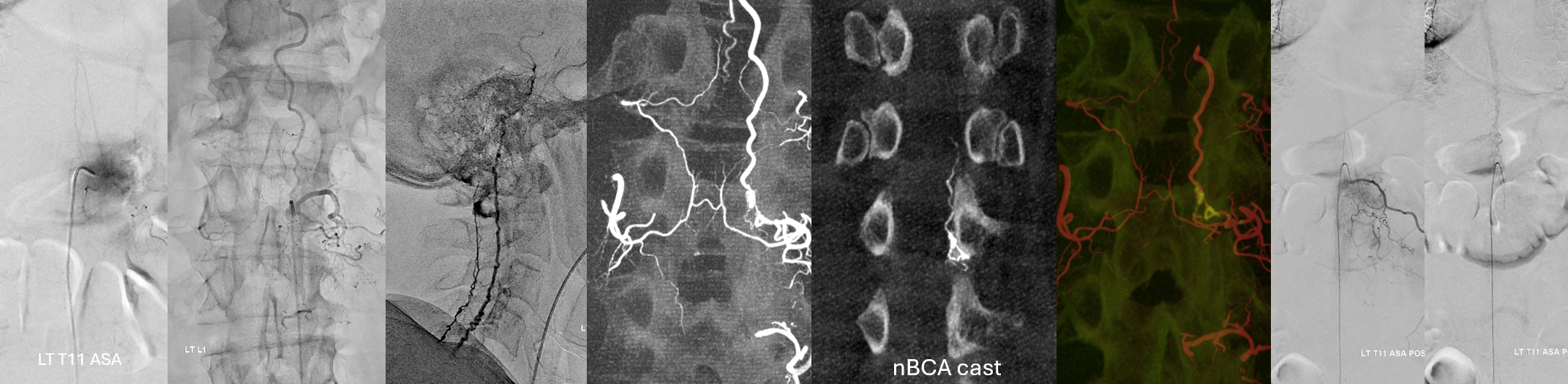
This case encapsulates all of dural fistula pathophysiology, and nearly all of modern treatment strategy.
Clinical presentations on neuroangio.org are purposefully withheld for reasons of confidentiality. What is interesting here is that the clinical status, while clearly symptomatic, was much better than expected based on the below images.
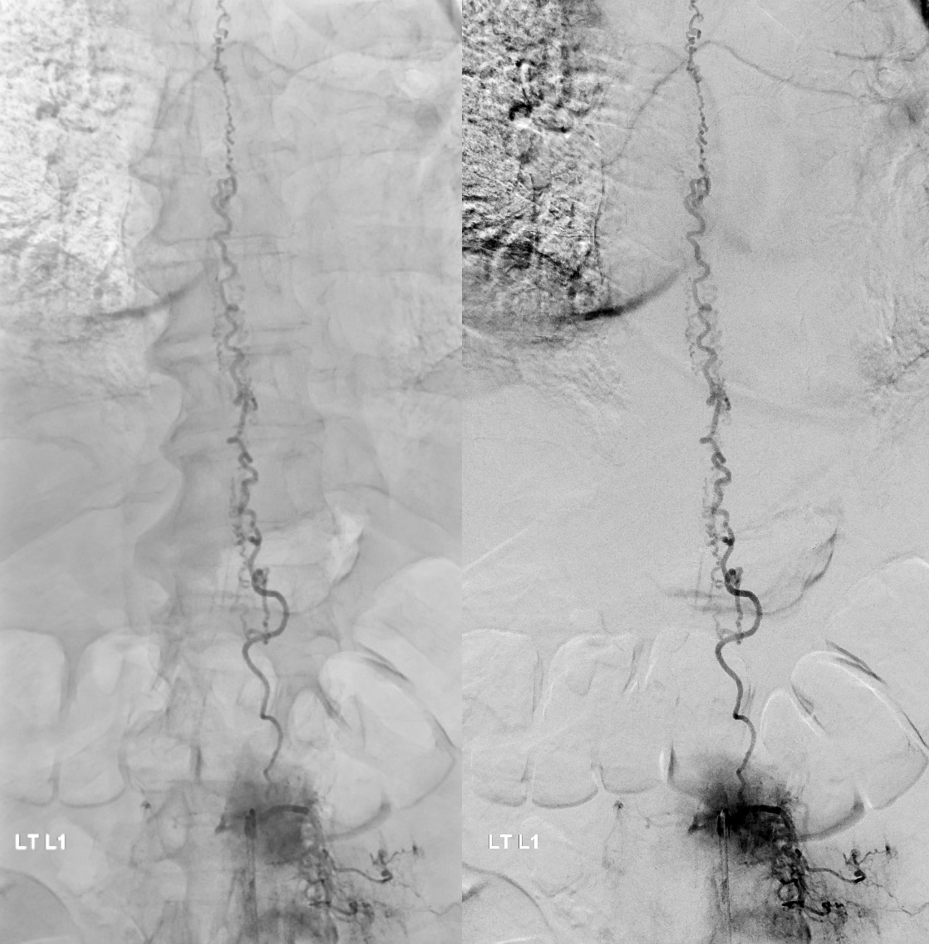
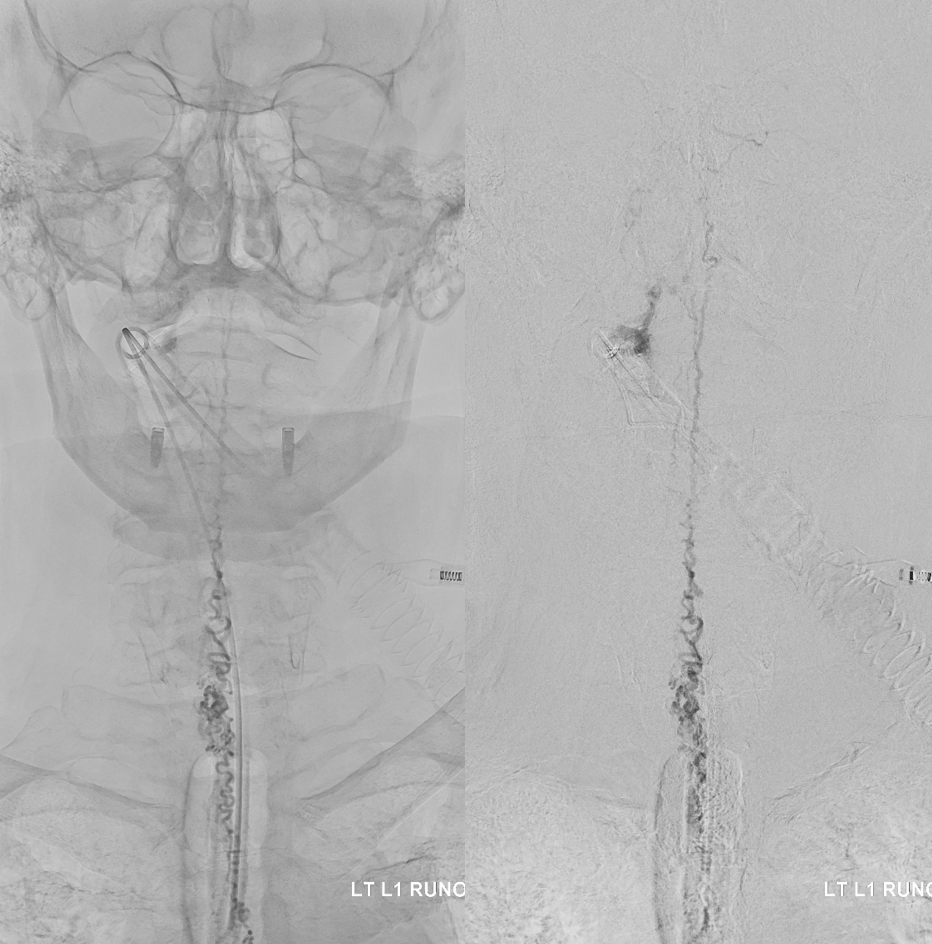
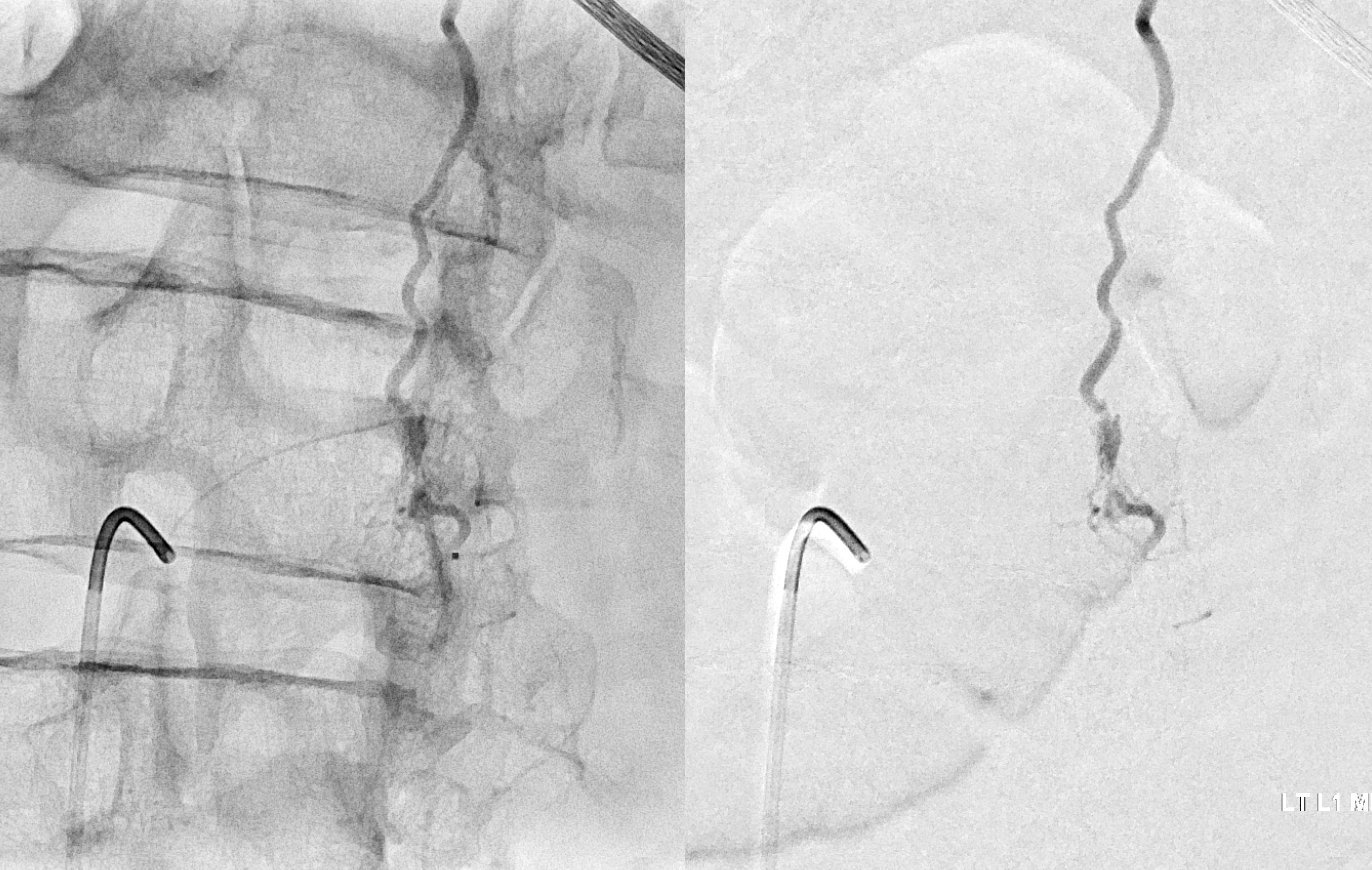
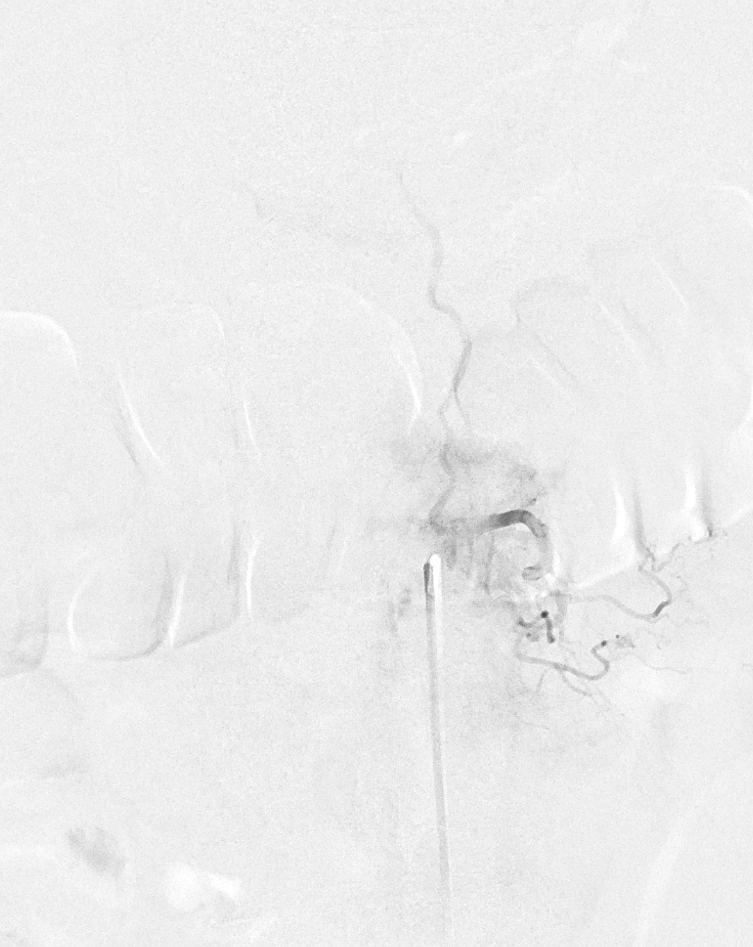
Fistula at left L1

