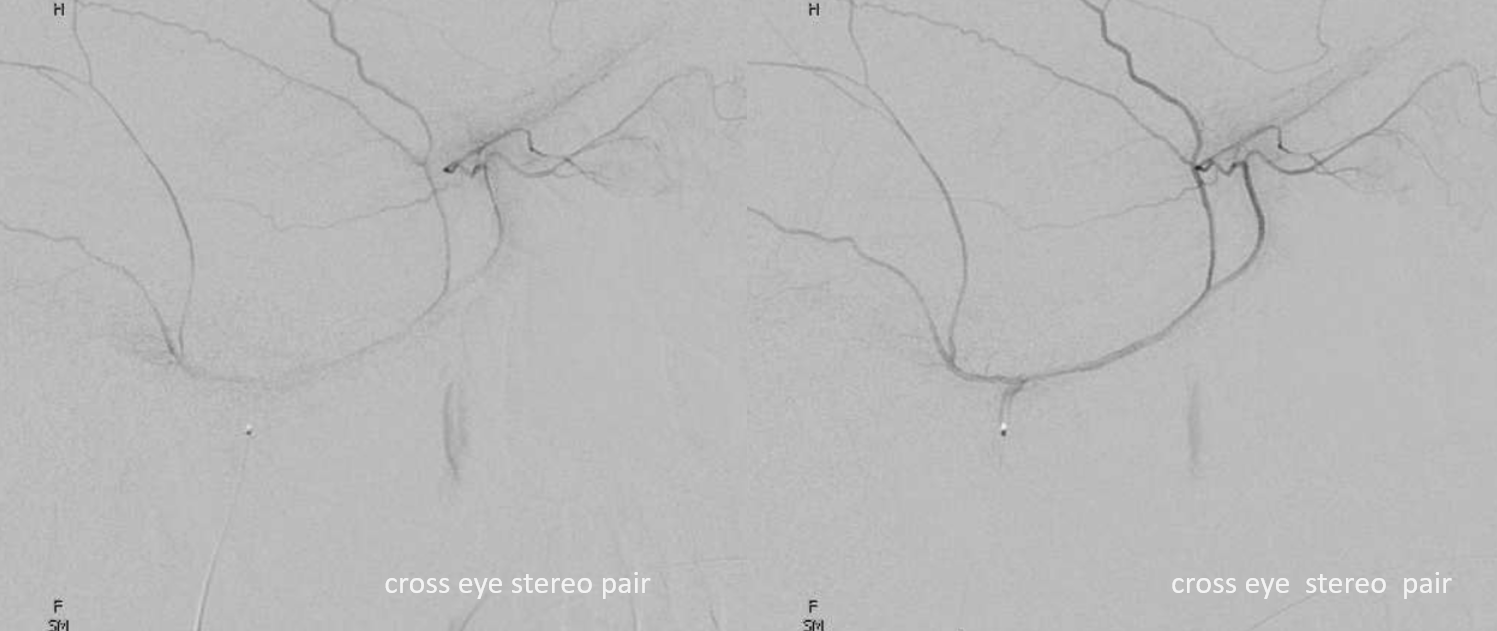
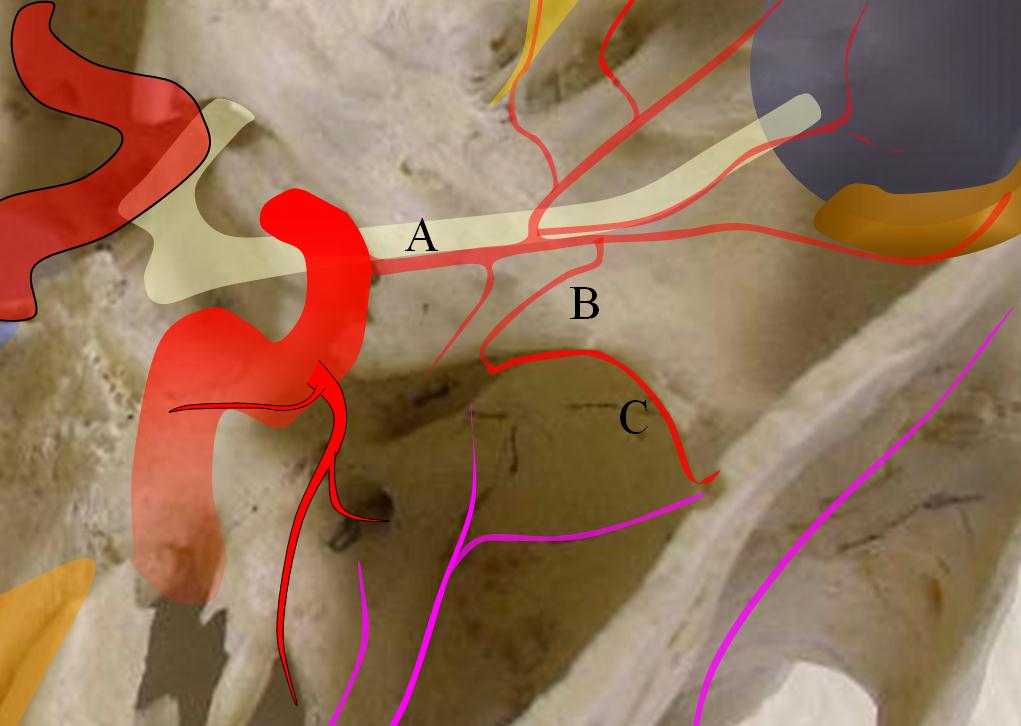
Recurrent Meningeal Artery
The flipside to above is MMA origin from the ophthalmic — it is by far more common than meningo-ophthalmic artery. There are several “sub=variants” and, like anything else, there is a spectrum — sometimes the recurrent meningeal branch only supplies the frontal territory of the dura, with “normal” IMAX origin of small MMA restricted to the temporo-parietal region. All of these variants imply inability to use the MMA for embolization without catheterizing the ophthalmic artery and risking blindness. Below the recurrent meningeal artery (B,C) is shown.
Angiographic example (ophthalmic = orange, recurrent meningeal = red, frontal branch = yellow). Notice that only frontal branch is supplied — a very common theme.
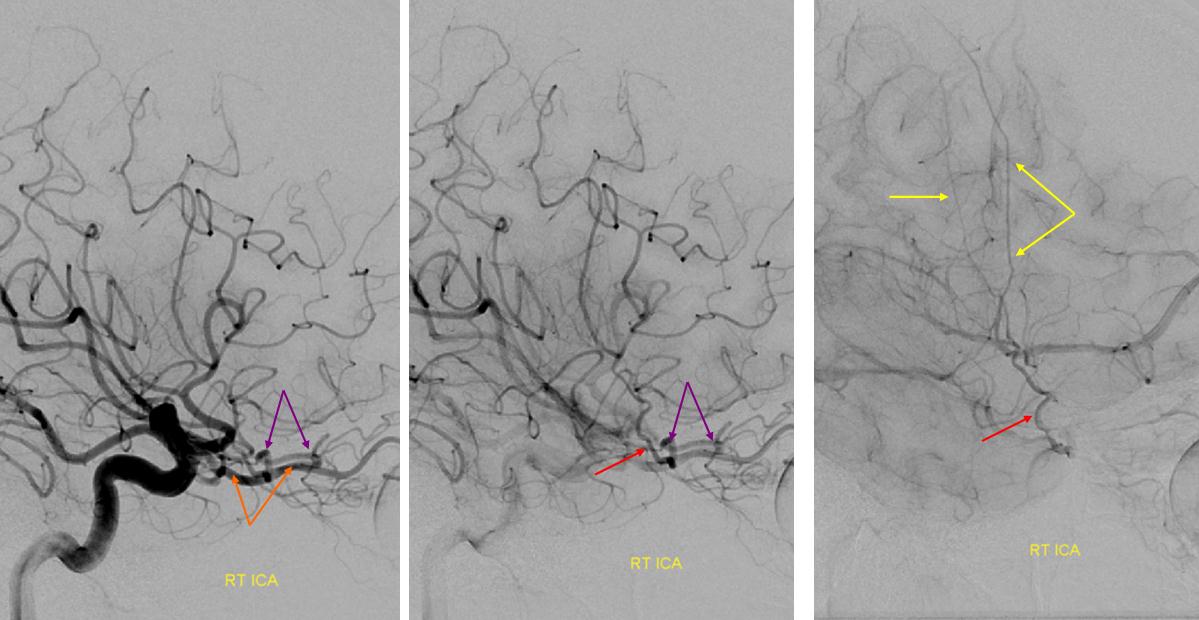
Recurrent Meningeal Artery 2 — another example of another “partial” recurrent meningeal with supply of frontal territory (white arrows) only. Parietal branch (black arrows) arises in usual manner. Notice that meningeal branch is best seen in late arterial/parenchymal phases of brain opacification, since its transit time is usually slower than intradural branches
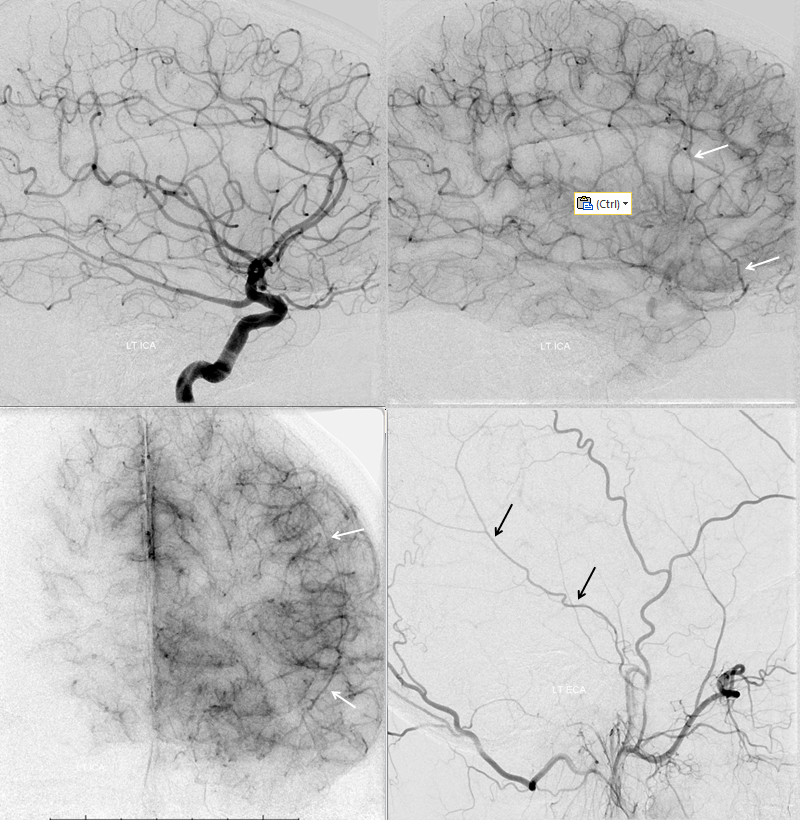
Recurrent Meningeal 3 — “complete” variant with full MMA territory supply. Pink – sphenoidal branch. white – frontal branch. black – parietal branch
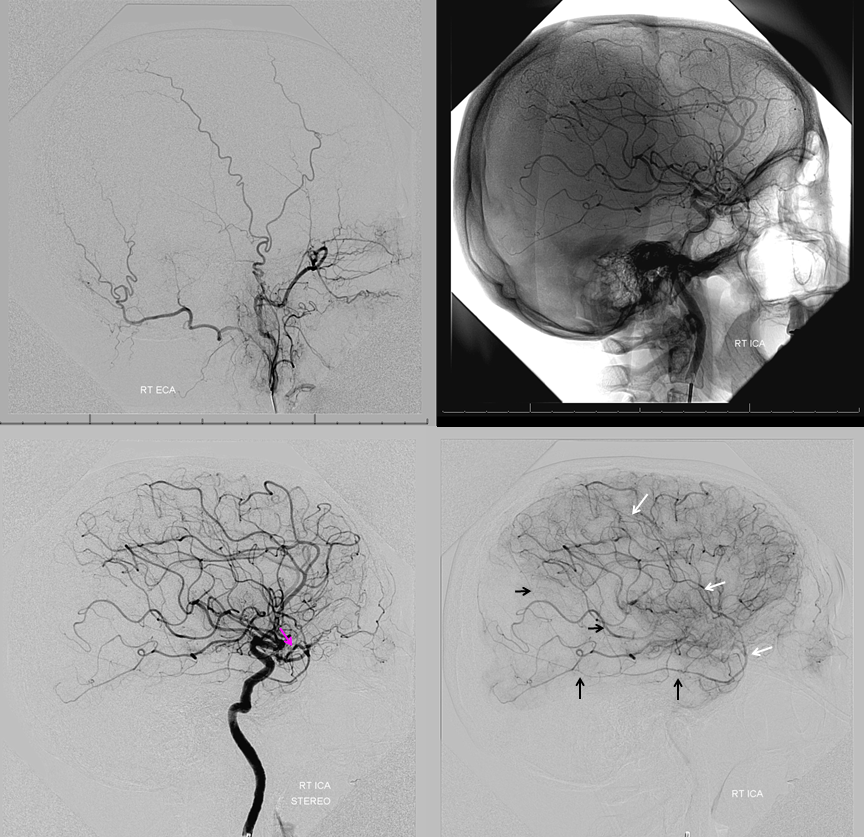
Frontal views
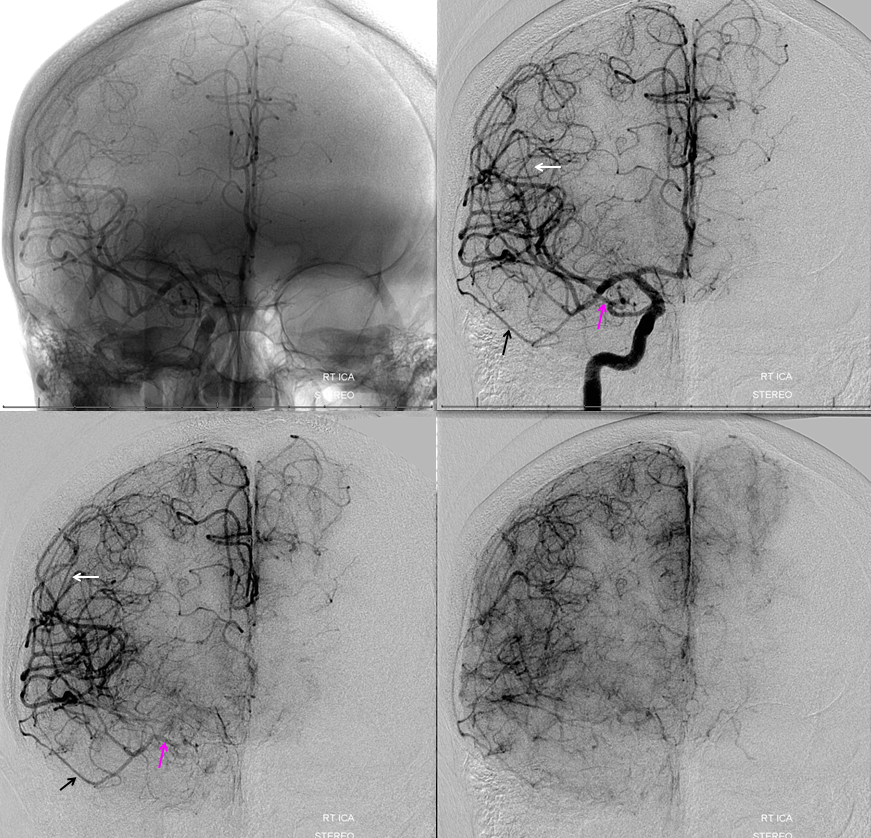
Recurrent Meningeal 4
On head CT, aberrant MMA origin can be inferred by absence of foramen spinosum on left (blue arrow on “usual” right side) The recurrent meningeal artery travels along the sphenoid ridge (orange arrows) and then over the convexity (red arrows). Again, only frontal branch is seen
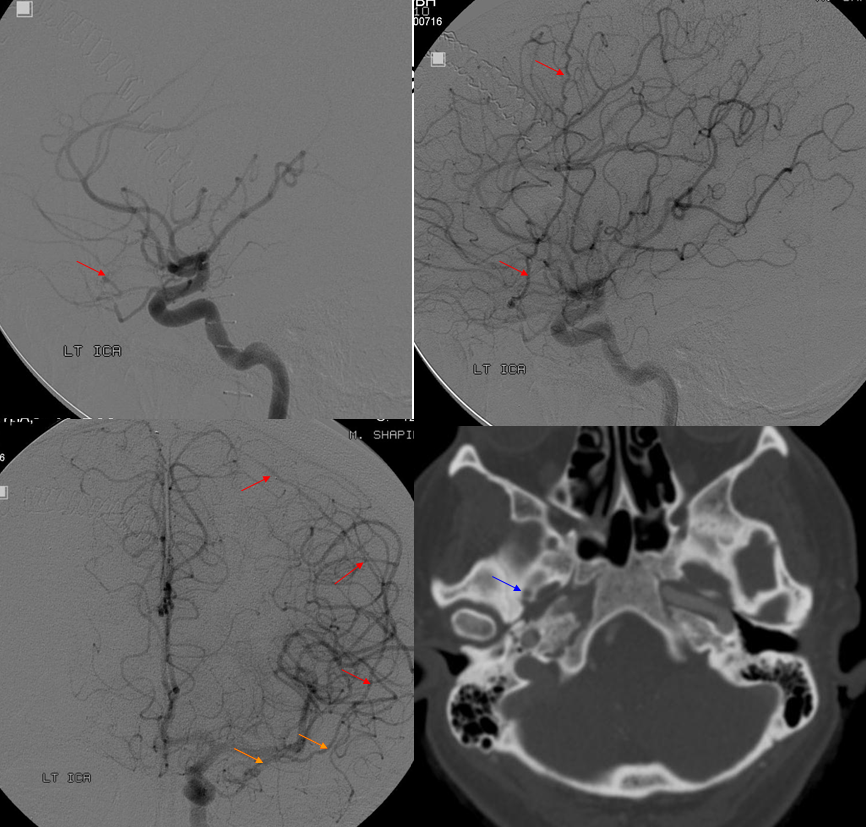
Recurrent Meningeal 5: Illustrating the spectrum principle — on the left, only frontal branch (dashed arrows) of MMA origin is from the ophthalmic, which is fortunate, as the posterior branch (upper right image) supplies a meningioma for preop embo. On the right, the recurrent meningeal supplies entire MMA territory (arrows), with nothing from ECA
