Spinal Intradural Fistula (a.k.a. Ventral Intradural Fistula, a.k.a. Pial Fistula, a.k.a. Type IV)
Please note: Related information, written specifically for patients and interested laypersons, is found in the Patient Information Spinal Fistula section
When a fistula develops between any artery supplying the spinal cord and a spinal cord vein, it is called an intradural (pial) fistula. It is not a spinal dural fistula, because dural fistulas form in the dura (usually in the nerve root sleeve, although Spetzler insisted on a subarachnoid location just within the nerve root sleeve) and DO NOT involve arteries that supply the spinal cord. The dural fistula becomes symptomatic as a result of spinal venous congestion, and not because the fistula directly involves a spinal cord artery. For details, see dedicated Spinal Dural Fistula page. Also, a spinal intradural fistula is not an AVM, because AVMs have a nidus, and a fistula does not. In truth, it is sometimes difficult to tell even on angiography whether or not a nidus exists — tortuous veins can look a lot like a nidus. The intradural fistulas range from single artery-to-vein connection to increasing arterial feeder complexity. This complexity is likely related to regional peri-fistulous anatomy dictating availability of additional arterial contributors, the size of fistula, and its duration of existence. However, meticulous microcatheterization does answer these questions and so ultimately intradural fistulas can be separated from AVMs by superselective spinal angiography.
A diagram of a simple spinal fistula between the anterior spinal artery and adjacent surface spinal veins is shown below.
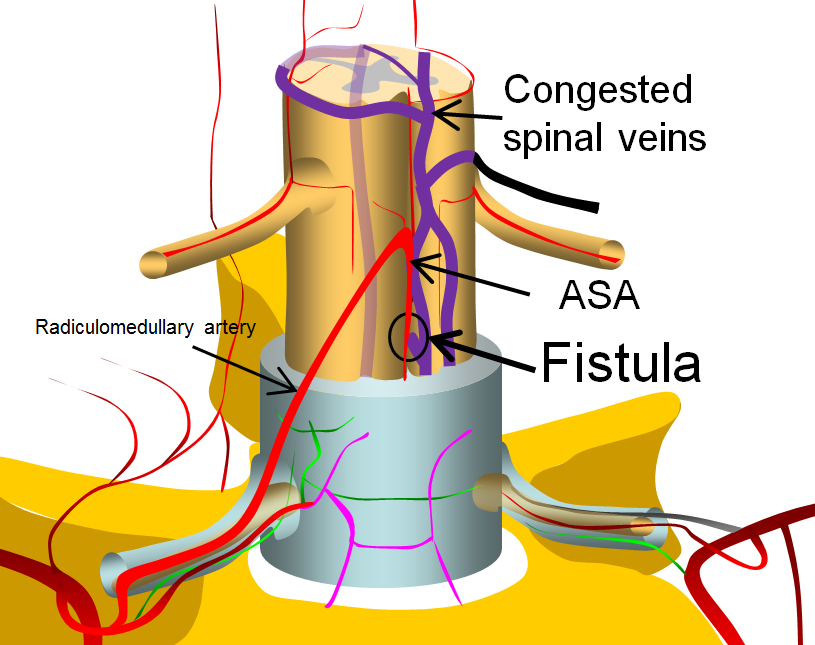
This image is modified from a larger arterial anatomy diagram, shown below, and more fully discussed in the Spinal Arterial Anatomy section.
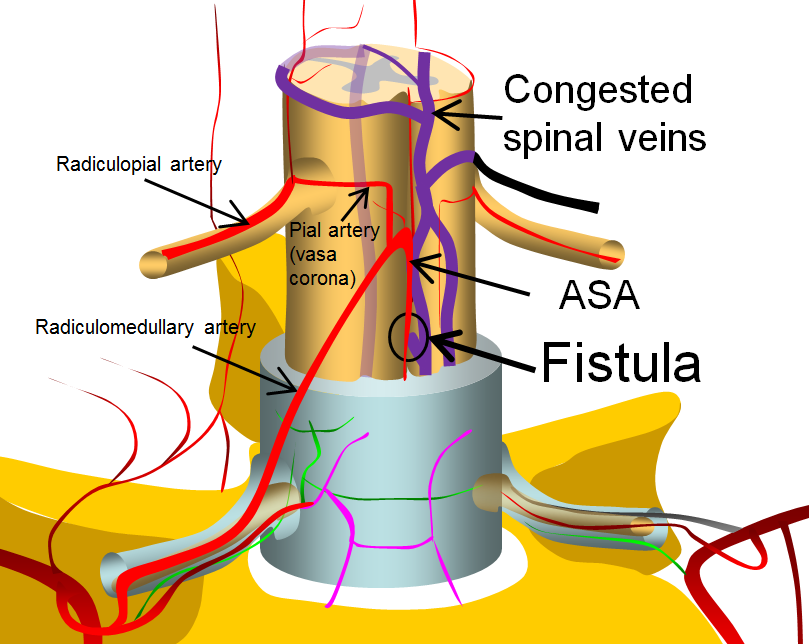
More complex fistulas can have both anterior spinal and posterior spinal artery supply, such as this diagram below. Notice that despite having multiple feeders, the fistula point is still a “single hole”. The difference is that a radiculopial artery was also recruited into supplying the arterial side of the fistula. This radiculopial artery is, generally, the safer embolization route, than the ASA.