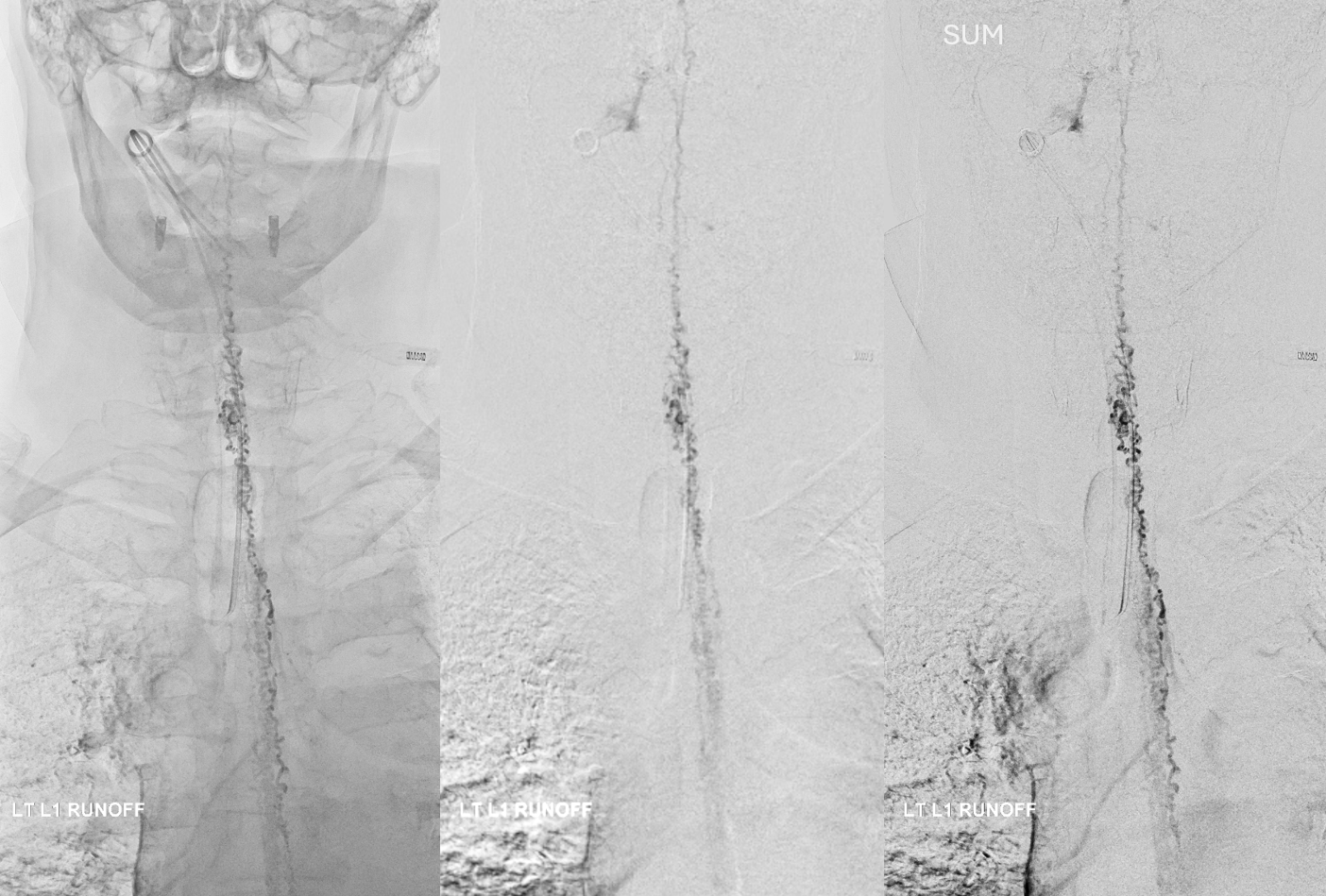
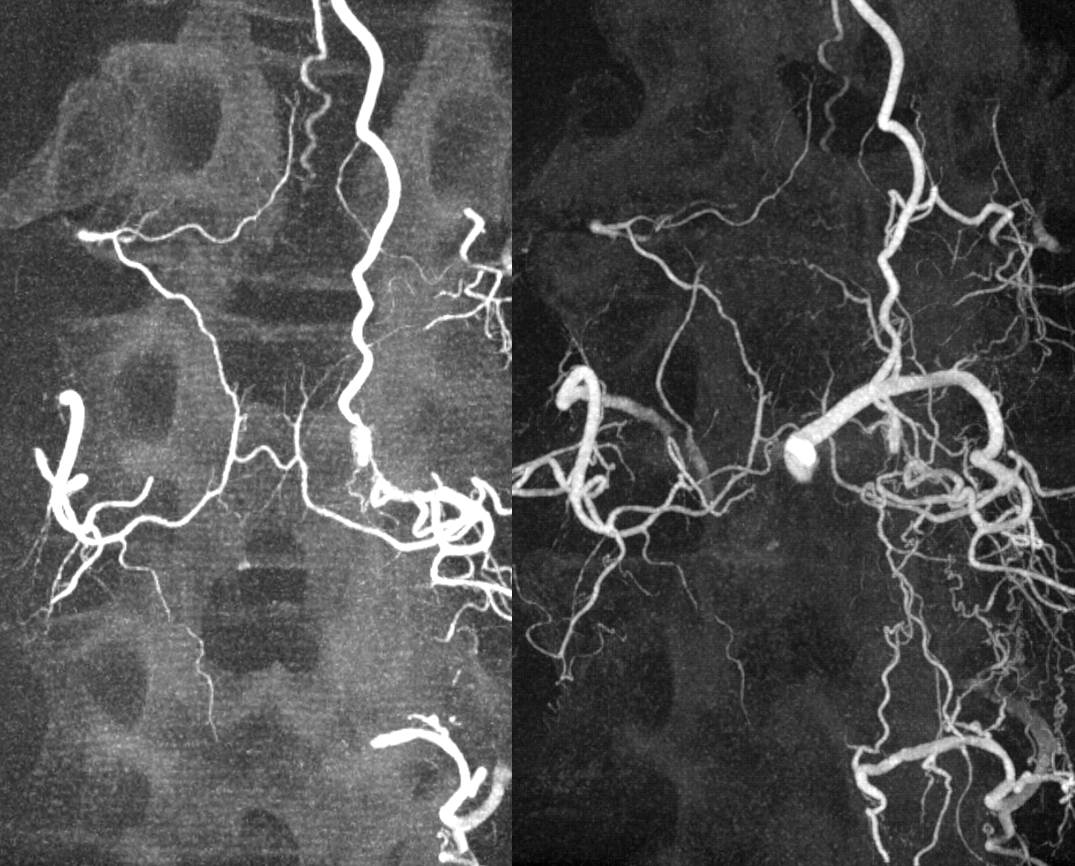
All dural fistula pathophysiology, and spinal too, consists of two key factors. One is fistula. The other is degree of venous congestion. Normal spinal venous anatomy consists of multiple radicular veins which drain the cord via nerve root sleeve / foramen into the epidural venous plexus. These veins are almost always deficient (missing from getgo, thrombosed, both?) in patients with spinal dural fistulas. Could it be that venous deficiency comes first, and fistula emerges later? Probably not, but interesting to consider. In any case, good spinal angiography involves imaging of fistula runoff to see extent of congestion and where drainage finally happens. In this case, there are no radicular veins in lumbar or thoracic cord at all. Not until the foramen magnum/C1/C2 do we see any outflow, and that is insufficient as well — with congestion extending all the way up to basal vein! Note perfect visualization of anterior pontomesencephalic and interpeduncular veins. When it finally reaches basal vein, the outflow is diluted by cranial venous tributaries.