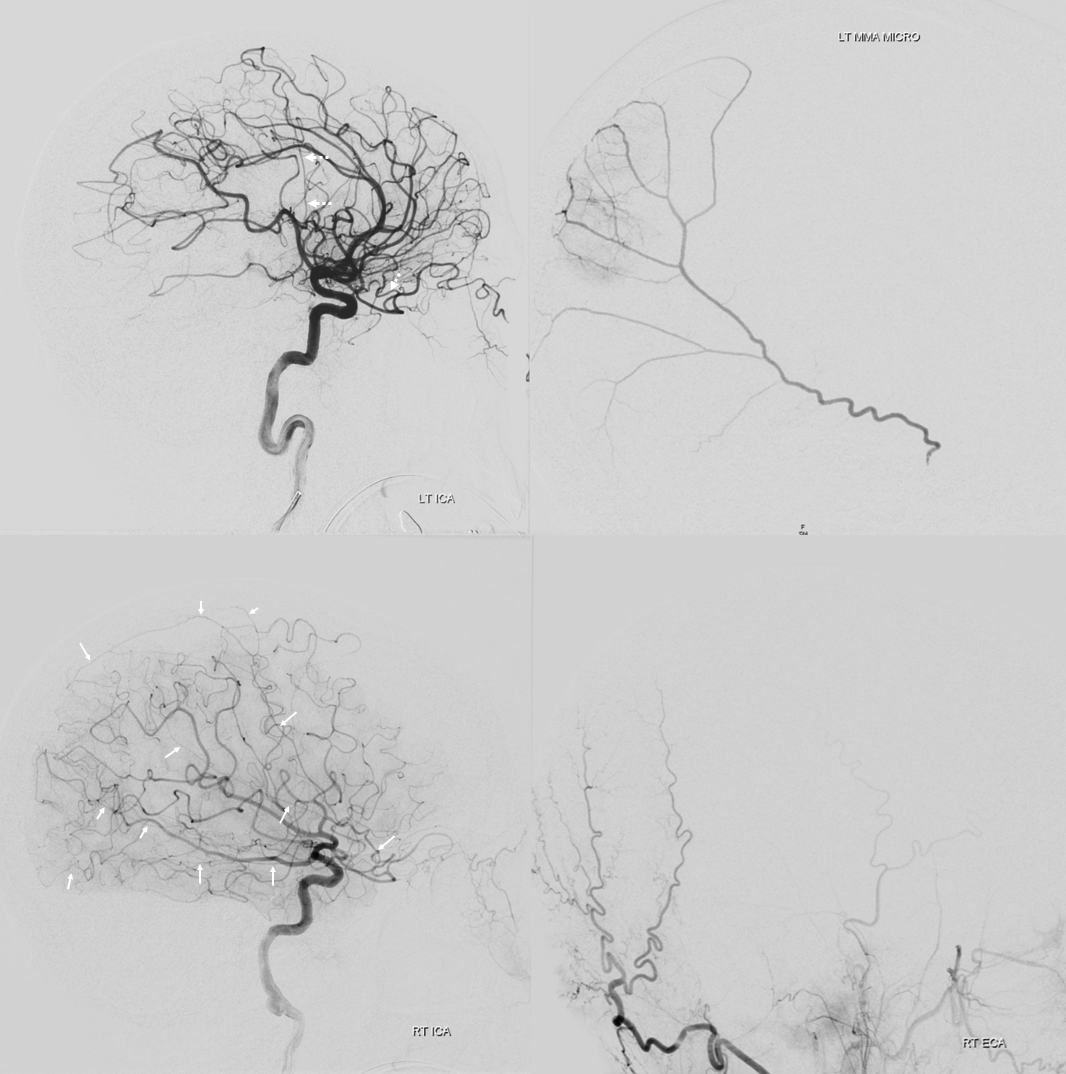
Recurrent Meningeal 6: Clinical Significance — see below case of Traumatic MMA fistula (red) arising from the Recurrent Meningeal Artery (yellow). Full case here
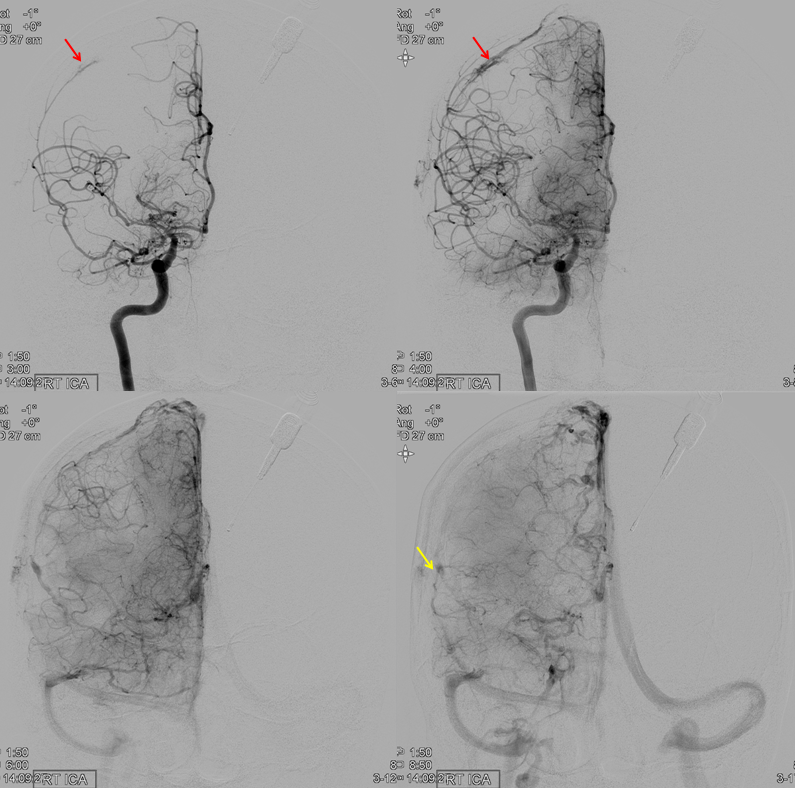
Co-dominance — this patient has a robust connection between “normal” ophthalmic artery and MMA. Like any ICA reconstitution by ophthalmic — the connections are always there. Here is a “normal” origin ophthalmic (red), giving off ethmoid brach (pink) with choroid blush of globe in venous phase (purple)
External carotid injection, lateral views, shows sphenoid branch connection with the ophthalmic artery and is branches (same arrow colors)
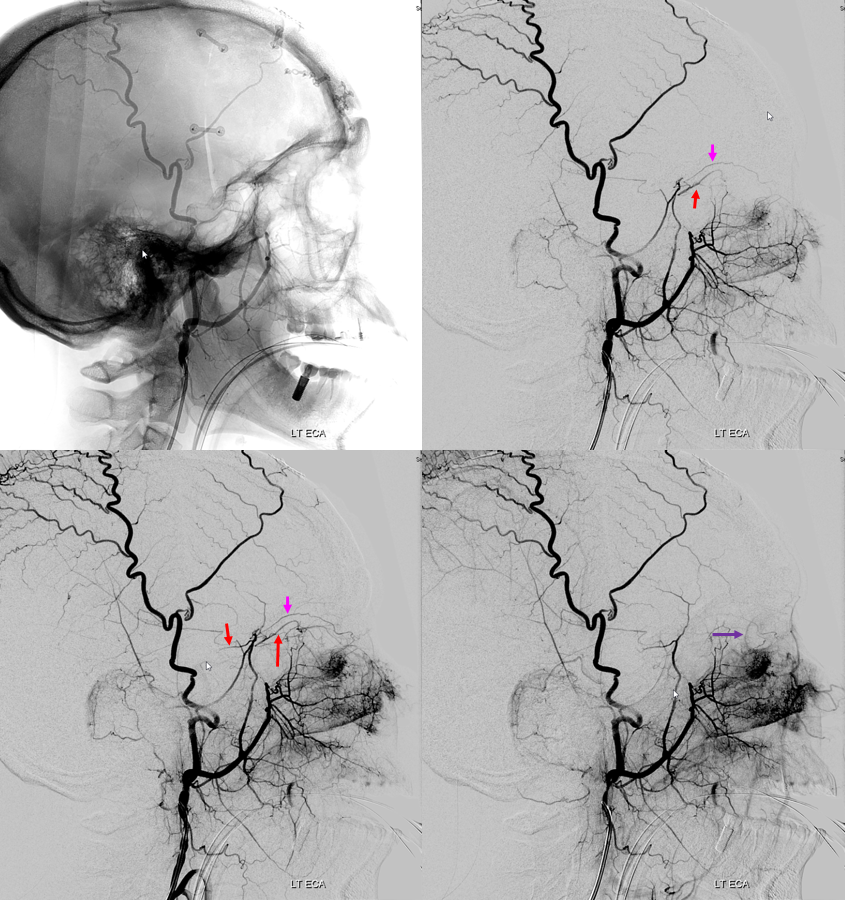
Frontal views (white arrow – sphenoid branch, yellow arrow — lacrimal branch)
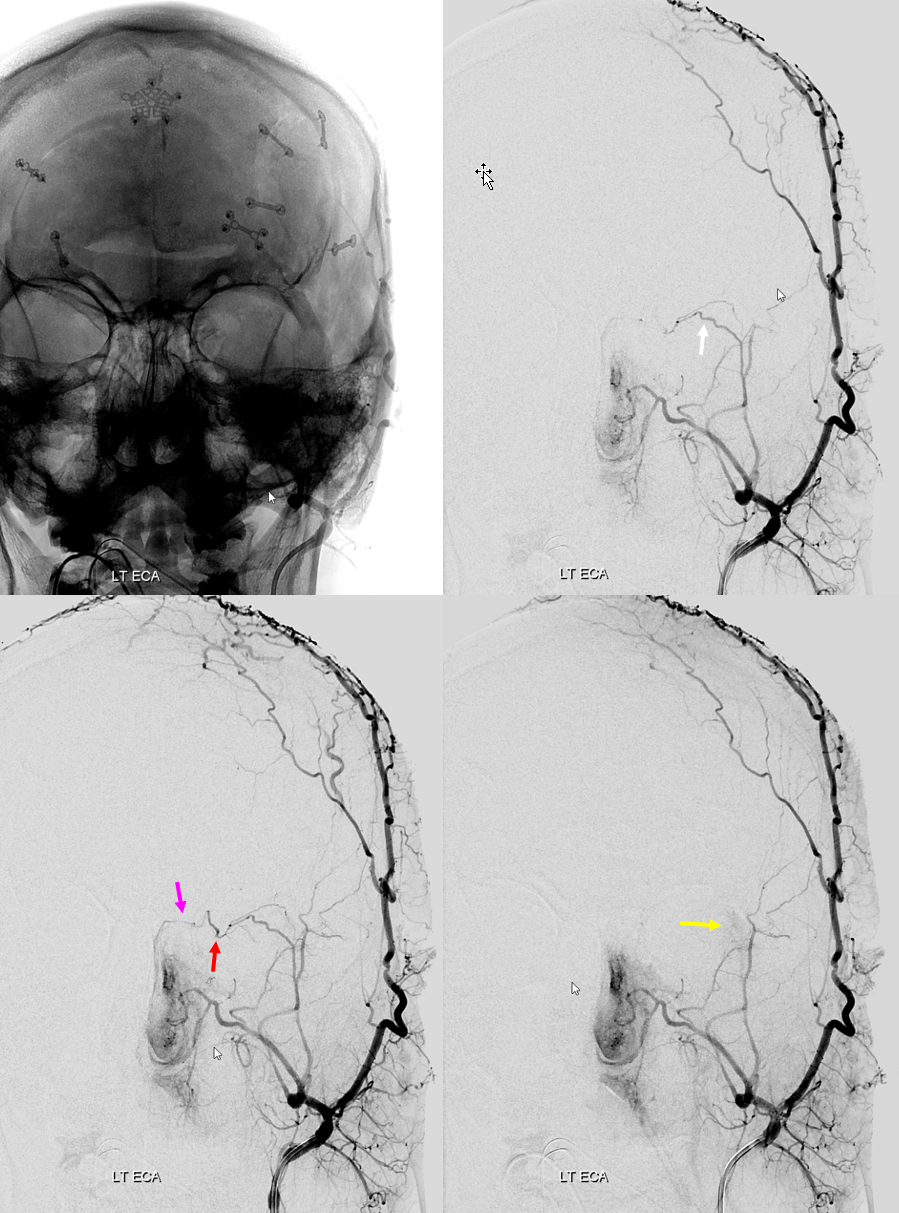
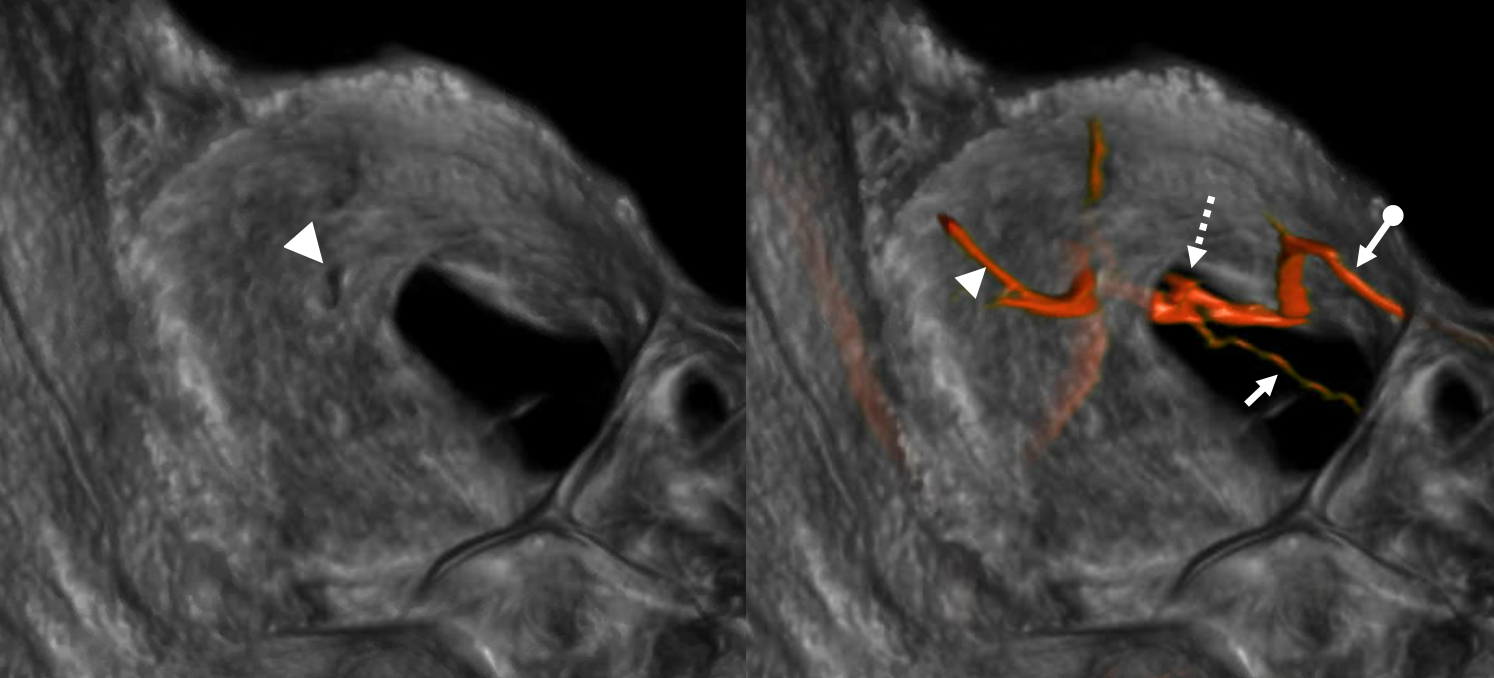
Functional Example — Occult Sphenoid Ridge Branch
Here is a great example of how to get into trouble. The anastomoses we know to exist can be occult, to be uncovered by changing hemodynamics — like the above example. Here is an MMA embo case. The MMA injection shows no obvious orbital connection
So, no problem here, right? The catheter is in the anterior division of the MMA, but not above the superior orbital rim — which is a marker of good orbital safety. Here is an MMA injection. Notice how there is already some Onyx material in the branch distally. During the second injection, “suddenly” — there is a heretofore invisible sphenoid ridge branch, heading towards the ophthalmic artery. What happened — the already distally present Onyx increases resistance in MMA vascular bed, with second injection now directed elsewhere — either reflux or some other branch, or rupture the MMA. As it happens, Onyx flows into the sphenoid branch. This was recognized and injection stopped.