Finally, very complex ones look really “busy” angiographically, and may be very difficult to differentiate from AVMs, as in the diagram below. Again, though having multiple feeding arteries, the fistula itself is a “single hole”, as most of them are, despite what they might appear.
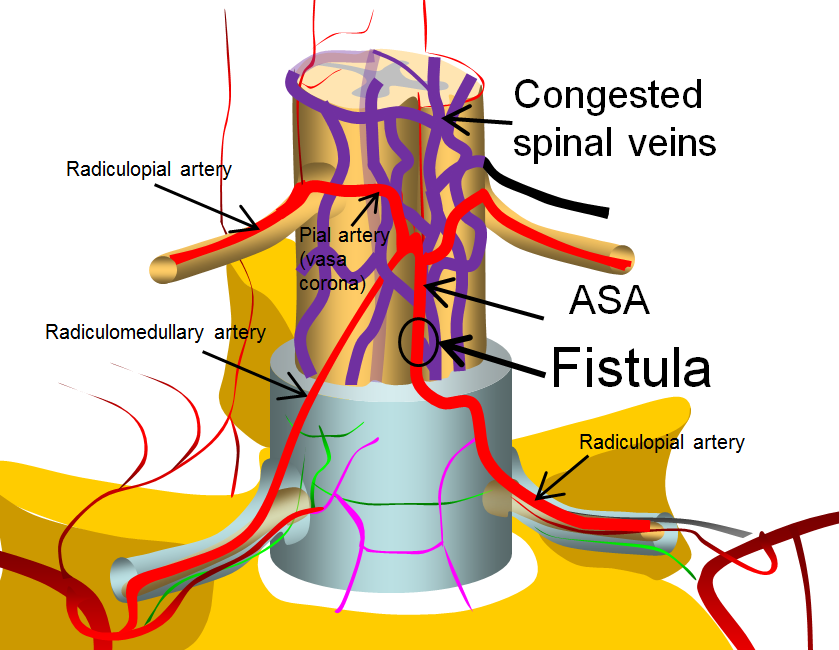
Again, what all of these fistulas have in common is 1) supply by artery or arteries of the spinal cord and 2) lack of nidus.
Etiology (why fistulas form) — just like brain AVMs, spinal dural fistulas, brain dural fistulas, and nearly everything we treat — the etiology is unknown. There are some theories but no proofs. However, there are important associations — genetic ones. A number of genetic disorders are associated with both spinal intradural fistulas and spinal AVMs. This is particularly true about the more complex fistulas with multiple feeding arteries. Sometimes, the spinal fistula is part of a larger regional vascular malformation (such as Cobb syndrome), and sometimes not. Also, there is nothing particularly special about the “spinal” part — the fistulas and AVMs associated with these disorders involve other parts of the body as well. Some of the more common disorders are:
Parkes-Weber syndrome: a triad of skin capillary malformations, arteriovenous fistulas (anywhere in the body), and limb overgrowth. It might be that limb overgrowth in some cases is a result of increased arterial inflow and impaired venous/lymphatic return in a limb affected by capillary malformations and arteriovenous fistulas. The fistulas seen in this syndrome can involve the spinal cord. Therefore, any patient with spinal fistula should at the least have a dermatologic examination, and perhaps a genetic one.