More info on radicular veins can be found in Spinal Venous Anatomy Page
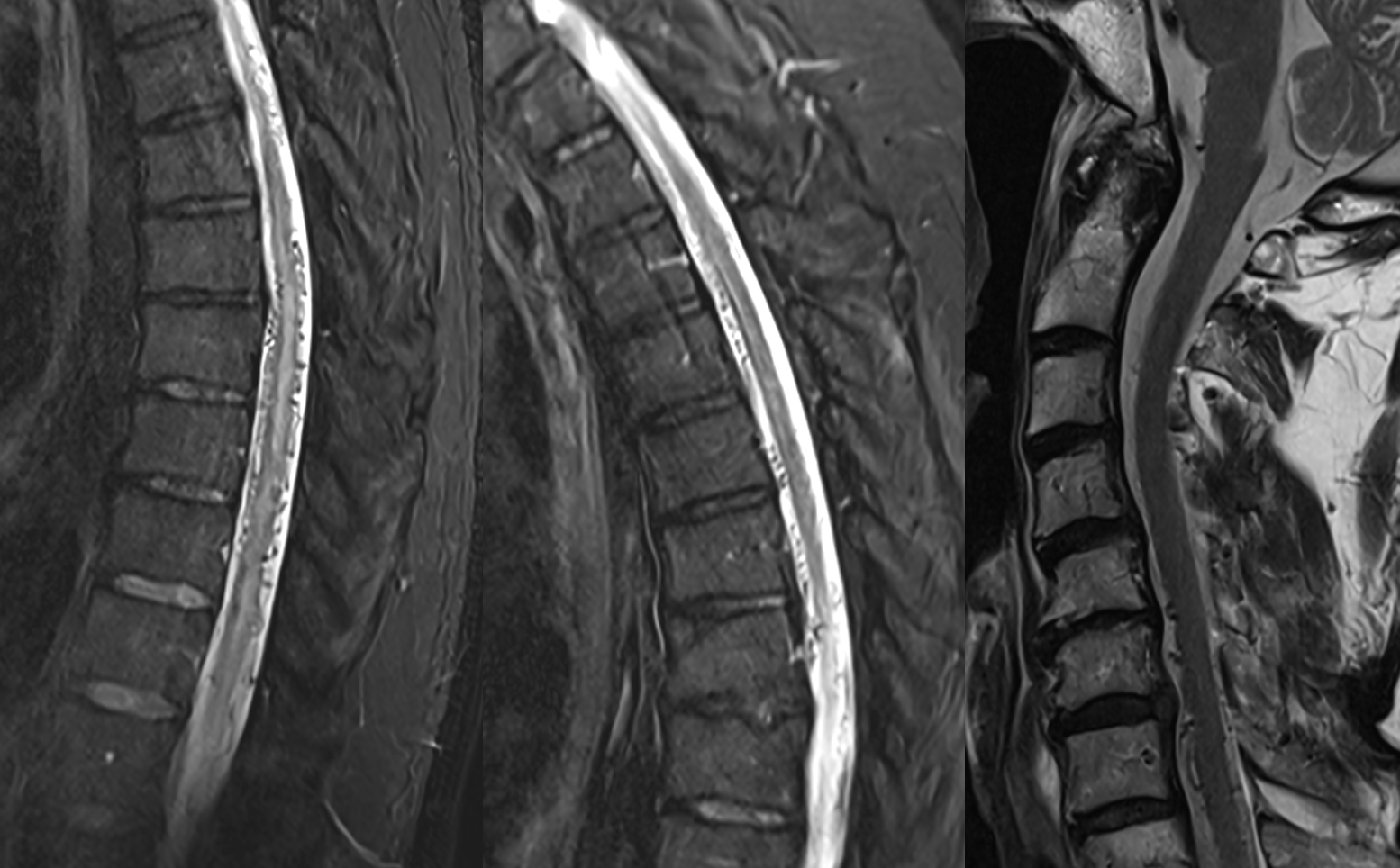
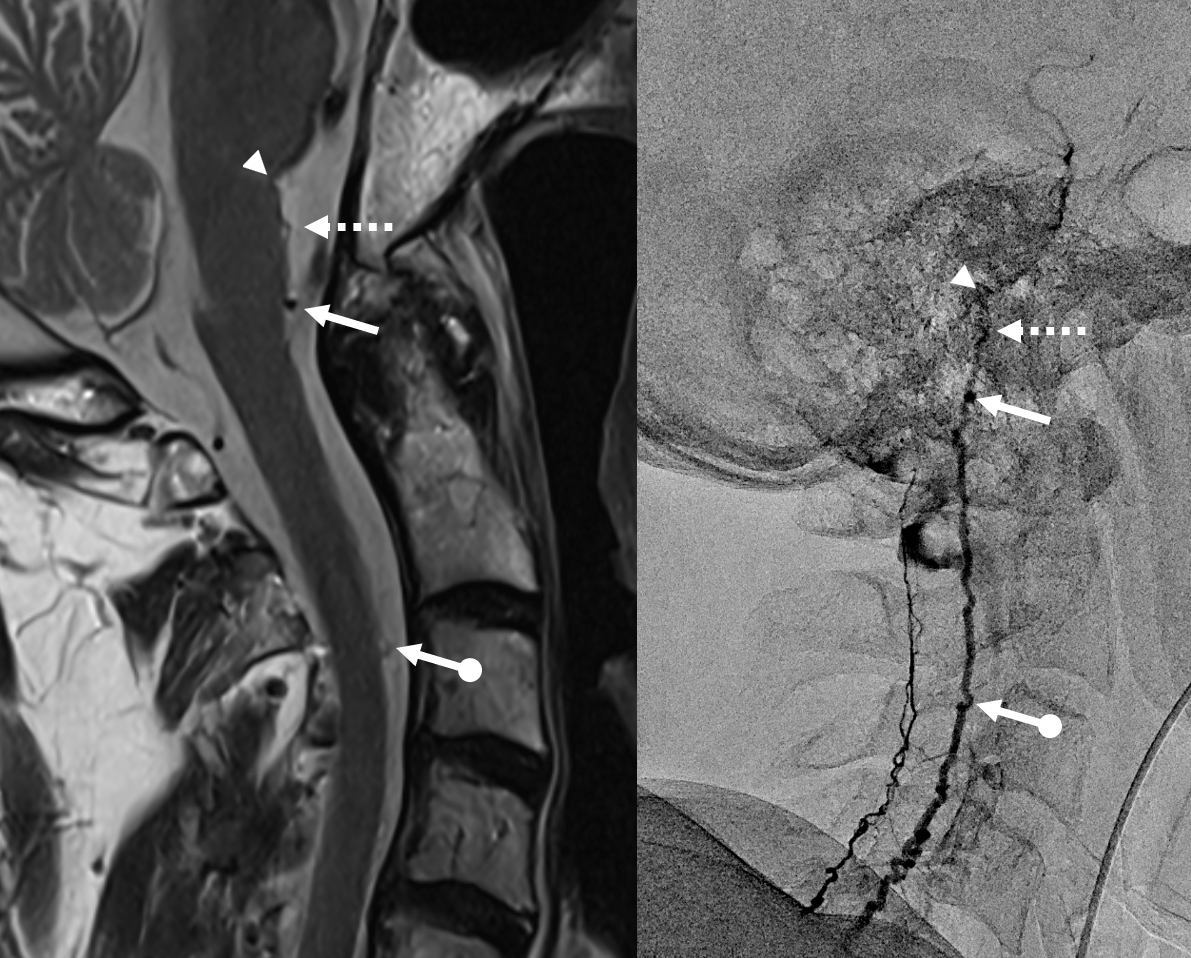
In retrospect, corresponding veins on MRI and angio
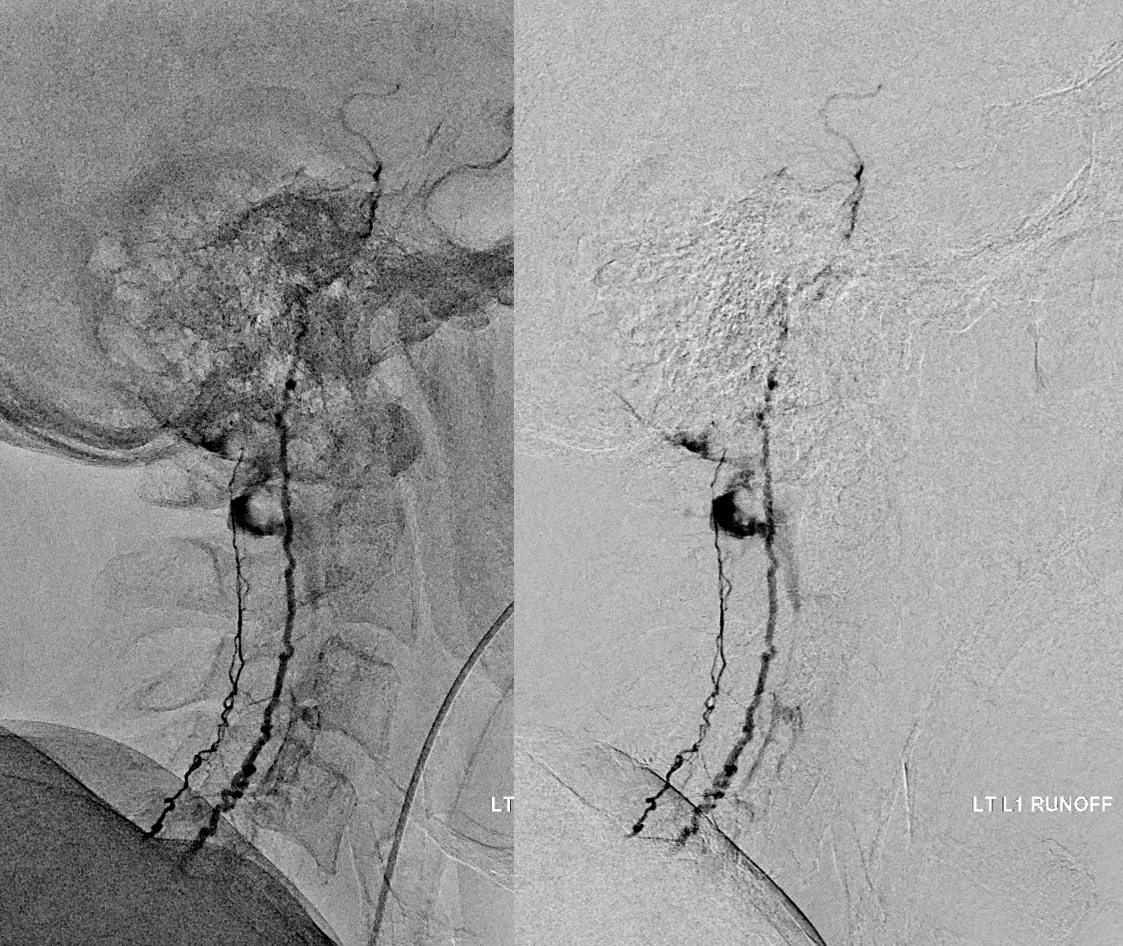
Very important to find both dominant radiculomedullary supply to the lower cord (aka Adamkiewicz) and posterior spinal arteries. In the Adamkiewicz injection, there is usually no venous phase due to fistula-related congestion. In this case, not only we dont see veins, we also don’t see the conus basket. That is frequently seen from posterior spinals, except in this case we did not see posterior spinals either. That is a concern since we don’t want to inadvertently embolize them. However, given the congestion the most likely explanation is that the basket normally opacifies from the Adamkiewicz / ASA, and is congested by fistula.