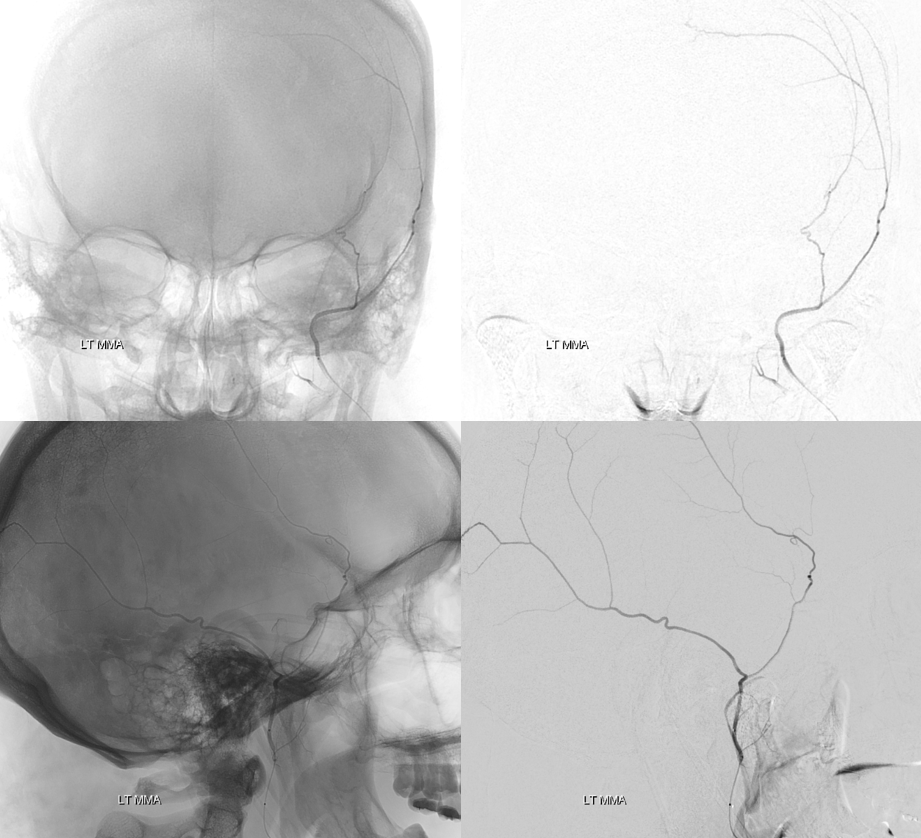
Distal MMA Branches
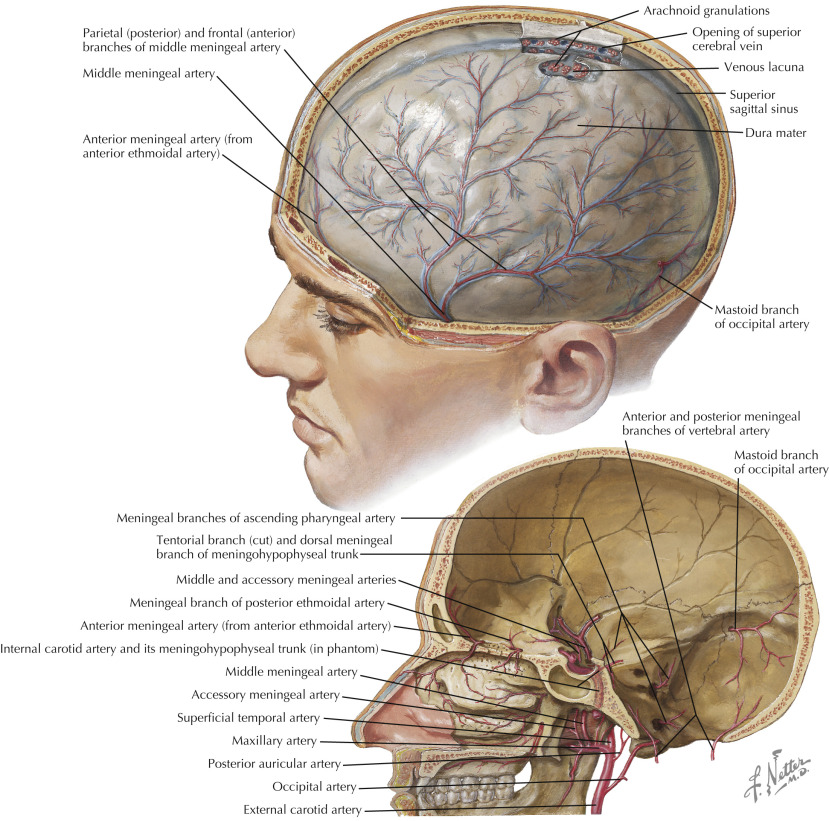
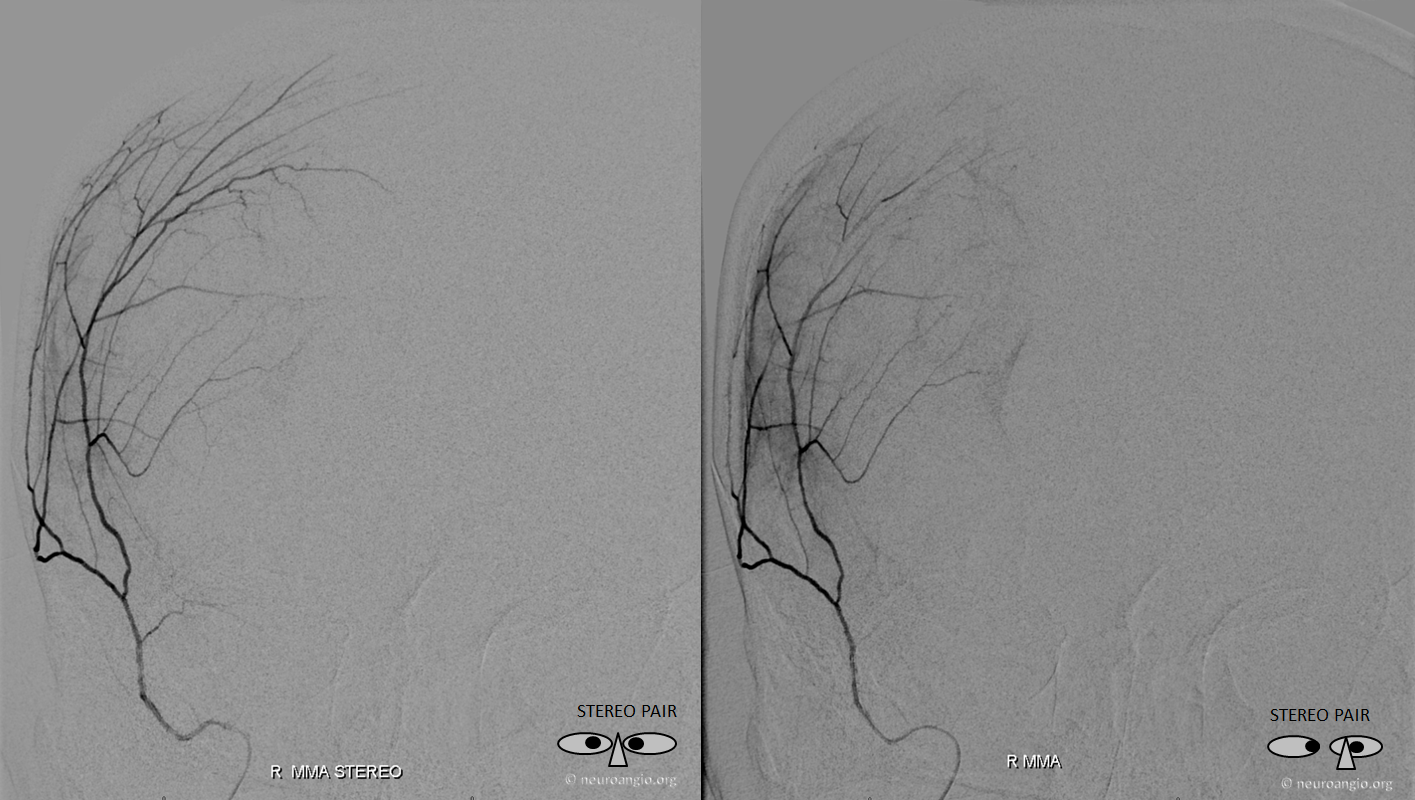
In terms of “dangerous anastomoses”, the most important ones are petrosal and sphenoid branches discussed above. Distal to the sphenoid, there are occasionally important things to consider (antosynagniosis, see below, for example) but generally speaking its very safe territory. If you want to see distal branches of the MMA, look at the inside of dry skull, where MMA makes groovy impressions. Netter has it best — see one of his awesome images below. Notice how the artery travels in the center of the larger vein (tram-tracking). We will get to this later.
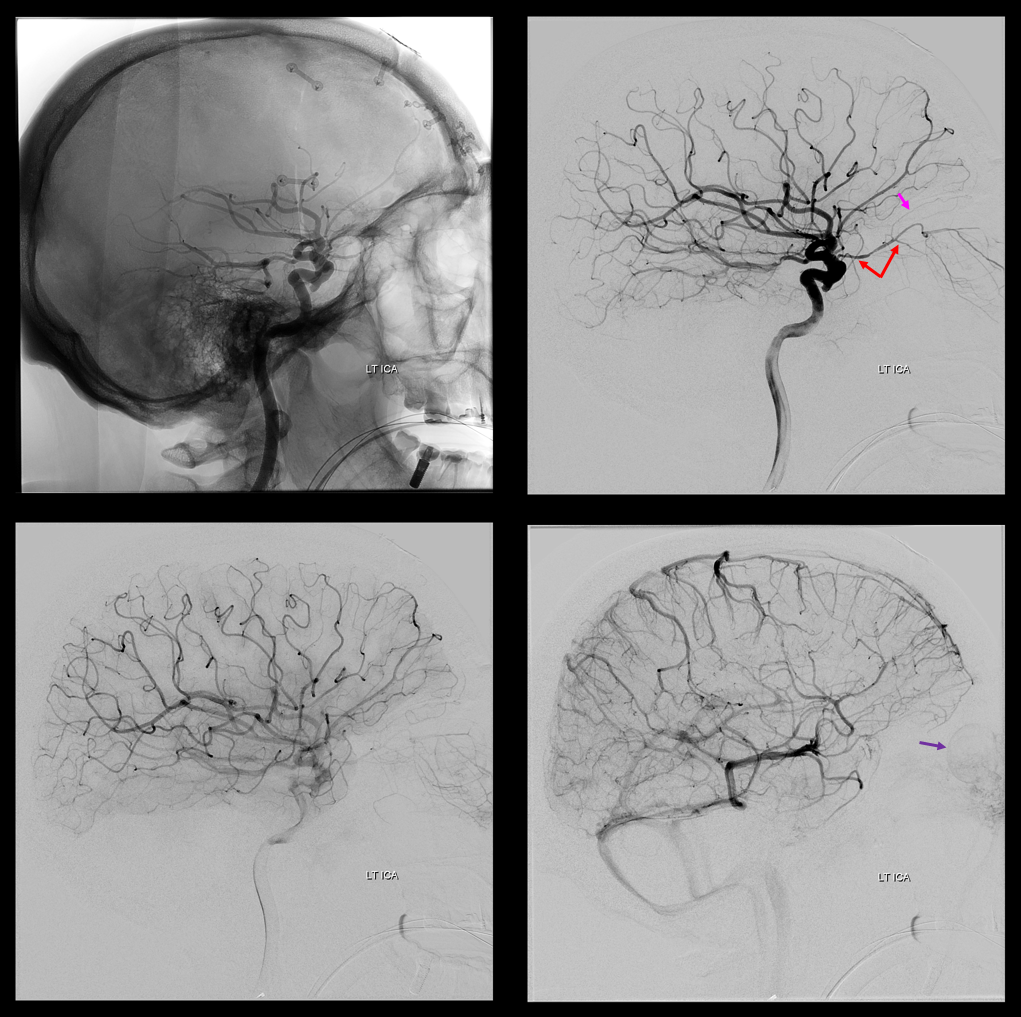
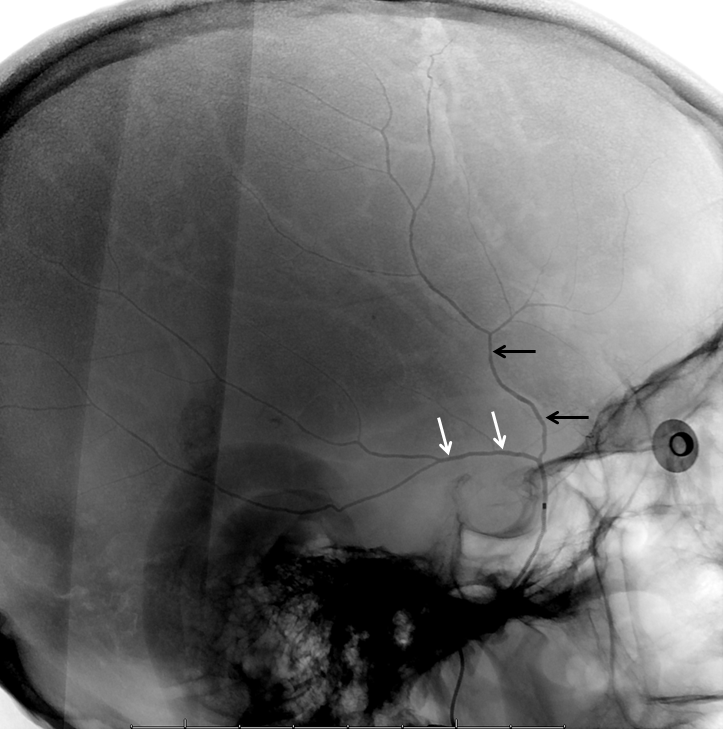
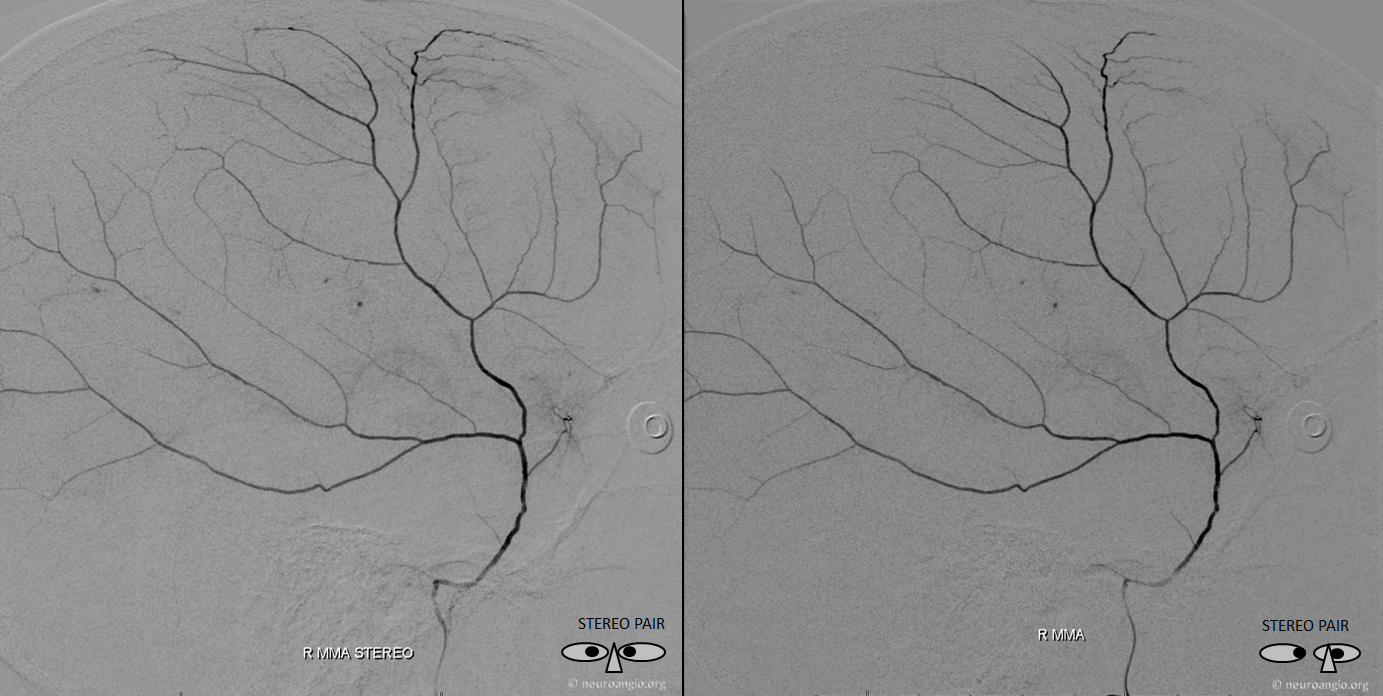
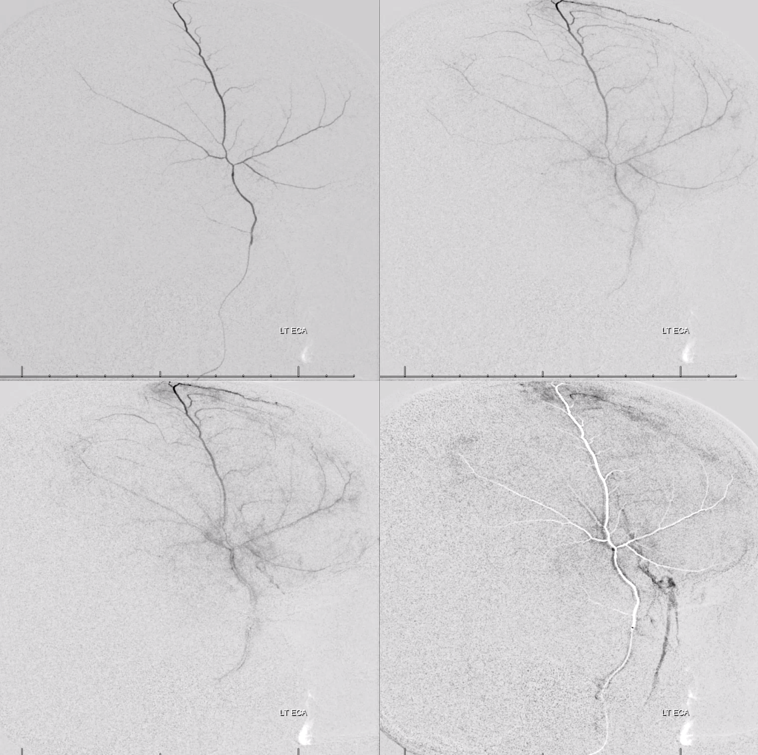
There are two main branches — anterior (frontal) and posterior (parietal). The important thing to understand is that meninges are extremely well vascularized. There are all kinds of connections between anterior, middle, and posterior meningeal arteries, etc. This forms the main part of next important section — the meningeal network. Here is an unsubtracted view of MMA branches, with grooves for them visible on x-ray. They are not the only grooves in the skull– diploic veins make wider, more conspicuous ones. See Diploic Veins page for more info. Frontal branch is black arrow, parietal is white
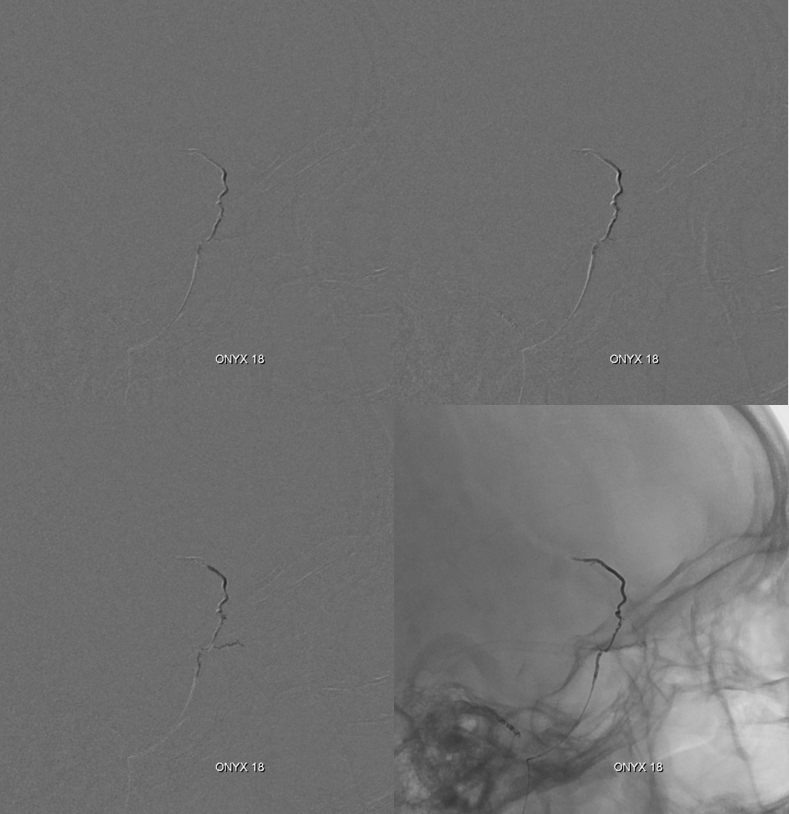
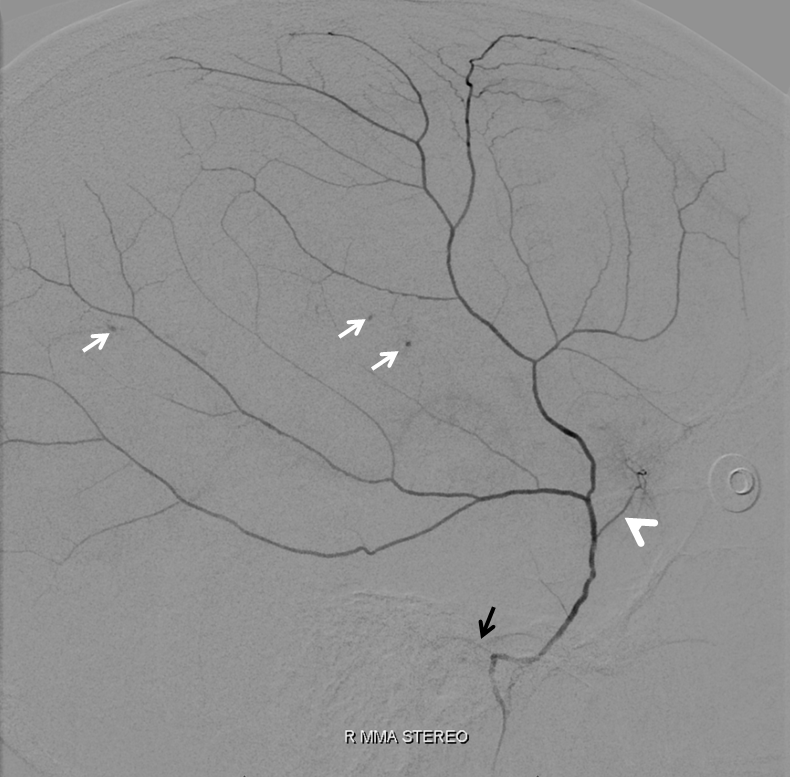