Capillary Malformation-Arteriovenous Malformation Syndrome (CM-AVM Syndrome): Finally, a syndrome with a semi-intuitive name. The capillary malformations refer to ones on the skin; the AVMs can be anywhere in the body. Although not part of the name, AV fistulas (spinal pial fistulas, for example) are part of the syndrome also. There is no limb overgrowth. As you can see, CM-AVM and Parkes-Weber are quite similar. In fact, some cases of Parkes-Weber and CM-AVM share the same genetic cause. It seems likely that both are in some way expressions of shared genetic aberrations.
Hereditary Hemorrhagic Telangioectasia (HHT), a.k.a. Osler-Weber-Rendu Disease (Rendu-Osler-Weber or ROW for the continentals): Skin telangiectasias (small AVMs) and AVMs in the lungs, liver, CNS, and pretty much anywhere else in the body. About 70% of patients with pulmonary AVMs will have HHT. Unlike Parkes-Weber and CM-AVM, HHT is hereditary with autosomal dominant mode of transmission.
Klippel-Trenaunay Syndrome: Consists of skin port-wine stain (usually on a limb), limb overgrowth, and venous abnormalities in the overgrown limb — most notably varicose veins. AVMs and fistulas are not part of this disorder, for which no genetic cause is known (in contrast with Parkes-Weber and CM-AVM Syndrome). It is probably incorrect to consider KT in the differential diagnosis of spine or brain arteriovenous shunts. However, some believe that Sturge-Weber Syndrome and KT Syndrome are expressions of the same disease involving either the brain or the extremities.
Klippel-Trenaunay-Weber syndrome: Confusion exists as to whether this is the same thing as Klippel-Trenaunay, or some kind of amalgamation between Klippel-Trenaunay and Parkes-Weber. There is simply no way to create law and order in the land of genetic diseases, certainly not vascular ones. To my genetically-disoriented mind, Parkes-Weber and KT are entirely different conditions — KT is a venous problem, and Parkes-Weber / CM-AVM are arteriovenous shunts.
Cobb Syndrome: Defined as a cutaneous vascular malformation (venous or arterial) and spinal AVM. Technically, if the skin component is not present, you can’t call it Cobb syndrome — but what it may mean is that the AVM stopped short of the dermis. The morbidity of the condition is really defined by what happens in the spinal canal. Most often, the spinal component is not a fistula, but an AVM, so technically Cobb syndrome is better placed on the Spinal AVM page, but I think it is important to discuss it here also.
Bottom line is that if you only remember Parkes-Weber, CM-AVM, and HHT when you see a patient with spinal intradural fistula or AVM, you will do better than most — just send them to a geneticist (after looking at the skin).
The frequent association of complex intradural spinal fistulas with underlying genetic disorders was noticed by the great Lasjaunias, who came to view the entire spectrum of spinal fistulas and AVMs through the lens of underlying conditions in his Spinal Vascular Lesion classification system, known as the Bicetre System (more on classification systems below). He advocated that any patient with a complex spinal fistula be evaluated by a geneticist, and held that even simple fistulas or AVMs might be incomplete expressions of some possible underlying genetic issue. This of course is difficult to prove, but we absolutely follow the recommendation for a genetic evaluation (please note that genetic does not mean hereditary. Most of the above conditions are believed to be spontaneous mutations).
Clinical Features
Hemorrhage. Unlike dural fistulas, which do not hemorrhage (I have never witnessed a case or heard of one conclusively established), about 1/3 of intradural fistulas present with bleeding. The possible reasons for this are several — first, intradual fistulas are usually much higher flow lesions than dural fistulas, particularly the complex kind. Second, the arteries involved are not located in the relatively tough dura. There may be other reasons also. Both spinal subarachnoid hemorrhage and intraparenchymal (intramedullary) cord hemorrhage (hematomyelia) can be seen. We have an extremely low threshold for performing a spinal angiogram for any intradural hemorrhage, subarachnoid or intramedullary.
Spinal hemorrhage usually presents as sudden, severe back pain (perhaps we should coin the term “worst back pain of life”, although one might expect stiff competition from especially nasty disks and vertebral body fractures…). The pain, however, is usually just the beginning — weakness, sensory deficit, paralysis, and/or loss of bladder/bowel function usually follow in the next minutes (at which point pain, tragically, improves). Exact nature of symptoms of course depends on the location and amount of bleeding.