For more anatomy background, including the basket, see Spinal Arterial Anatomy page
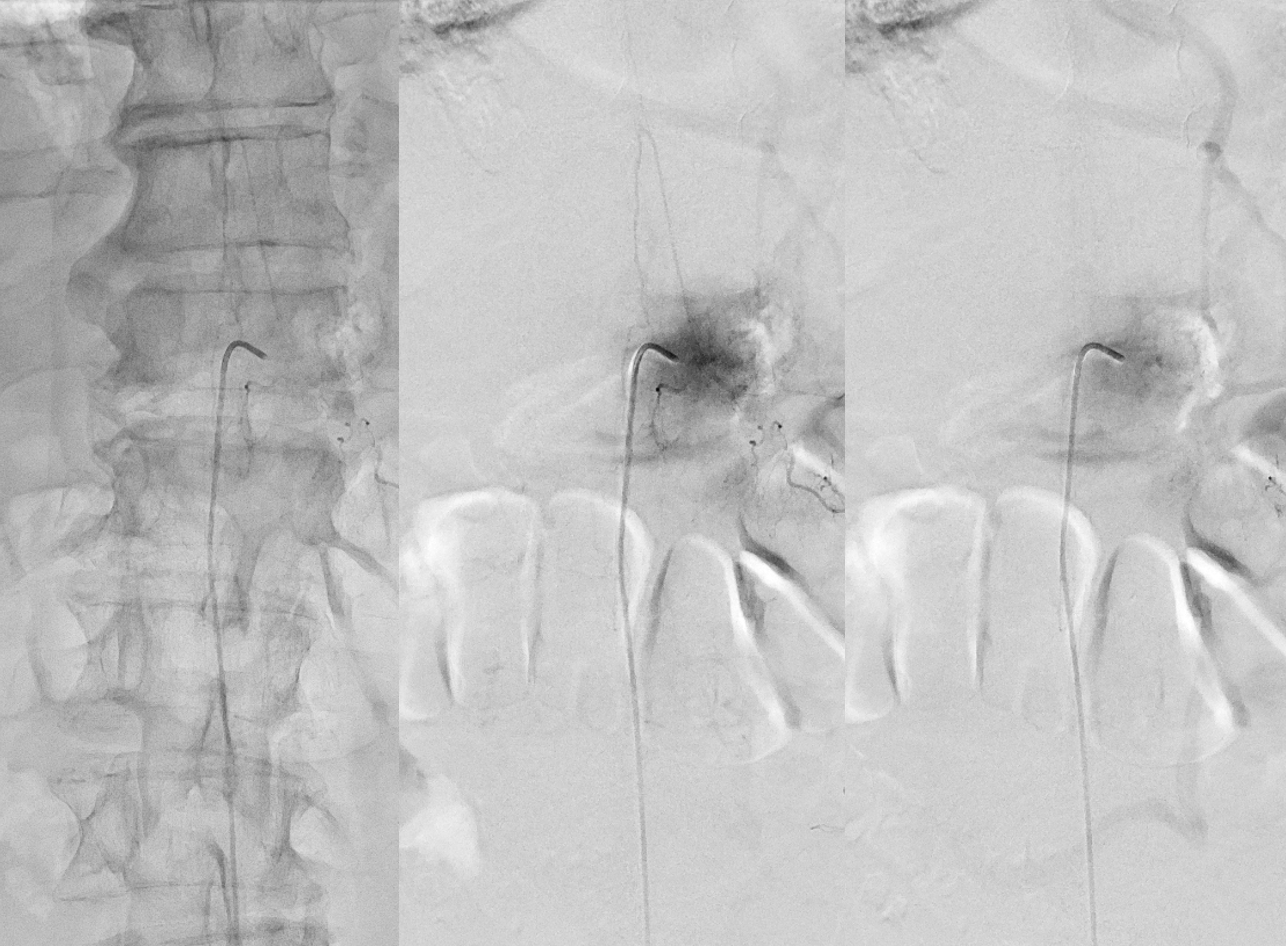
Now to treatment. Hands are raised to get optimal Cone Beam CT view of the fistula (see Cone Beam CT Comprehensive Section, particularly Spinal CBCT Page, on details of how to do this). In this case, we do a dual volume 7 second unbinned “micro” acquisition, with injection of 100% contrast at 1 cc/second for 10 seconds with 3 second delay. Reconstructions are MIP and VR
VR
Headway duo near fistula. There are several other inflows, and the position is not optimal
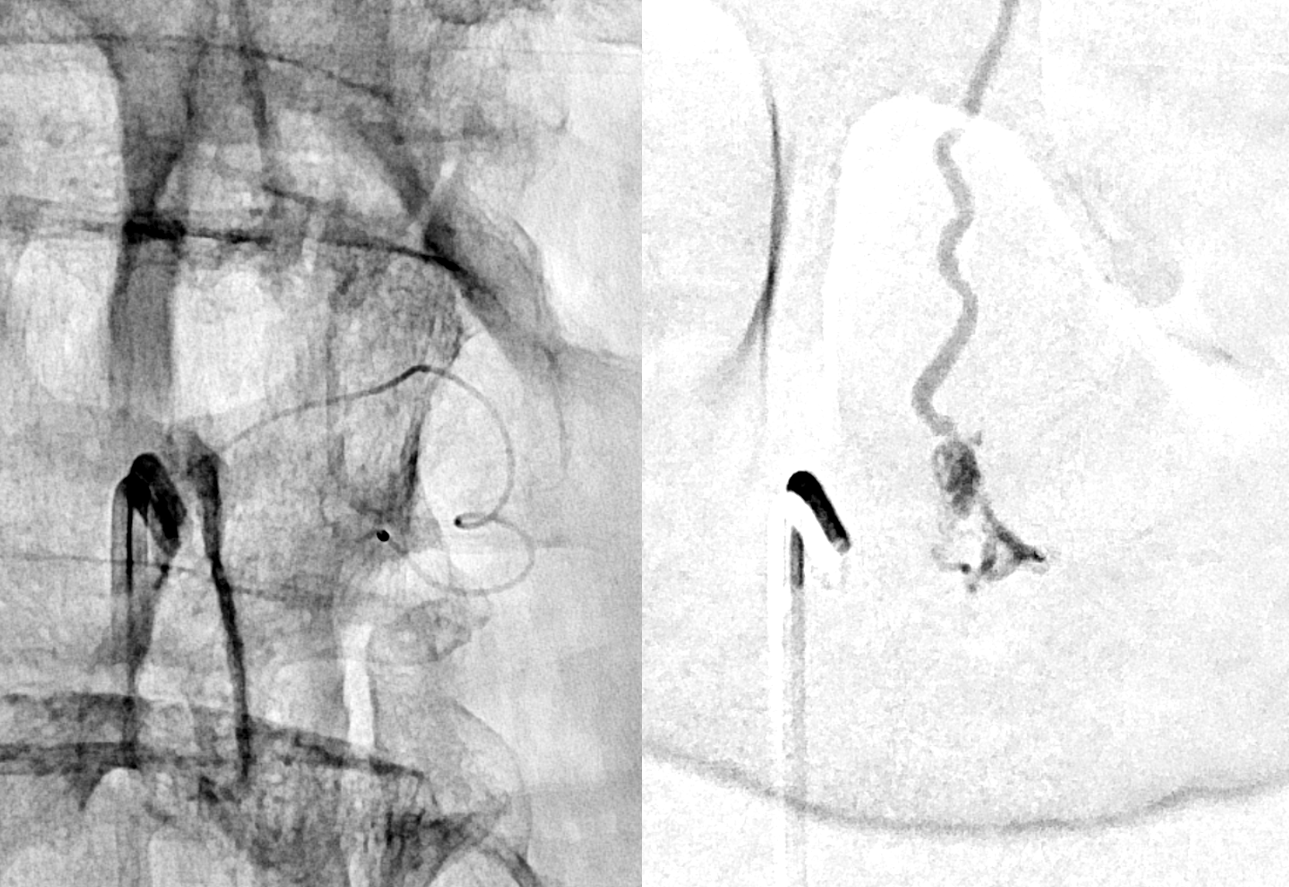
Below is a better position. The fistula is at the level of the ball of vessels above the microcatheter tip. Several tributaries are present, which is not great, as they will tend to polymerize nBCA before it reaches the vein. Fortunately the radicular venous segment is long, before it reaches cord surface veins, so there is a lot of safety. This is not the case with upper thoracic fistulas, and we don’t want to spill glue into cord surface veins. It promotes thrombosis, which is erroneously known as the Foix-Alajouanine syndrome. The actual syndrome was described by Foix and Alajouanine as the clinical end stage of untreated spinal dural fistulas — ascending paralysis, followed by respiratory dysfunction, aspiration, and death. It was not an iatrogenic problem. The name was adopted to describe post-treatment acute deterioration due to thrombosis of enlarged cord veins after closure of the fistula and reduction of venous flow, in part because clinical presentation is comparably horrible. Typically happens some 6-12 hours after treatment, and almost always within 24 hours. Treatment is with aggressive emergent anticoagulation, and outcome is variable.