Lateral DSA of MMA in a patient with a subdural hematoma, where the vascular network is particularly obvious, including several areas of obvious leakage (arrows). Also notice small petrous branch (black arrow) and sphonoid branch (white arrowhead). Better get that microcatheter more distally before taking this MMA down.
Beautiful cross-eye frontal stereo of same patient
Lateral cross-eye stereo
Meningeal Circulation — Meningeal Venous Phase and Veins
Almost nothing is ever said about meningeal veins in the literature. Veins get the diss in general, but for the meningeal system the shaft is particularly bad. And chances are they are just as important as veins elsewhere, and ignoring them is just as silly.
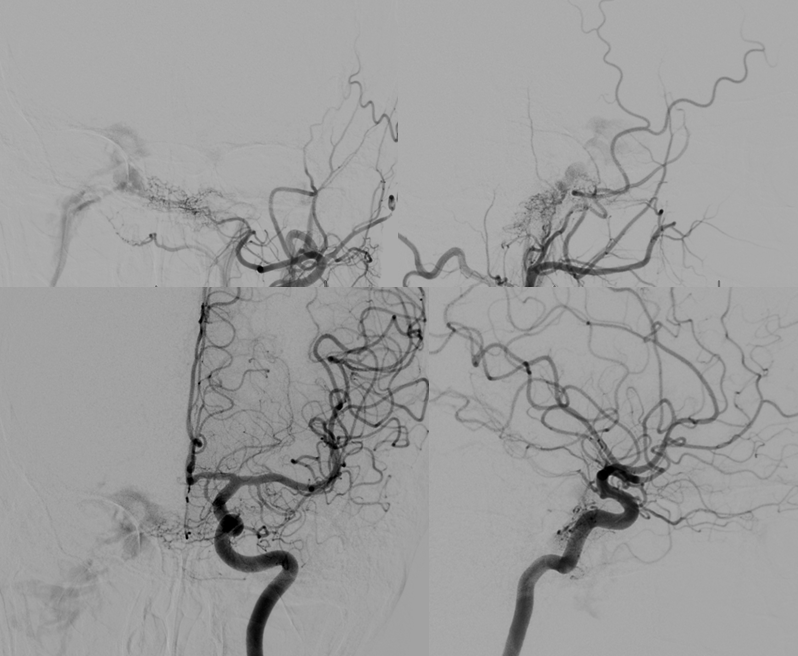
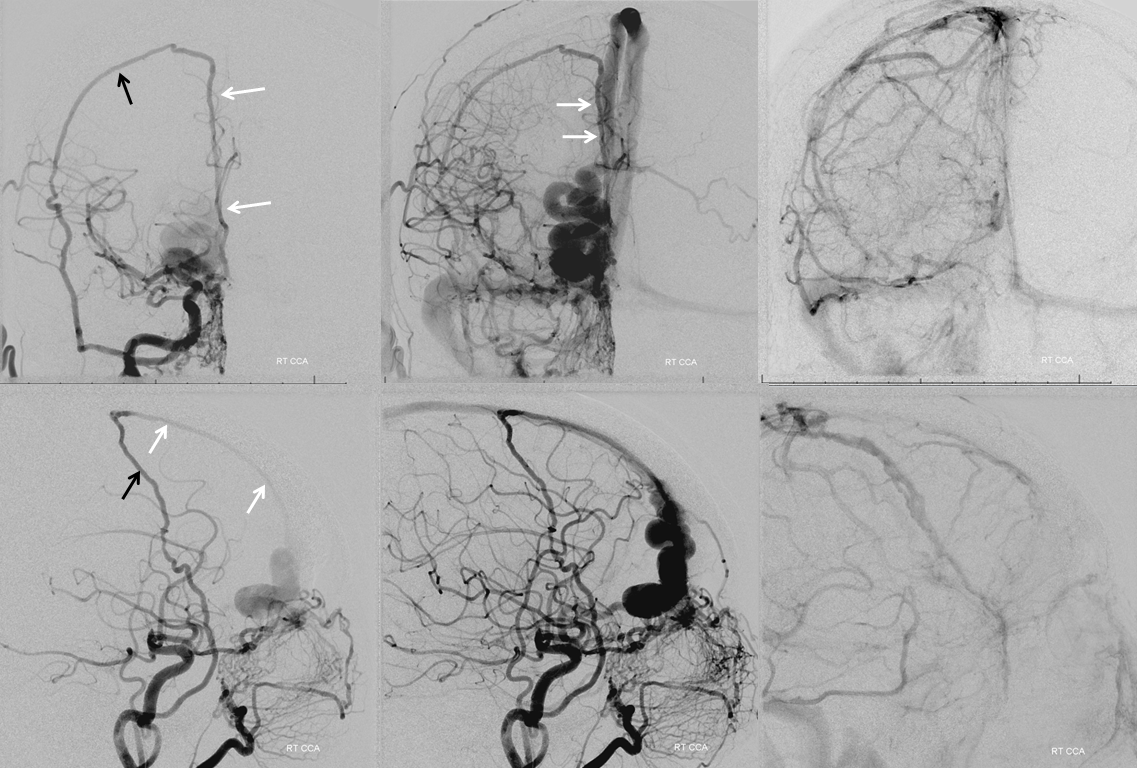
Below is a representative arteriovenous injection sequence, frontal and lateral views, in a patient presenting for MMA embo for subdural hematoma. The catheter is located in the frontoparietal division. Notice connection with the anterior meningeal/SSS frontal branch (white arrows), the characteristic tramtrack appearance of the middle meningeal vein (dashed arrows) and additional drainage of the meninges likely via an emissary vein towards the deep temporal space (open arrows). Not all meninges drain into the middle meningeal veins — some do, while others find other routes. Notice the characteristic hypervascularity of the meninges in the subdural hematoma state. The double=mask technique in the right lower lateral image superimposes white arterial phase on the dark venous phase.
Same image, now arrows
Same patient, with catheter in the petrosquamosal branch. Here, contrast extends transiently towards the midline of the proximal superior sagittal sinus branch (black arrow). Venous phase shows drainage of the dural into the sigmoid sinus.