A very important point: Large amounts of spinal subarachnoid bleeding, particularly if the source is in the cervical spine, will extend into the cranium. When this happens, back pain will evolve into a terrible headache, with meningeal signs, etc. In such cases, a brain subarachnoid hemorrhage workup is often the starting point. Head CT shows hemorrhage, and a bloody tap will show up if one does an LP. In these cases, a cerebral workup is negative. Do not miss a spinal source! We always look for at least cervical anterior and posterior spinal arteries in ALL cases of angio-negative brain SAH where the predominant location of blood is in the posterior fossa — about 10% will have a spinal source. The anterior spinal artery does not always originate from the vertebral artery — potential sources include ascending cervical, deep cervical, and supreme intercostal arteries — see Spinal Arterial Anatomy for details.
The remaining 2/3 of patients present with symptoms of spinal venous congestion — similar to those with dural fistulas. Intradural fistulas, by definition, open into spinal cord veins, which often leads to the kind of global venous hypertension seen in cases of dural fistula. Such symptoms often have a conus feel, and include pain, progressive myelopathy, bowel/bladder/sexual dysfunction, saddle anesthesia, other sensory issues, and the like — just like a dural fistula.
MRI appearance
This can be easily deduced based on clinical symptoms. Hemorrhage is hemorrhage, parenchymal or subarachnoid. Nonhemorrhagic presentations of spinal vascular congestion look very much like spinal dural fistula. Remember to look outside the spinal canal! Are there other vascular lesions — in the vertebral bodies, paraspinal soft tissues, skin perhaps? An especially knowledgeable physician should look at the patient’s back for vascular birthmarks.
Spinal Vascular Malformation Classifications
Nothing has been so far been mentioned about the various spinal vascular malformation classifications. A classification is only helpful when it accomplishes a purpose. In the early days of spinal angiography, when pioneers like Djindjian, Doppman, De Chiro, Aminoff, Logue, and Thron recognized and understood the pathophysiology and pathoanatomy of the spinal dural fistula, a classification was a way to define heretofore unrecognized diseases — which were seen as a kind of terrible spinal vascular mess with poor long-term prognosis (see landmark study by Aminoff and Logue).
Spinal dural fistula only became a recognized disease about 50 years ago — until then it was best described as Foix-Alajouanine syndrome — essentially a very advanced dural fistula (the extent of confusion which existed on the topic still echoes in the complete lack of literature agreement as to what Foix-Alajouanine syndrome actually is, and what underlying disease it represents. For the record, it is a description of progressive ascending myelopathy in patients with untreated dural fistulas — essentially a monograph on the natural history of the disease, ending in quadriparesis and death, as most recently pointed out by Ferrell, Tubbs, Acakpo-Satchivi, Deveikis, and Harrigan). Until the now-recognized diseases such as spinal dural fistula or spinal intradural fistula had any recognized names, a classification was the way to give them names and separate them by various (usually angiographic) properties. Now, I believe the situation is different. Just like we do not classify right lower quadrant disease as “Type 1 — appendicitis”, “Type II — diverticulitis”, “Type III — mesenteric adentitis”, “Type IV — ascending colon carcinoma” so there may no longer be particular reason to classify spinal vascular malformations. We can simply give each patient a unique diagnosis — indeed, spinal dural fistula is as different from spinal pial fistula as appendicitis from diverticulitis. Finally, there are some conditions, such as the spinal epidural fistula, which are not part of most classifications, but are nevertheless just as real as the juvenile AVMs. In the process of our evolving understanding of spinal vascular disease, many names have been proposed for the same entity, and grading of severity introduced on top of those. The resulting confusion was attacked by introduction of yet more classifications, with predictably opposite results. A table, shown below, loosely associates some of the more or less known classification systems, with the accompanying disclaimer. The interested reader can search the web for original articles, some of which have been liked throughout this text.