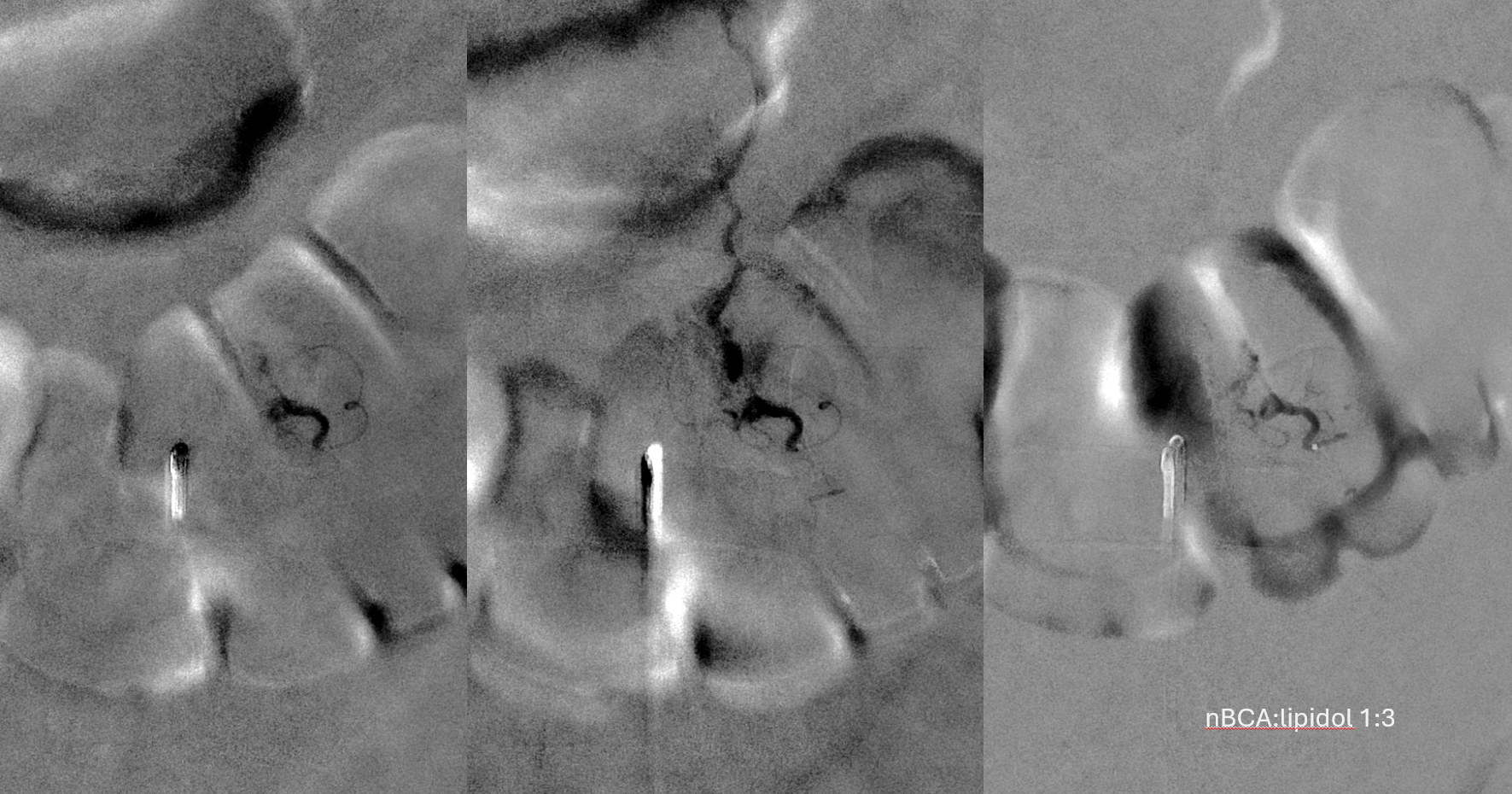
A 1:2 nBCA:lipidol dilution was chosen because of additional arterial inflows over which we had limited control (we usually use 1:1). Result seems good
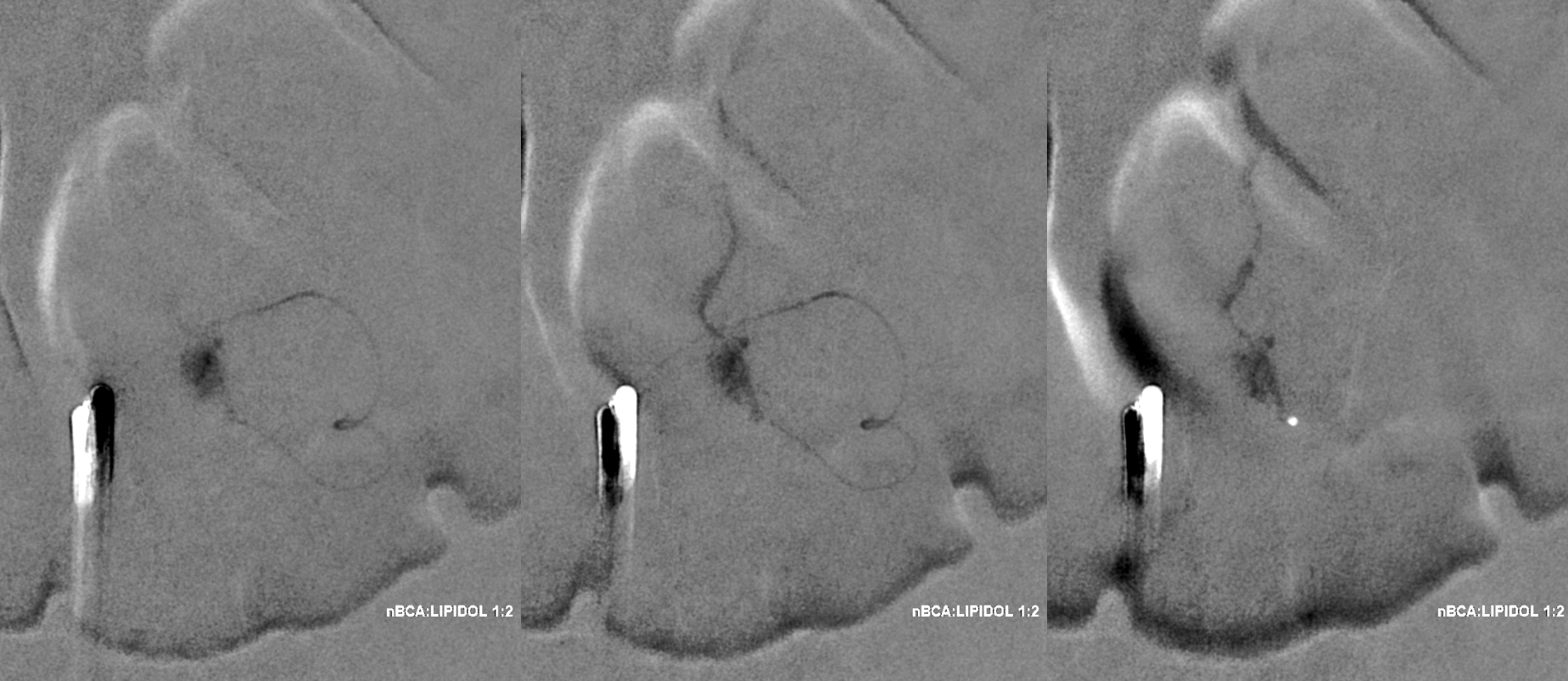
Not so fast. Fistula still alive — which is exceptionally rare after putting this much glue into the vein. Yes, glue is diluted but still its plenty. There is also heparin on board — we pre-treated given massive venous congestion and concern for thrombosis. Still, this is extremely rare. Much slower, but still alive. It is a mistake to hope it thromboses later. Very likely it will not, and hoping it will only contributes to the reputation that endovascular is less effective than surgery.