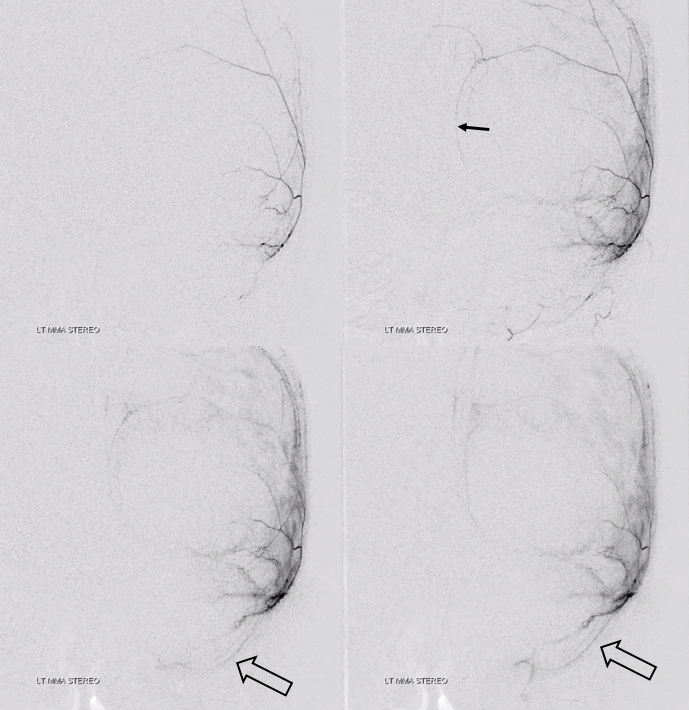
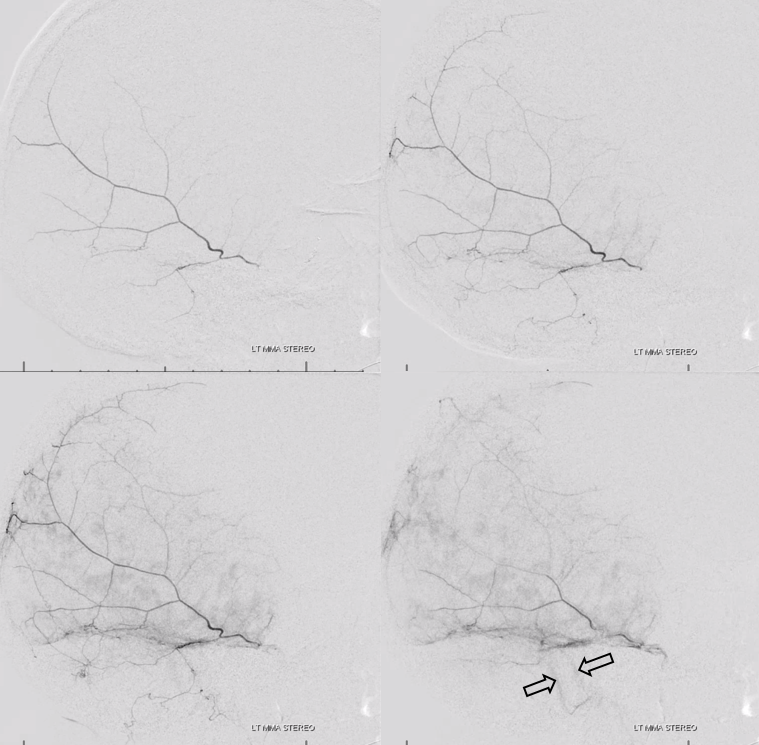
Unsubtracted views. Distal tip is solid arrow, proximal dashed arrow. Notice that despite “proximal” appearance of the distal tip in the lateral view (apparently not yet beyond the expected location of the facial nerve arcade / stylomastoid foramen), the frontal view shows that the distal tip is quite lateral, well beyond origin of the “dangerous” petrous branch — which typically comes off very proximally, very soon after MMA emerges from spinosum. The frontoparietal branch has already been embolized with particles and closed with a coil. Notice post-embolization contrast stasis in the subdural collection, outlined by the oval.
Arterial Variants
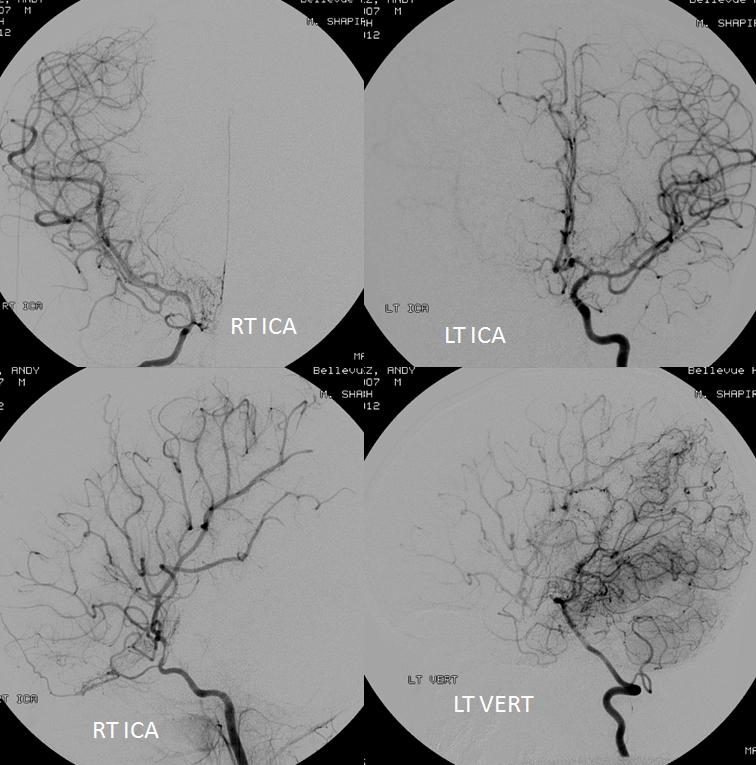
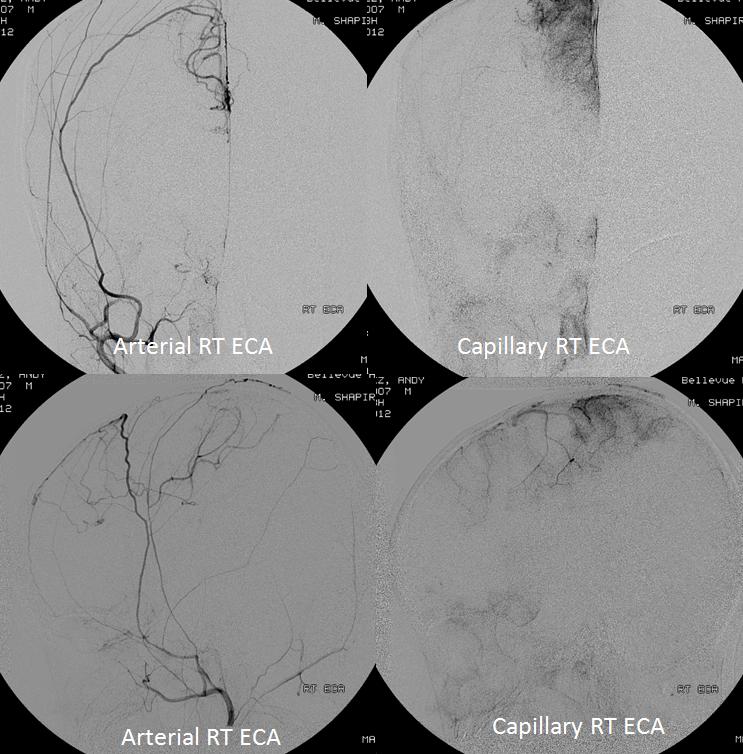
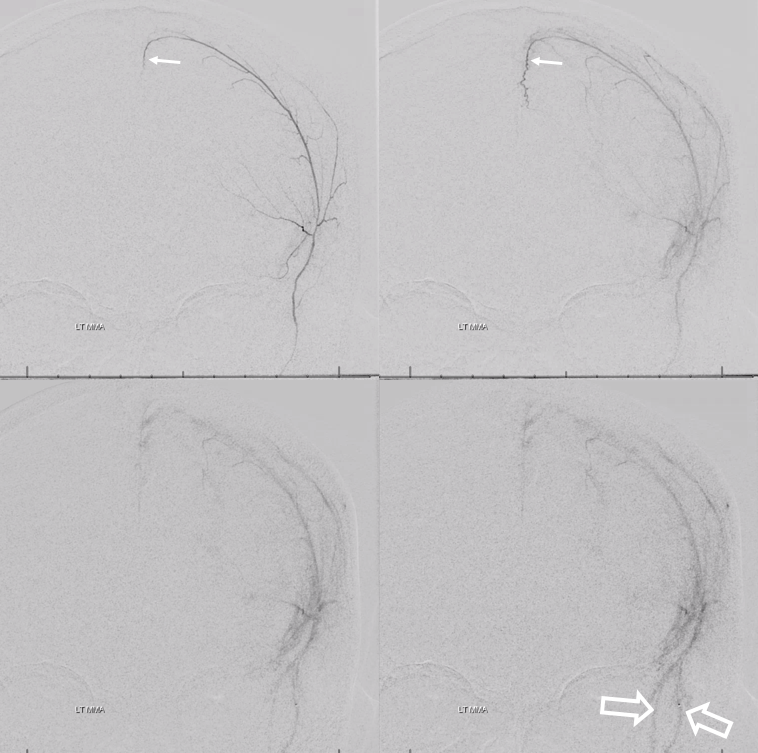
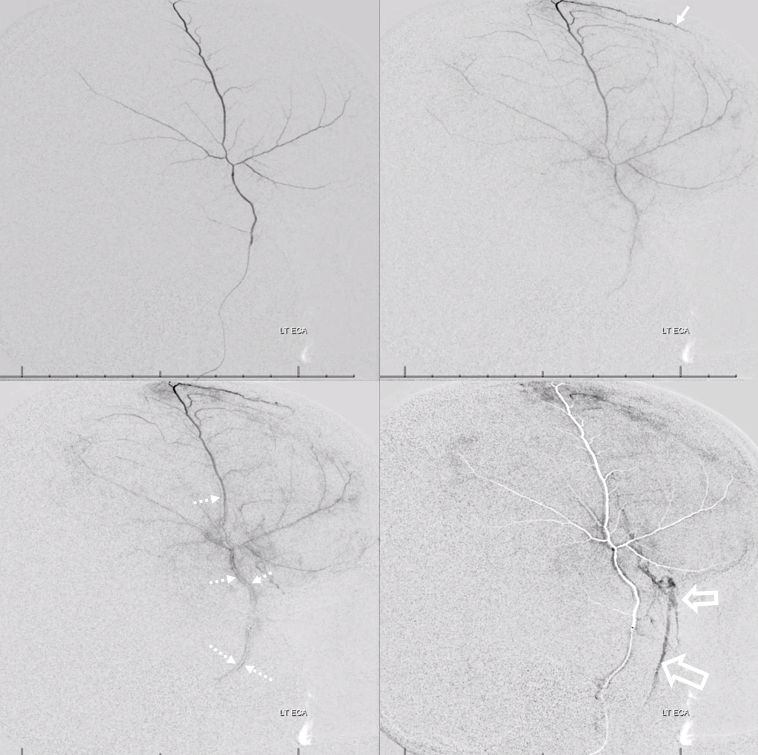
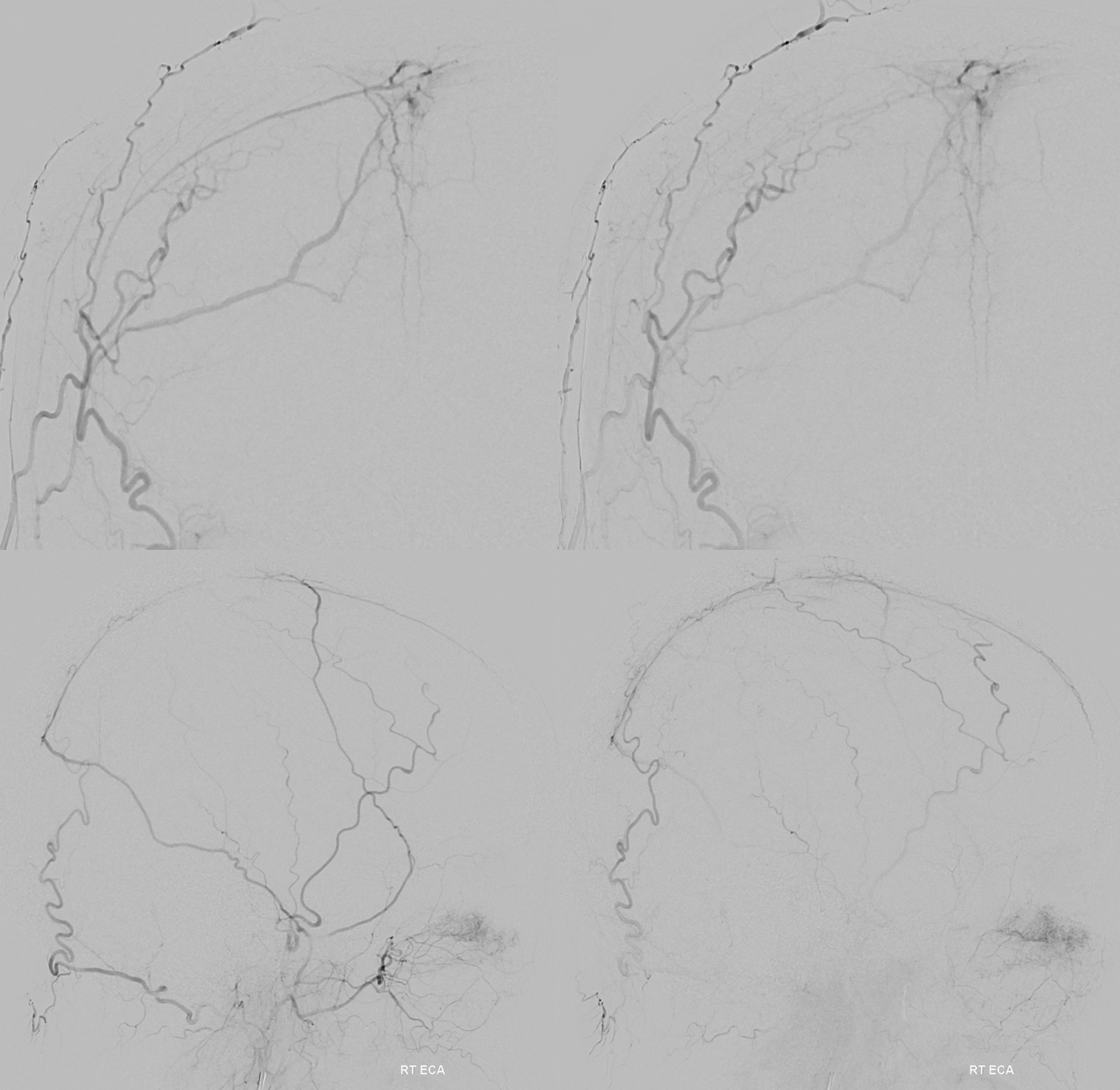
Variants — of course, like anything else, there are variants. Here there is one MMA trunk rather than two. ICA injections shows no additional MMA contribution to frontal territory
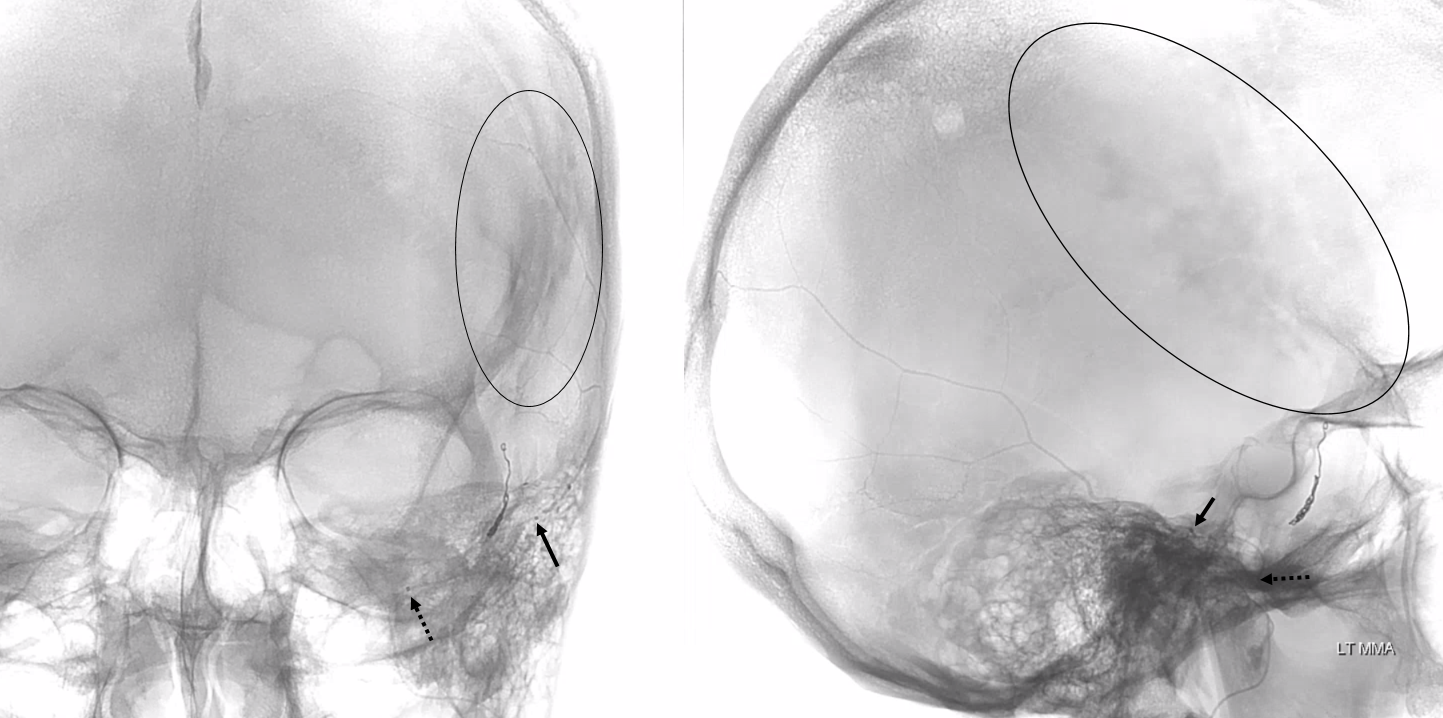
Petrosquamosal Branch — frequent supplier of sigmoid fistulas, and as “safe” an embolization territory as they make ’em
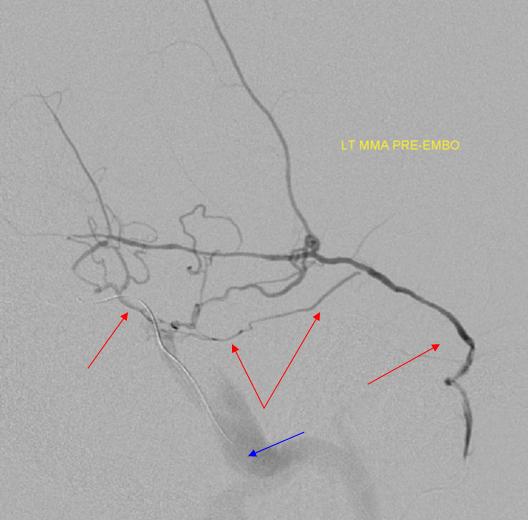
Selective injection of the petrosquamosal branch demonstrates a fistula in the sigmoid sinus region opacifying the sinus (blue arrows). The fistula was embolized with glue from the branch with the leftmost red arrow.