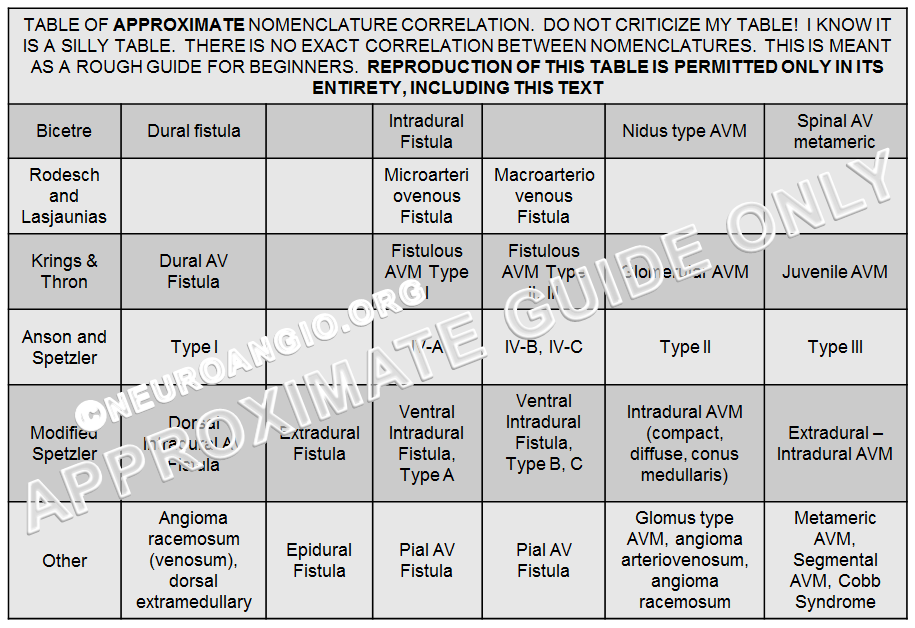
The Bicetre classification system, perhaps, is one with an enduring clinical purpose — to associate spinal pial fistulas and AVMs with known genetic (both spontaneous and hereditary mutations) disorders. For example, complex intradural fistulas (a.k.a. VI-B, VI-C, macroarteriovenous fistula; Fistulous AVM Type II, III; Ventral Intradural Fistula Types B, C; pial fistula) are frequently seen in patients with Parkes-Weber or HHT syndrome. The utility of such a system is to prompt a genetic evaluation in a patient with no known genetic disease, based on presence of a suggestive spinal lesion. It is also useful for research purposes in all manner of known genetic vascular conditions, and potentially in discovering new ones. The classification did not gain wide acceptance. I do think, however, that neurointerventionalists should play a more global diagnostic role in the spine by alerting our referring physicians to the potential of an underlying genetic disorder in patients with complex intradural fistulas. Many physicians outside of the neurointerventional community are not aware of the existence of spinal (or brain) pial fisulas, and certainly the majority do not know of the potential association between spinal fistulas and genetic disorders, and it is our duty to inform them of this.
Angiographic evaluation
The purpose of diagnostic angiography is to establish a definitive diagnosis and provide sufficient information about the lesion to enable a meaningful discussion of treatment options, with corresponding risks and advantages. This is especially critical in the spine, where the margin of treatment error is particularly slim. This requires expert understanding of spinal vascular normal and pathologic anatomy. For the spinal pial fisula, this means identifying every possible contribution to the fistula, positively identifying regional anterior and posterior spinal arteries, and recognizing the status of veins in terms of congestion, embolization “safety”, etc. Spinal fistulas can have one or more feeding pedicles — finding one does not mean that others do not exist. This requires a complete spinal angiogram — which for example includes median sacral and bilateral internal iliac arteries (where spinal fistulas are known to be overlooked). The fistula, by definition, will be supplied by either anterior spinal artery, posterior spinal artery or arteries, or both. Depending on that, treatment risks can be assigned — posterior spinal or radiculopial arteries are generally safer to embolize than anterior spinal ones.
Embolization
The goal of endocascular treatment for the spinal intradural fistula (and any other fistula) is to close the actual fistula point, while sparing as whatever necessary adjacent arterial and venous angioarchitecture exists. In practice, durable closure requires use of a liquid embolic agent — nBCA or Onyx. For spinal works, most groups still prefer nBCA, as do we. Unless the fistula point is completely closed, the fistula will recur in a predictable sequence of clinical improvement, followed by re-appearance of symptoms as collaterals reconstitute the shunt.
The venous “safety” question is particularly important. Spinal dural fistula venous safety is represented by the length of the bridging vein — longer in the lumbosacral spine, and quite short in the mid-thoracic segments — paralleling the length of the nerve roots, even though these veins are not supposed to be, strictly speaking according to Lasjaunias and Krings, “radicular”. For the pial fistula, the venous safety is usually short — the fistula empties essentially directly into cord surface veins, and their integrity is critical to physiologic drainage of the spinal cord. Embolizing these veins actually makes the situation worse, similar to early surgical results of venous stripping before the true pathoanatomy of the spinal dural fistula was recognized. So the key is usually to take some vein (enough to make sure the glue sets in the fistula itself), but not too much. From a personal perspective, I think its important to appreciate that all embolization, even in the best of flow arrest circumstances, has a sizable component of unpredictability. The final success is only enhanced by considering of how the embolization might not go according to the plan. Much of course depends on the exact angioarchitecture of the fistula. Various embolization techniques have been described.