Re-catheterization (left and center) and additional nBCA injection — even more dilute now that the vein outflow is relatively protected. We think this is good now.
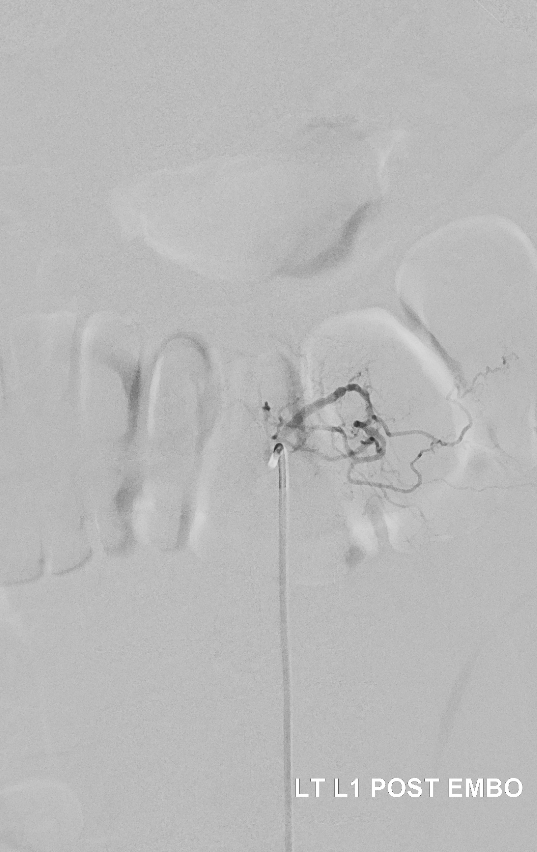
Post. It is important to control from other levels (left T12, left L2, and right L1) — not shown.
Key image here — injection of Adamkiewicz now shows return of the prodigal veins to the venous phase of the cord. Image on left also shows the now liberated basket.
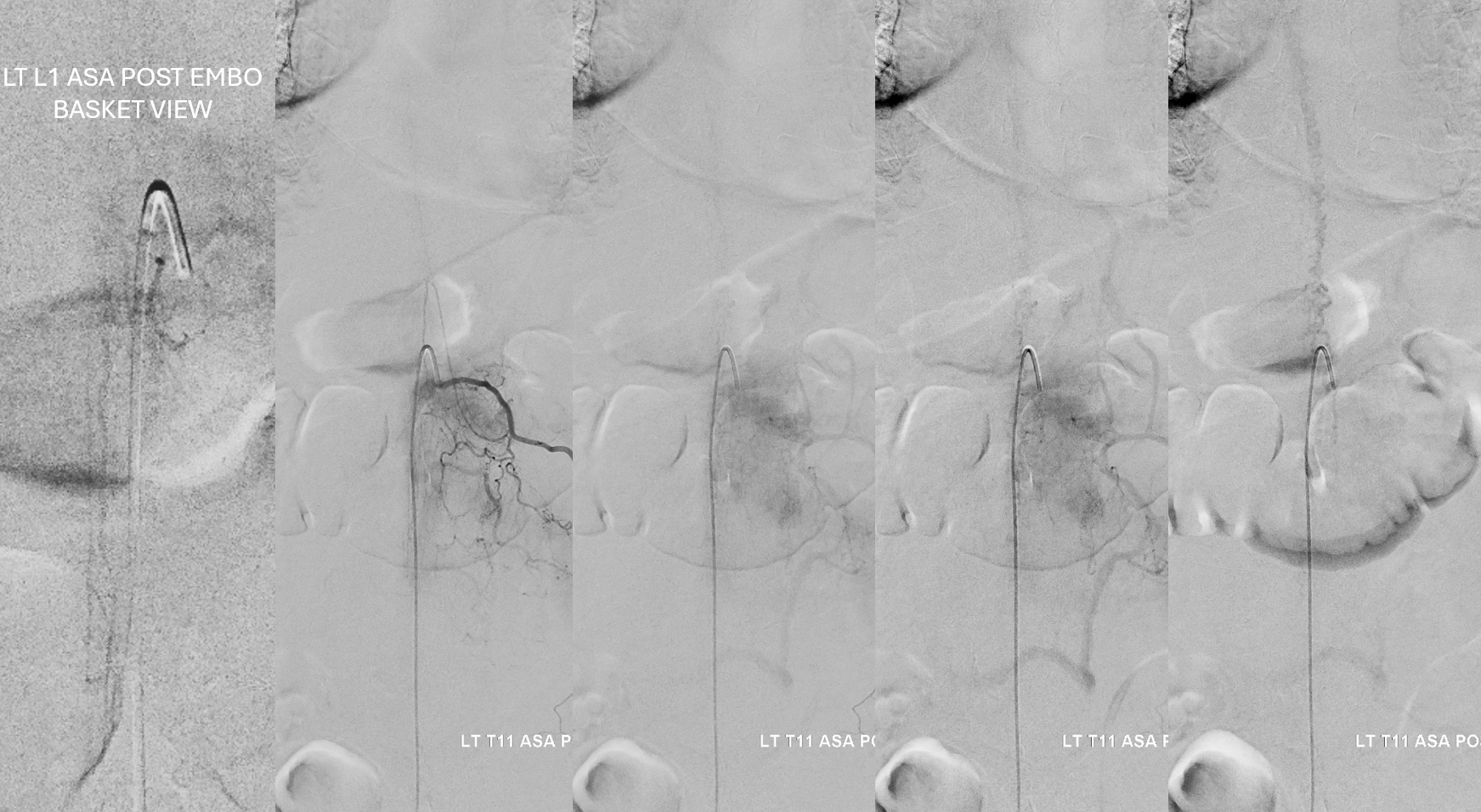
pre and post Rx ASA views side by side
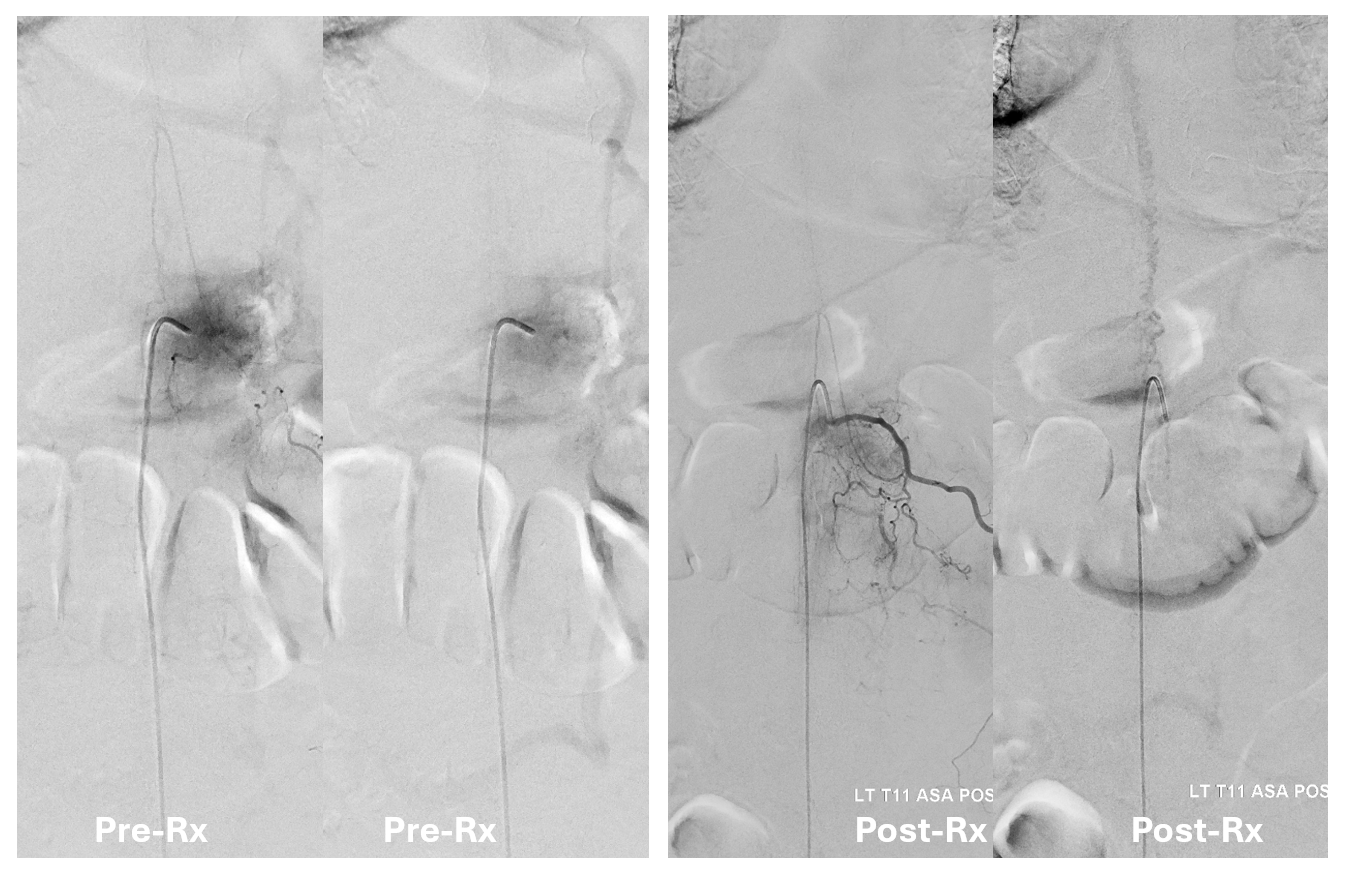