Meningeal vascular network
The key thing to understand is that the dura has huge capacity for vascular augmentation, as shown by myriad vessels seen in dural fistulas. There are important arterial branches in the walls of dural sinuses that are key to understanding dural fistulas, but are heretofore unnamed and get little attention despite having a constant, defined location and important role in regional pathology. They are however very real and seen in all kinds of settings, including normal, fistulas, hypervascular tumors, etc. These are illustrated in the figure below and, for clarity, given names such as “superior sagittal sinus artery”, “transverse sinus artery”, “sigmoid sinus artery”, “straight sinus artery.” Lets see if they stick.

Below are some examples, and links to others, to show how this works.
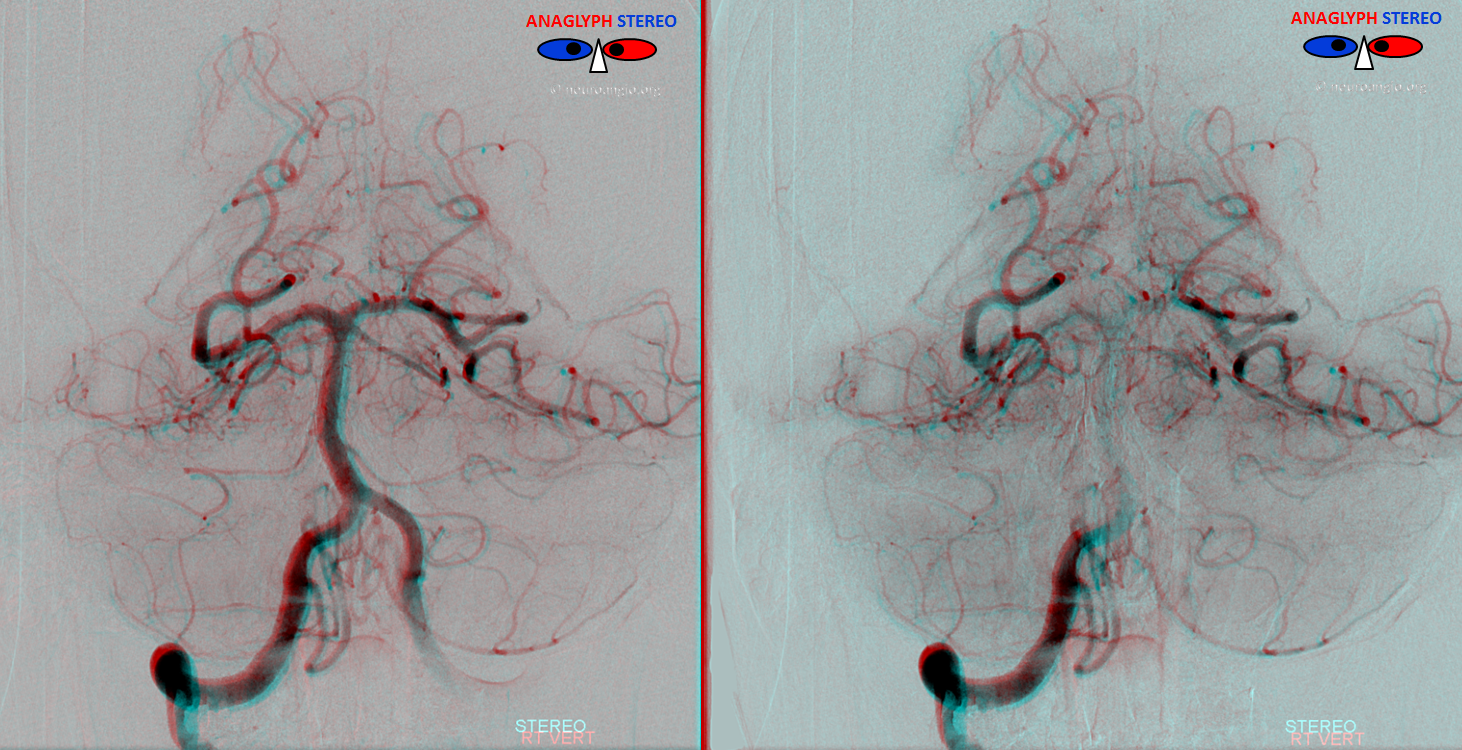
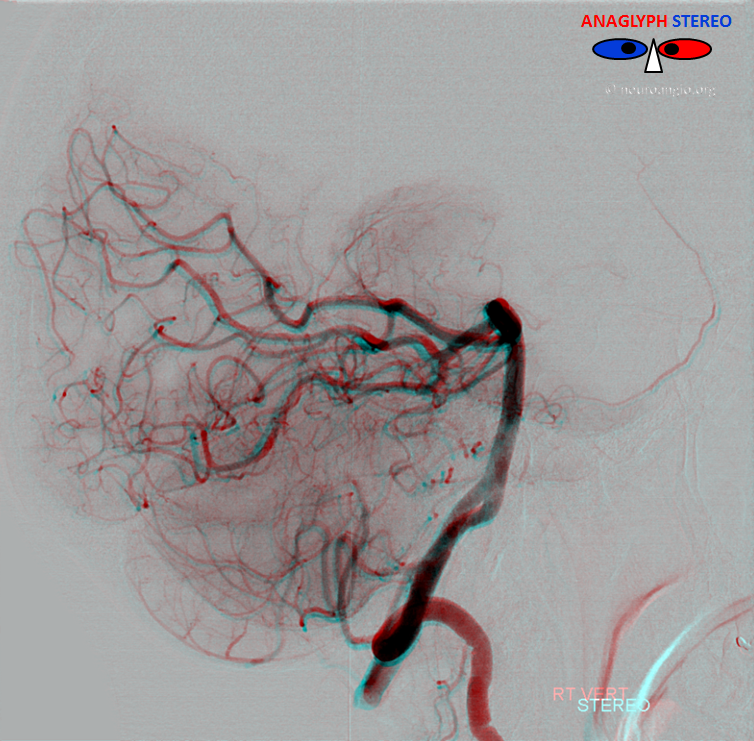
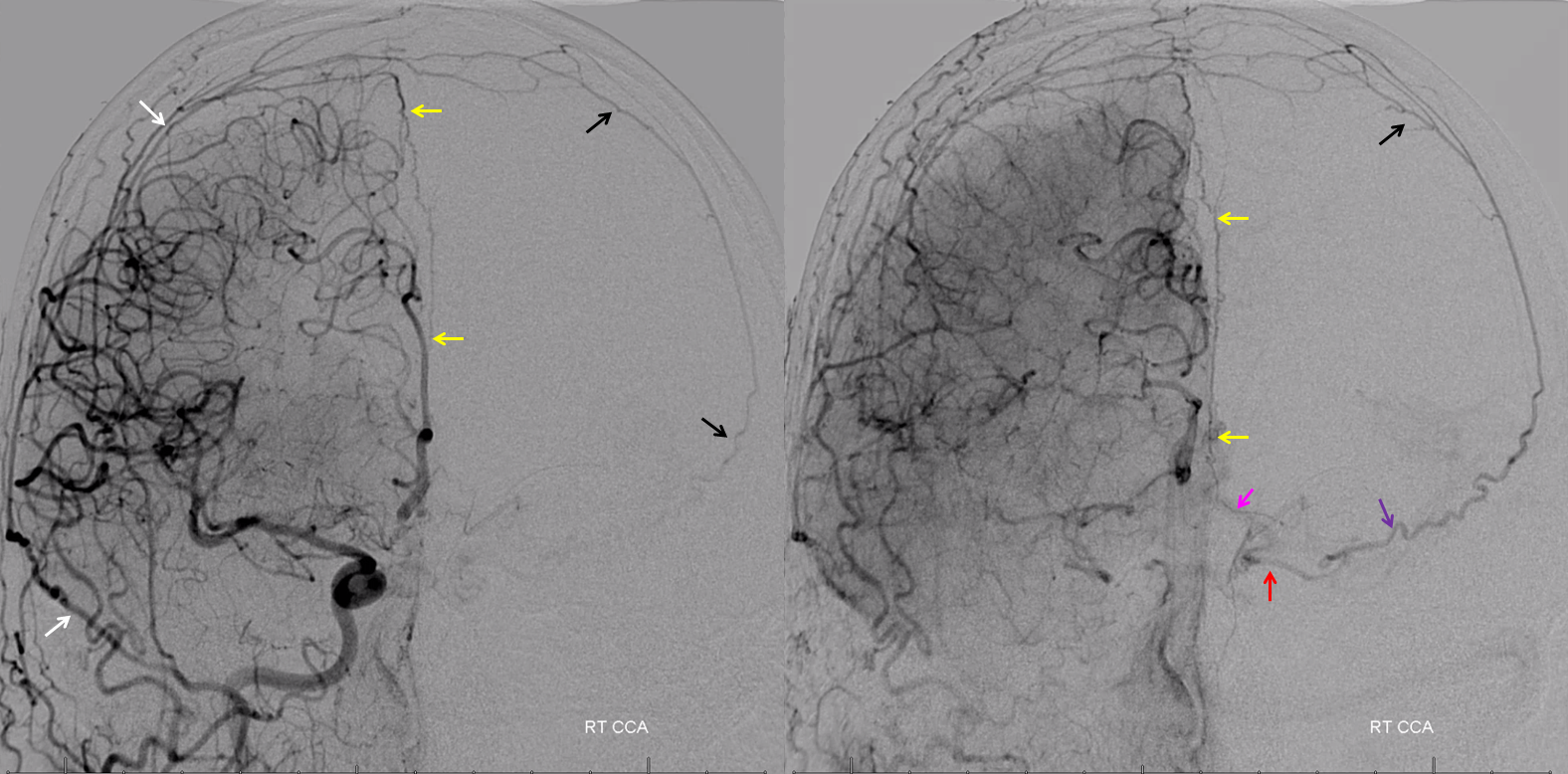
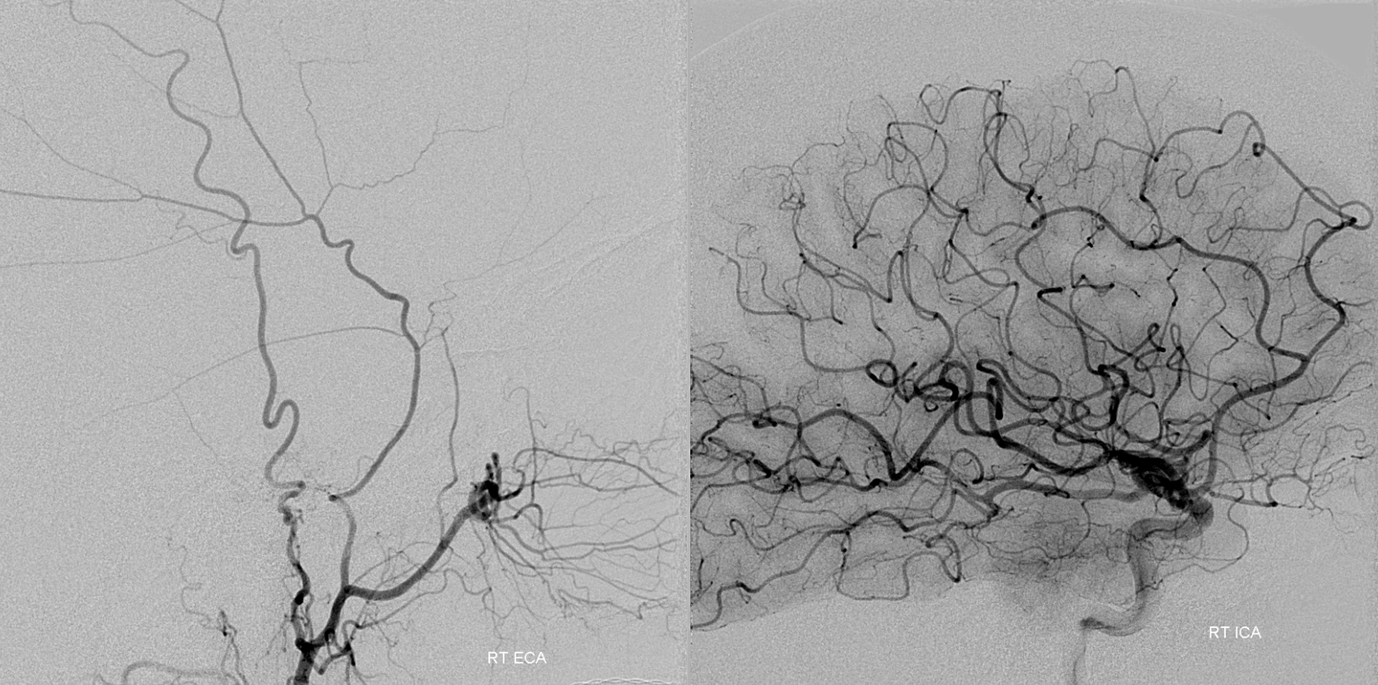
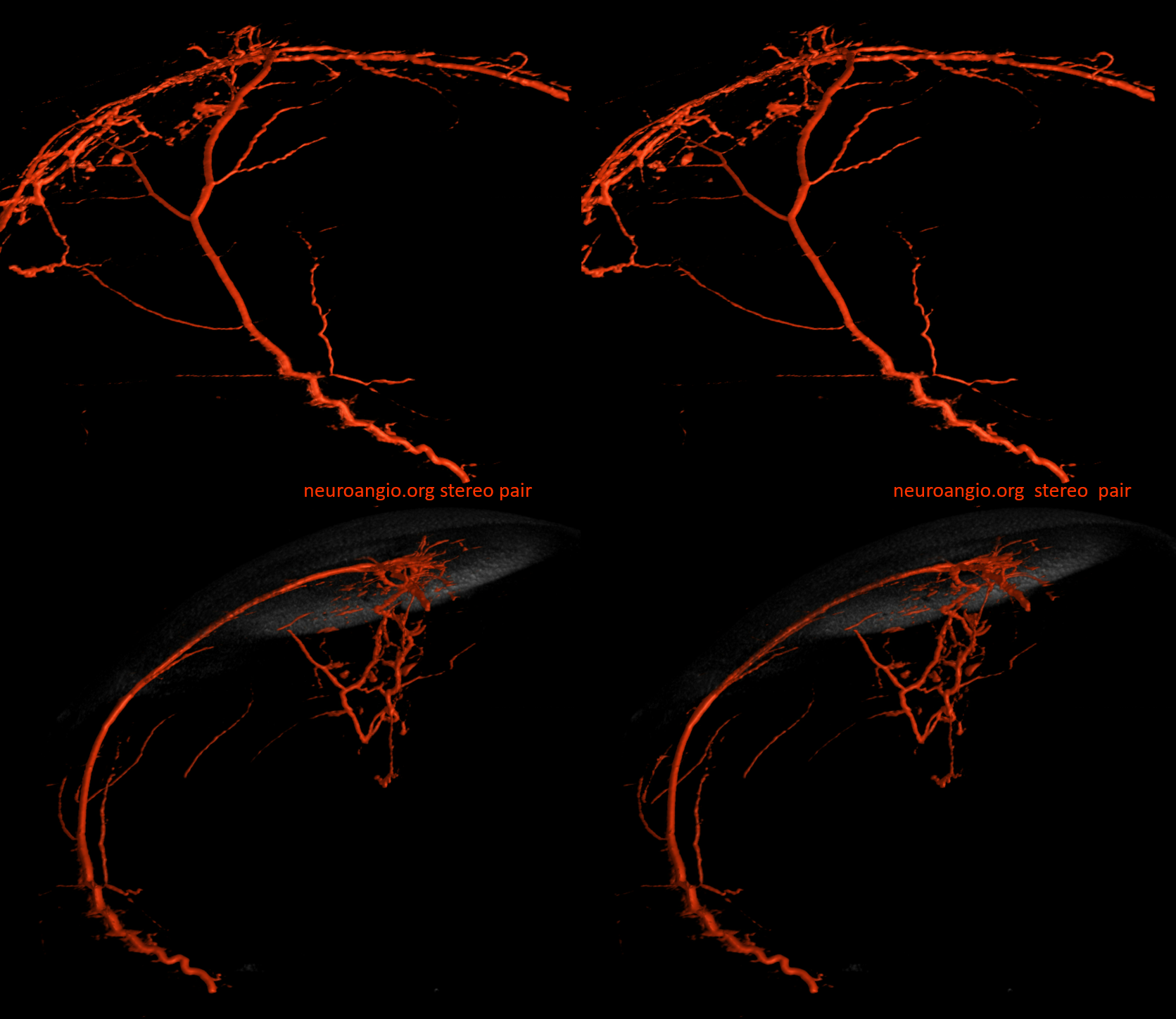
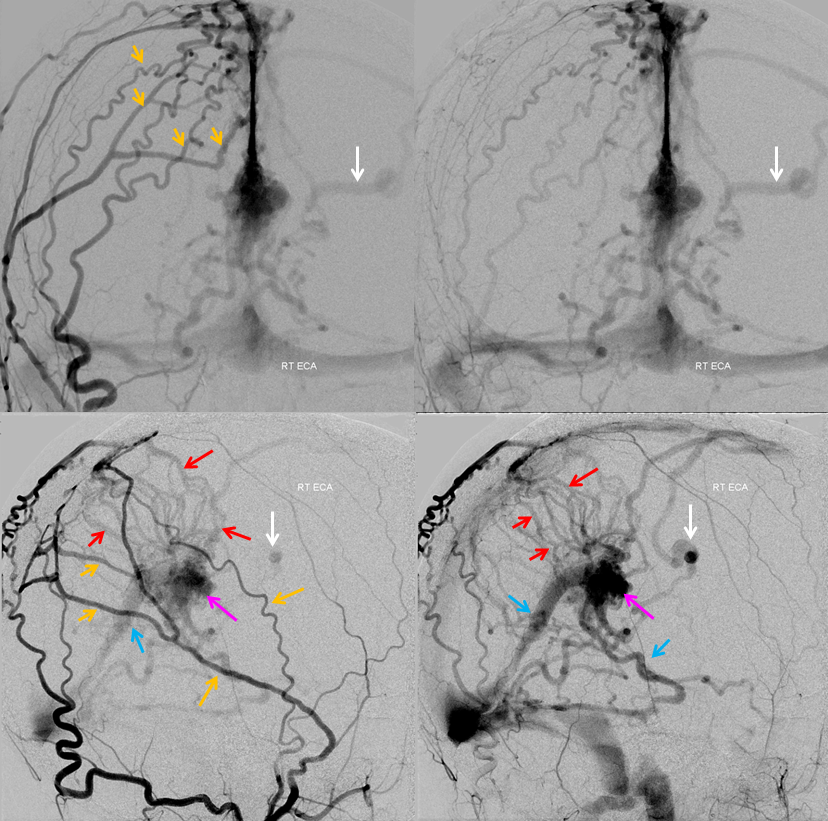
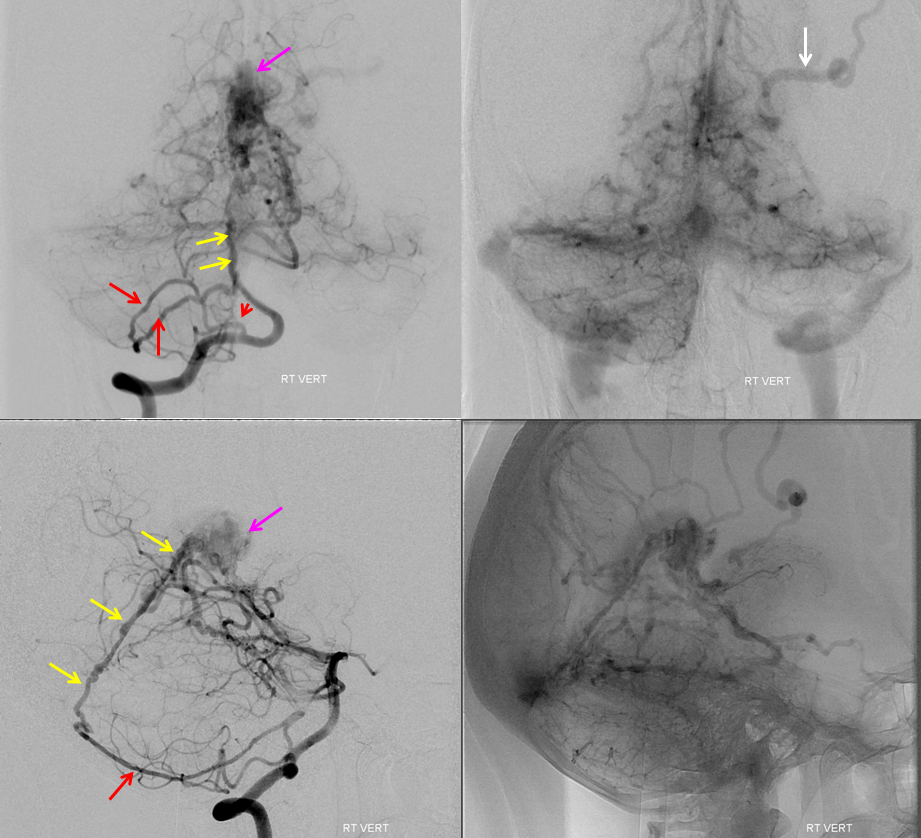
In this patient post bilateral pterional craniotomies with secondary MMA sacrifice, the whole territory fills from posterior meningeal arteries. The artery of the tentorium cerebelli (red) gives rise to the posterior meningeal artery, whose territory has considerably extended due to bilateral MMA sacrifice, demonstrating an impressive meningeal network (yellow arrows)
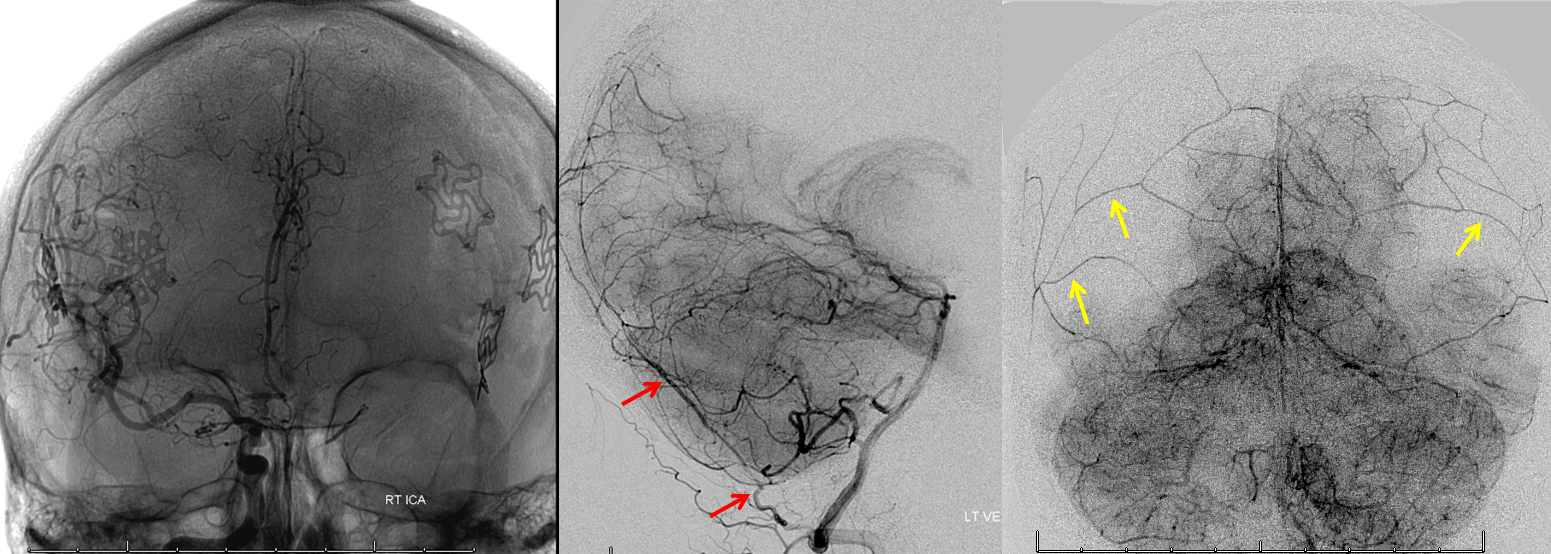
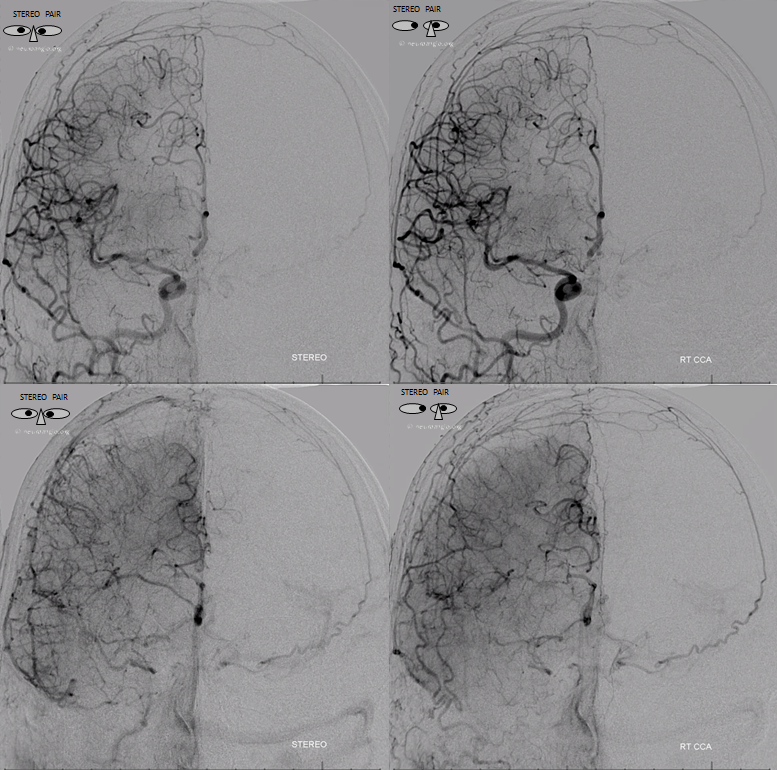
Same, in beautiful stereo
Examples of dural arteries in walls of sinuses