Illustrative Cases
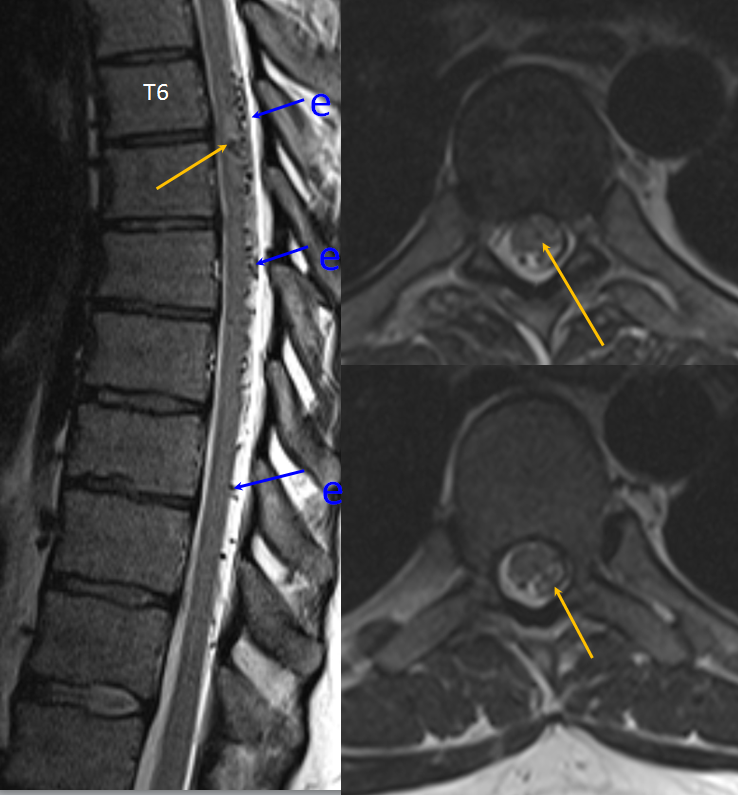
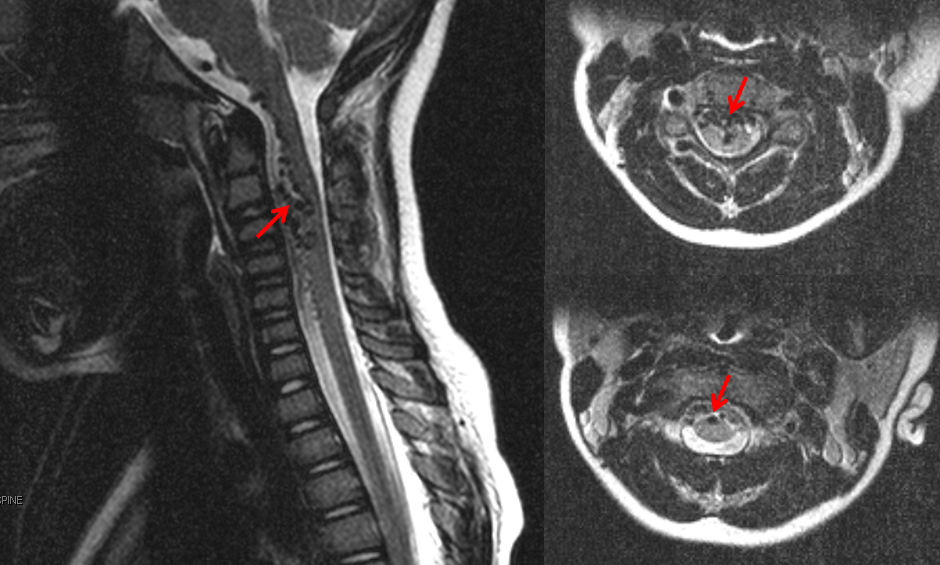
Mid-sagittal T2-weighted MRI of a patient with a spinal pial fistula. Notice concentration of veins over the thoracic spine, with sparing of the conus and lack of conus signal abnormality. These findings should suggest to the astute clinician that this may not be a typical spinal dural fistula. Note also focal T2 hyperintensity in he cord above a mass-like vascular structure (yellow arrow), which prompted concern for presence of an aneurysm.