It is critical to understand cord venous drainage in post-dural fistula patients is never normal. The radicular veins are still missing. This may in part be responsible for the incomplete clinical recovery, although the main reason is probably cord damage before fistula occlusion.
Cone Beam CT shows nBCA cast in the fistula basket and radicular vein. Fusion of pre and post Rx CBCTs is nice
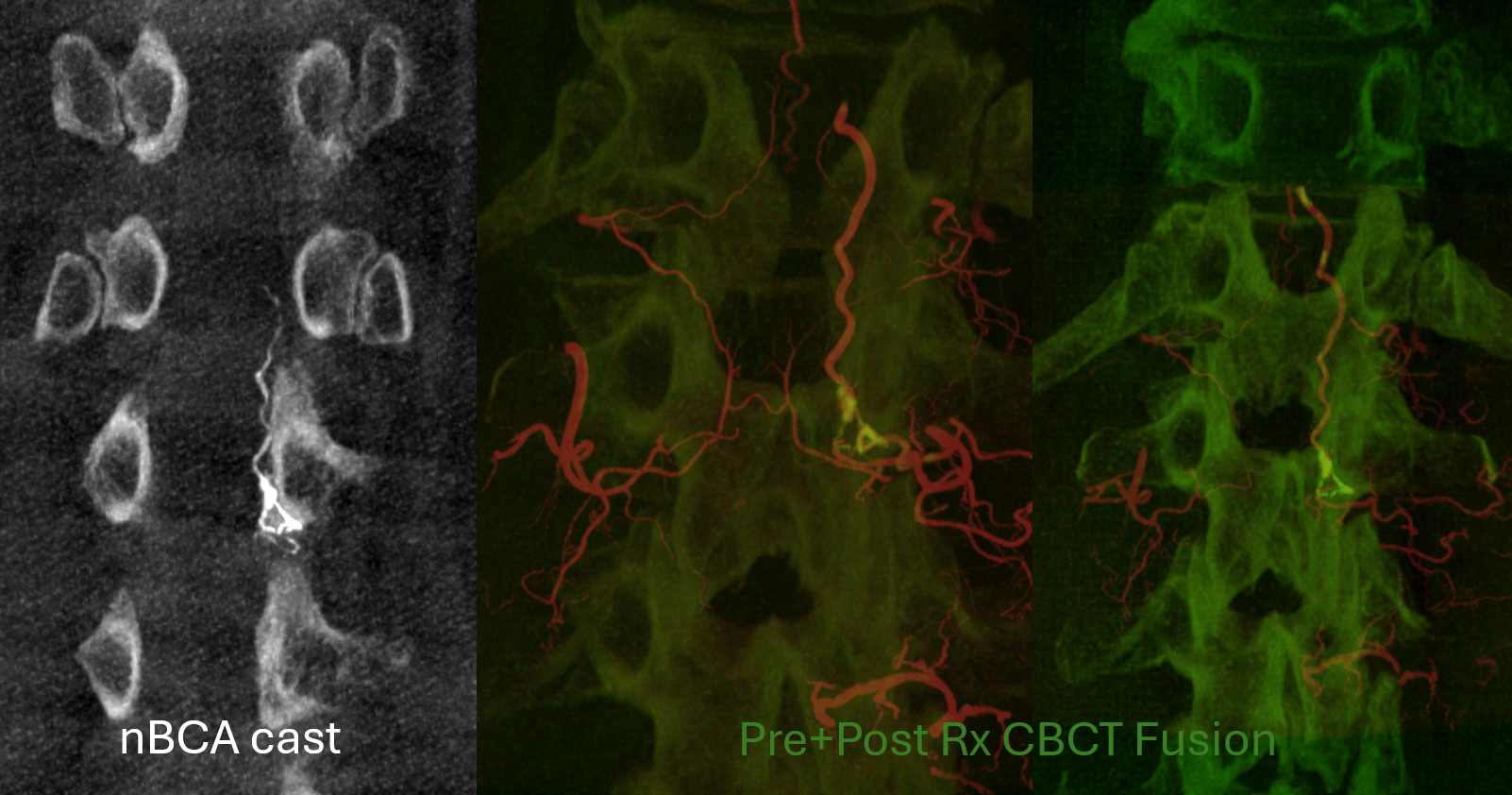
More cool fusions

Pretty much all of fistula pathophysiology is illustrated in this case. And a lot of treatment points too. More cases are in the Patient Information Spinal Dural Fistula Page as well as Spinal Dural Fistula Treatment Guide Page and on Case Archives page.