Normal and pathologic cases. Much easier to see when there is a shunt of course. However important to appreciate consistency of this nework in normal states
Ethmoid Fistula supplied by Superior Sagittal Sinus Artery (white), opacified via frontal branch of MMA (black). Full case is here
Sigmoid Sinus Fistula
Pial and posterior meningeal supply to sigmoid sinus fistula (pink) opacifying arteries in wall of the sigmoid sinus (white) and transverse sinus (black). Full case here
Sigmoid Sinus Fistula 2
Another patient with a sigmoid sinus fistula — multiple contralateral posterior meningeal and cutaneous occipital branches converging on Artery of Transverse Sinus (white) leading into Artery of Sigmoid Sinus (black) that forms a common arterial collector opening into the fistula (pink)
Torcular Fistula
Complex Sigmoid / Torcular fistula (top row) with supply to the torcular fistula (pink) via paired Superior Sagittal Sinus Arteries (white arrows) opacified by parietal branches of the MMA (black arrows)
Multiple arteries in wall of the falx cerebri — “falx cerebri arteries” (red arrows) supplying a falcotentorial junction fistula (pink). Full case is here
Same case, with posterior meningeal artery (red) opacifying the artery in wall of the straight sinus — “straight sinus artery” (yellow). Fistula point is pink again.
AVM
Sump from AVM reverses flow in the ophthalmic artery (red) with primary retrograde supply via the sphenoidal artery (orange) branch of the MMA (yellow). As a consequence of AVM reorganization, an autosynangiosis of the mid-sagittal anterior frontal convexity developed, supplied by two Superior Sagittal Sinus Arteries (blue arrows).