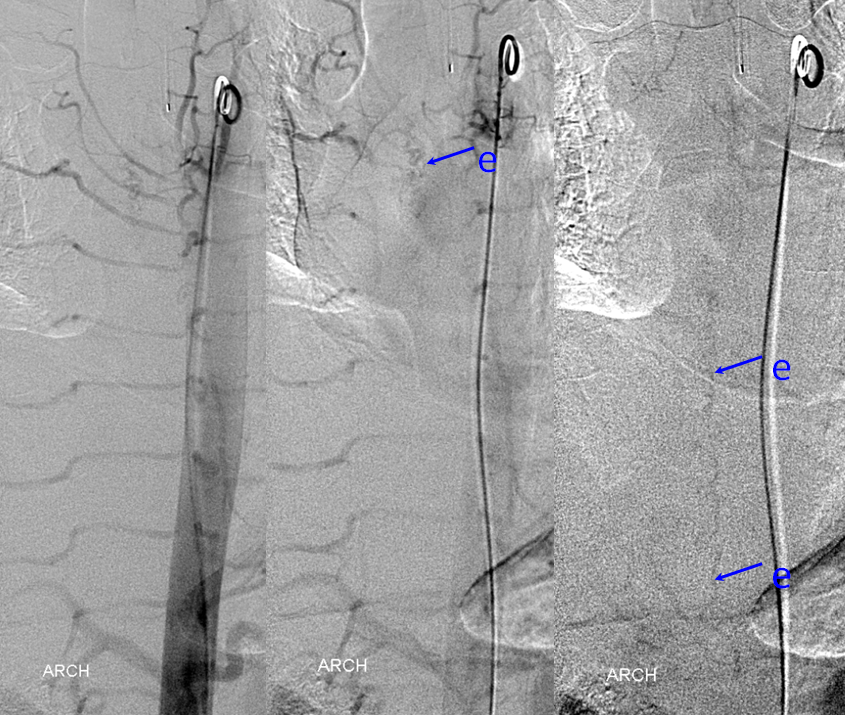
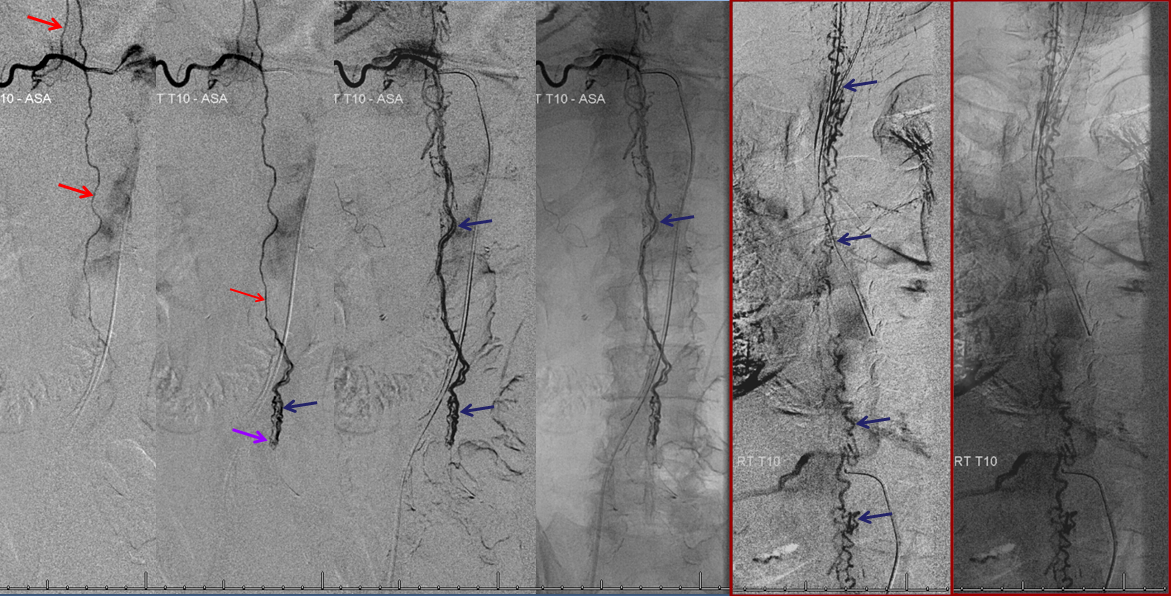
Aortic runoff is very valuable for obtaining a “lay of the land” view of things. In this case, dilated spinal veins (e) are crystal clear in late arterial and parenchymal phases, which suggests a high flow fistula, again poining away from the more classic dural fistula, and allowing to start the angiogram at suspected location of the shunt.