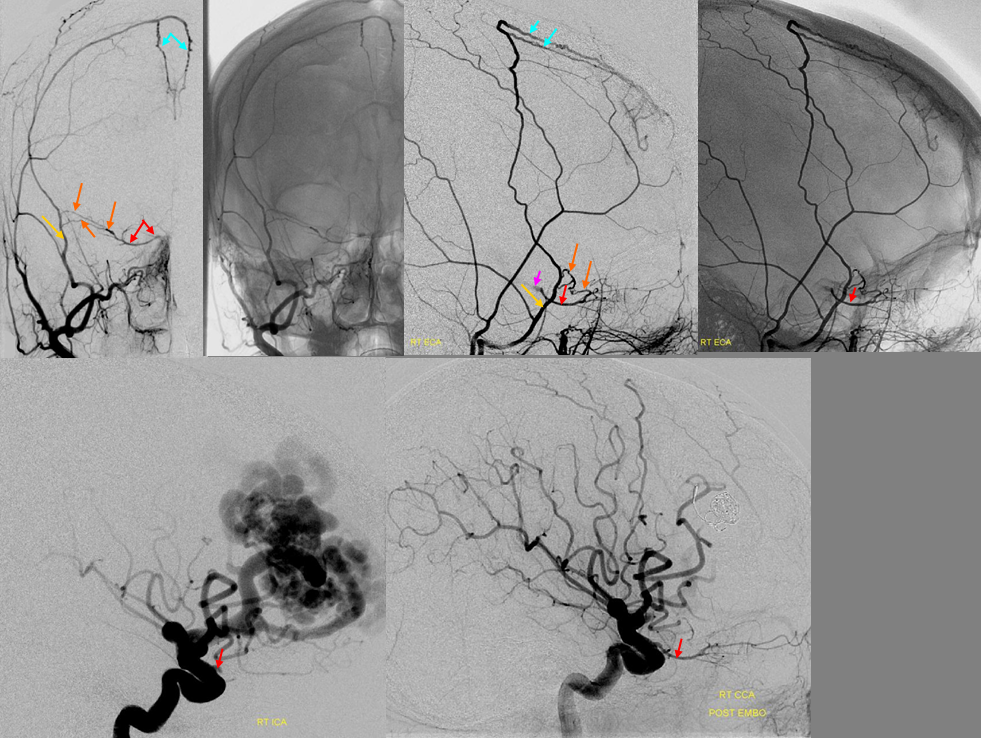
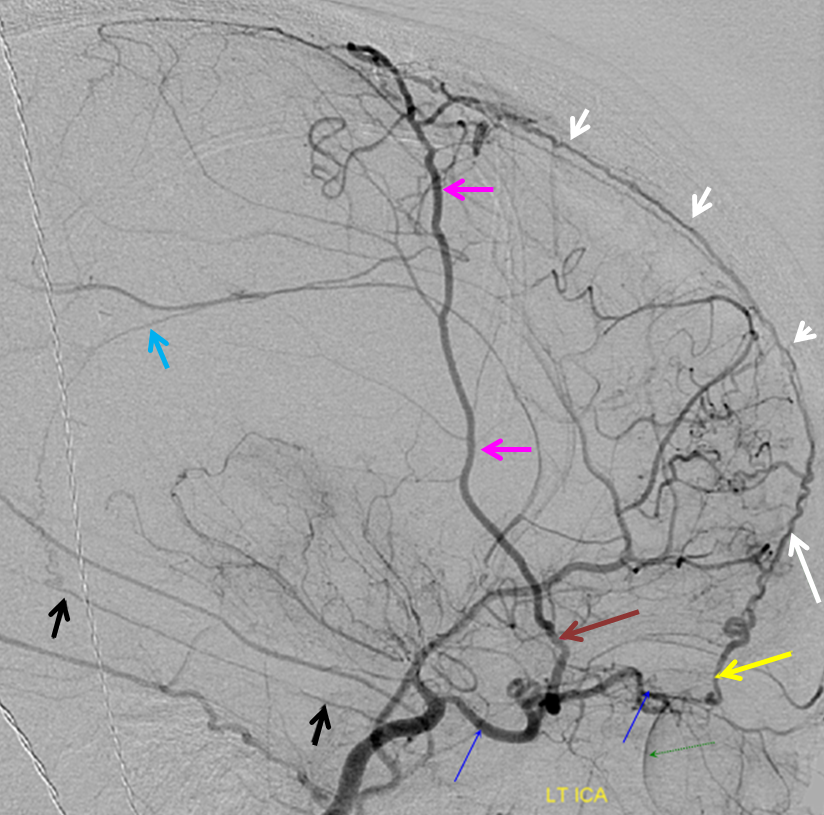
Post-radiation vasculopathy — a sad case of total brain irradiation in a child, treated abroad for a presumed malignancy. Years later there is extensive Moya-Moya like change. There are multiple meningeal autosynangioses, with a prominent anterior meningeal artery (the anterior part of Superior Sagittal Sinus artery — white arrows) forming a network with frontal branch (pink) of MMA (recurrent meningeal variant, sphenoid branch = brown arrow). Also notice medial tentorial (Bernasconi-Cassinari) branch (black arrows) of MHT supplying dural branch in the wall of inferior sagittal sinus (light blue arrow). The separate pericalossal arteries are faintly seen slightly higher
Surgical Dural Synangiosis
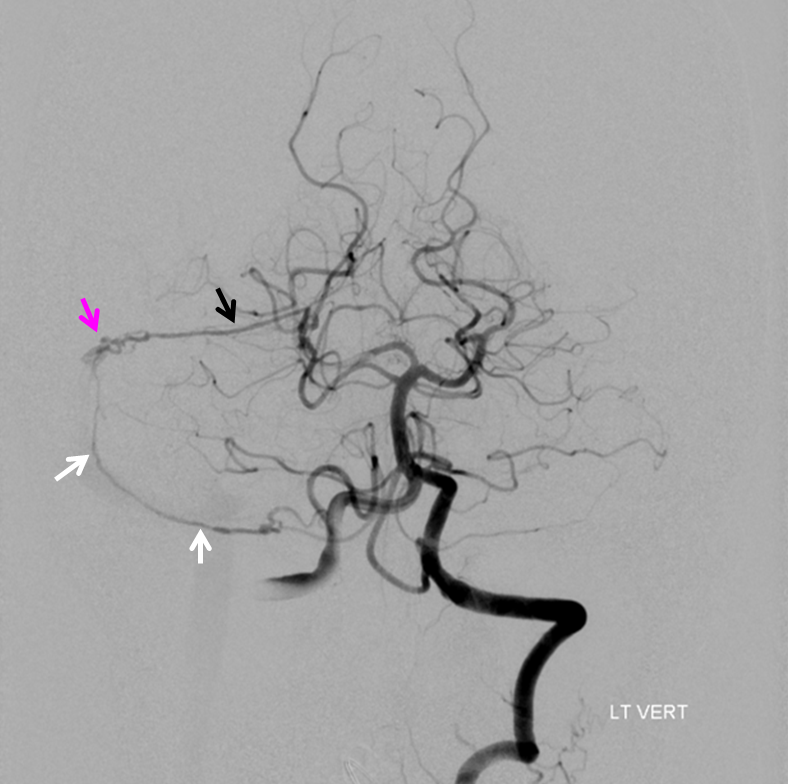
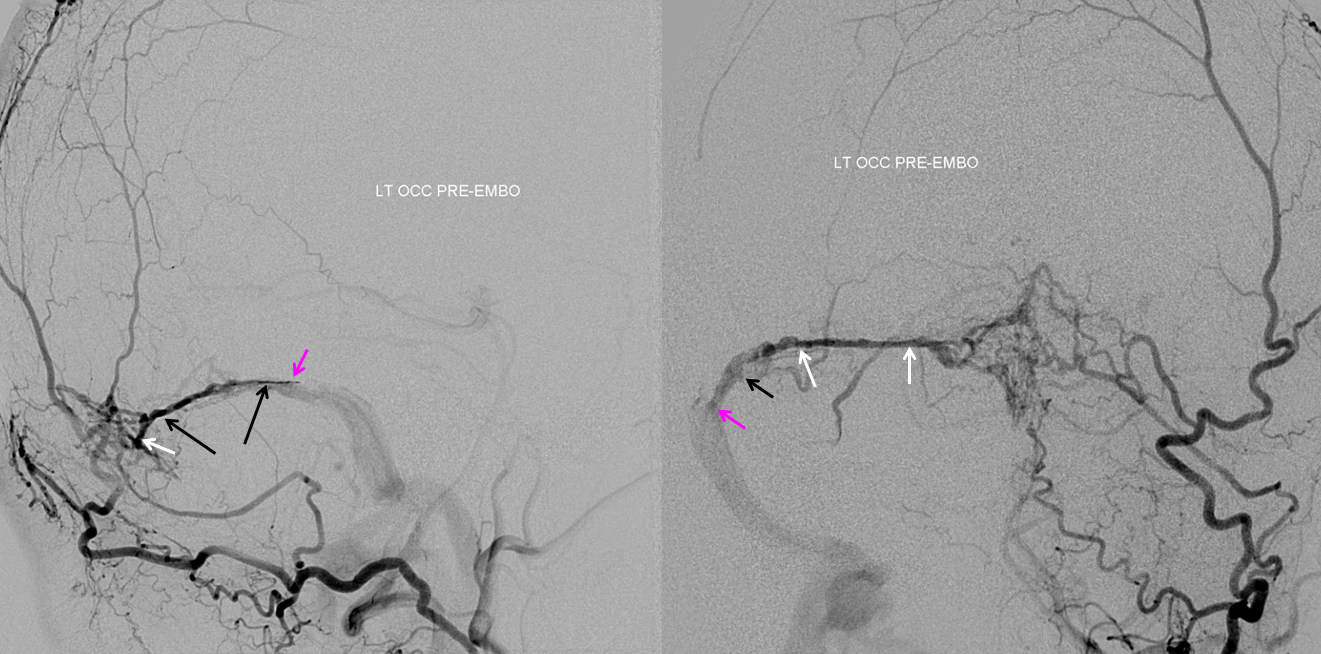
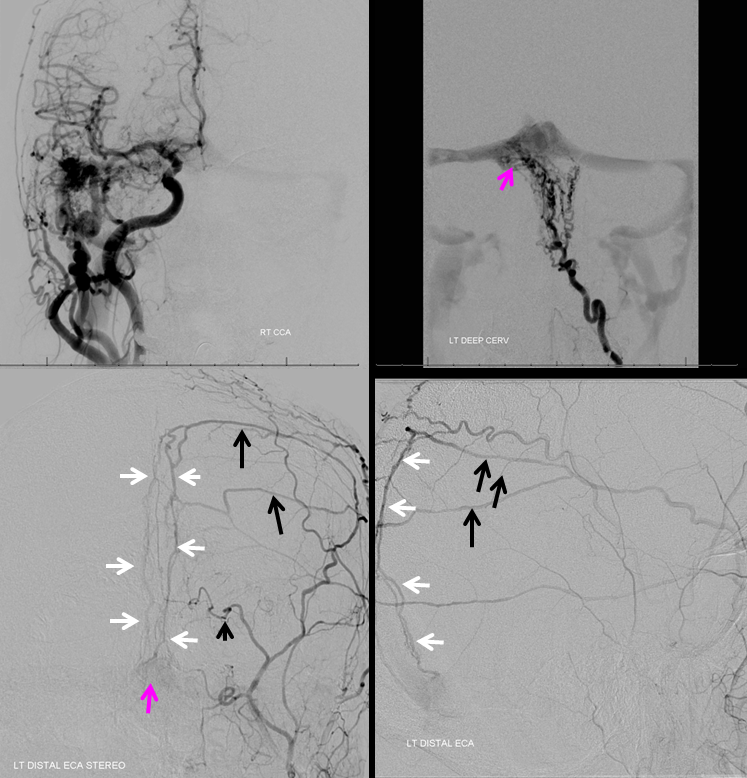
Patient with bilateral Moya-Moya, post bilateral dural synangiosis and burr hole (see Intracranial Collateral Pathways for the remainder of this case). Stereo image pair on top, with dural/ arterial vasculature in white, and venous outflow in black, outlining an arterial arcade which runs within the dural sheath of the transverse/sigmoid sinuses, partly made up by the jugular branch of the neuromeningeal trunk of the ascending pharyngeal artery. Anastomoses with middle meningeal and occiptal branches are clearly demonstrated. These arteries frequently participate in supply of sigmoid sinus dural fistulas.
. 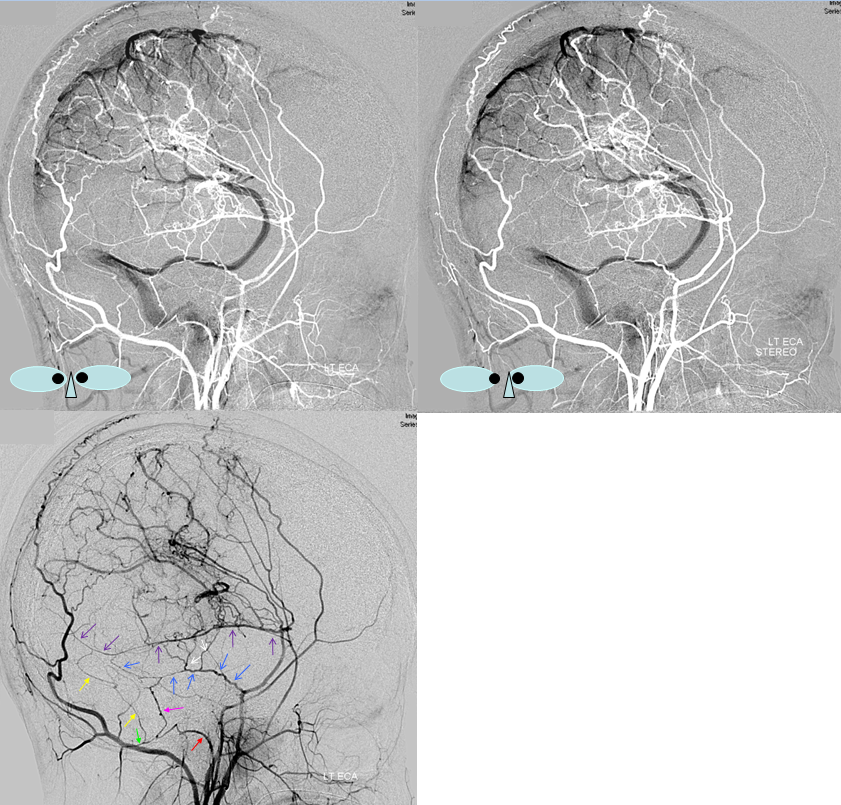
•Red – ascending pharyngeal artery, neuromeningeal trunk
•Pink – superior sigmoid sinus branch, supplied by jugular division of the neuromeningeal trunk
•Blue – middle meningeal artery, basal tentorial branch
•Purple – middle meningeal artery, petrosquamosal branch
•White – anastomotic connections between basal tentorial and petrosquamosal branches
•Green – transmastoid branch, occipital artery
•Yellow – inferior sigmoid sinus branch
Autosynangiosis
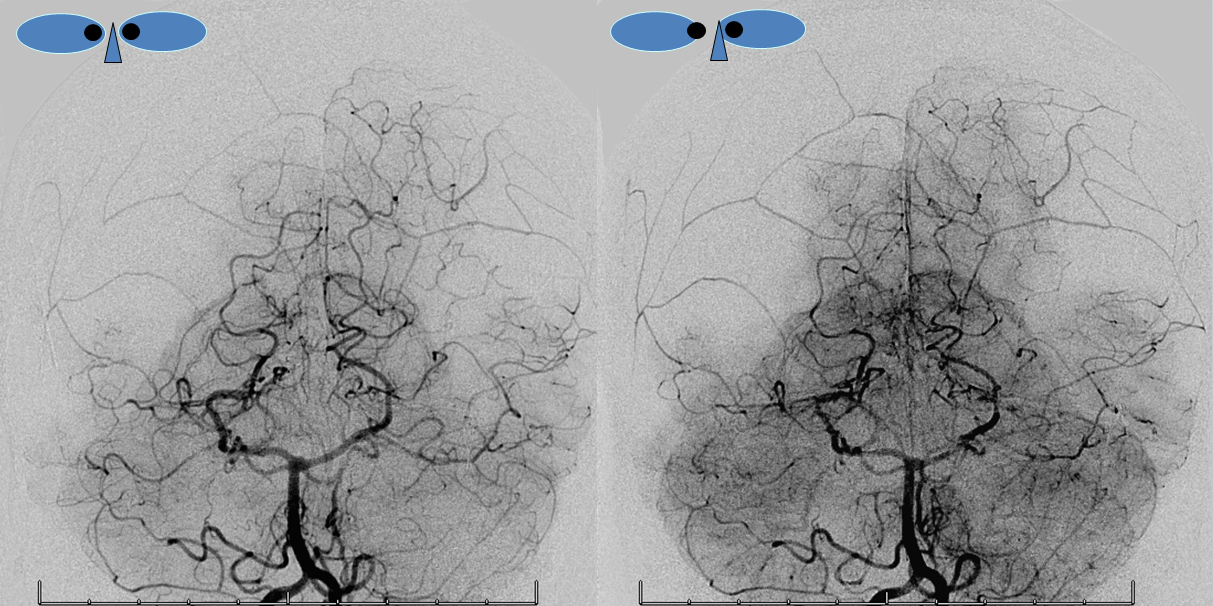
Chronic cortical ischemia promotes natural recruitment of meningeal vessels to help supply the brain, known as autosynangiosis, in contrast to the surgical synangiosis which aims to accomplish the same purpose by overlaying dura, muscle, drilling holes, etc in the head. This process is more robust in younger patients, often encountered in angiographic evaluation of Moya Moya disease. Here is an example in a 4 year old with Moya Moya with severe ACA territory disease, and secondary MMA autosynangiosis helping out this territory.
ECA, MMA authsynagniosis
Notice also a faint autosynangiosis between ethmoid arteries from the ophthalmic and frontal lobe base.
Cases Involving Middle Meningeal Artery
- Angiography after Burr Hole for EVD — another score for the angiogram
- Sigmoid Sinus Dural Fistula with Direct Cortical Venous Drainage (Cognard IIb)
- Sigmoid Sinus Dural Fistula with Direct Cortical Venous Drainage and Extensive Venous Infarction (Cognard IIb)
- Direct Occipital Dural Fistula Embolization (Cognard IV)
- Falcotentorial Fistula Angiogram and Gamma Knife Radiosurgery
- Sigmoid Sinus Dural Fistula with Venous Infarction and Scepter SC assisted nBCA embolization
- Ethmoid Dural Fistula Transvenous Embolization
- Sigmoid Fistula Superselective Transvenous Embolization 1
- Sigmoid Fistula Superselective Transvenous Embolization 2
- Superselective Transvenous Embolization of Complex Jugular Bulb (Condylar) Fistula
- Sphenoparietal Sinus Cognard IV Borden III fistula — ANAGLYPH page
- Petroclival Meningioma Embolization (including MHT access)
- Petroclival Meningioma Embolization (ILT and MHT access)
- Petroclival Meningioma Major ILT Embolization
- Sphenoid Wing and Petroclival Meningioma Embolization (MHT, accessory meningeal artery access)
- Persistent Stapedial Artery ICA Reconstitution in Acute Ischemic Stroke
- The Nonhappening Epidural Hematoma — Post-traumatic Dural Fistula
- Post-traumatic dural fistula with parenchymal, subarachnoid hemorrhage (companion case of The Nonhappening Epidural Hematoma)
- Post-traumatic fistulas
- Another Post-Traumatic Middle Meningeal Artery Fistula
- Post-Traumatic Recurrent Meningeal Artery Fistula
- Epidural Hematoma and Middle Meningeal Artery Fistula