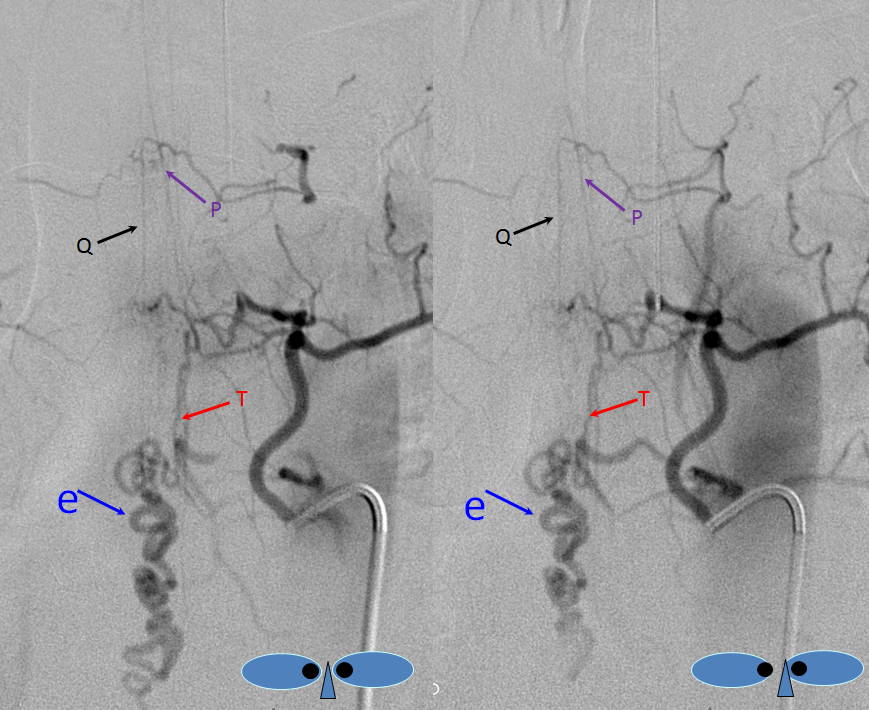
The fistula is found at the right T4 segmental artery level. The fistula is supplied by the posterior spinal artery (red arrow, T), opening into the same dilated paraspinal veins seen on the MRI and aortic runoff. Conveniently located at the same level is a radiculomedullary artery (P) opacifying regional anterior spinal artery (Q), showing conclusively that the anterior spinal aretery does NOT supply the shunt. This is very strong grounds for suspecting a pial fistula, since most intramedullary AVMs are going to have anterior spinal artery supply — this ASA is responsible for most of cord tissue, and therefore will be involved in nearly all parenchymal AVMs. The picture below is one of pial (intradural) fistula located on the posterolateral surface of the cord. Enjoy the stereo.
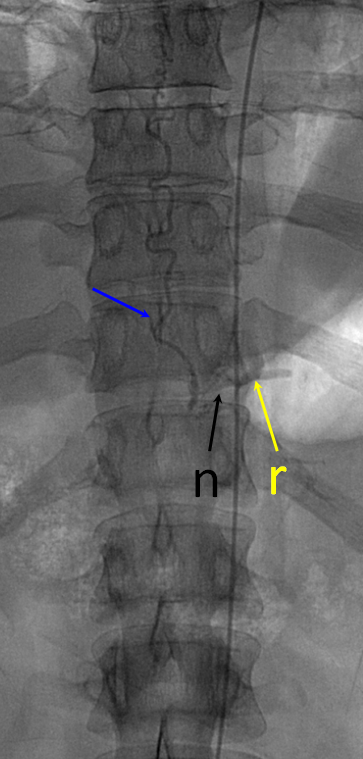
Defining venous runoff is critical. In this case, caudal drainange of spinal surface veins extends to a briding vein at the left T11 level, where the fistula ultimately decompresses via foraminal veins (n) into paraspinal veins (r)

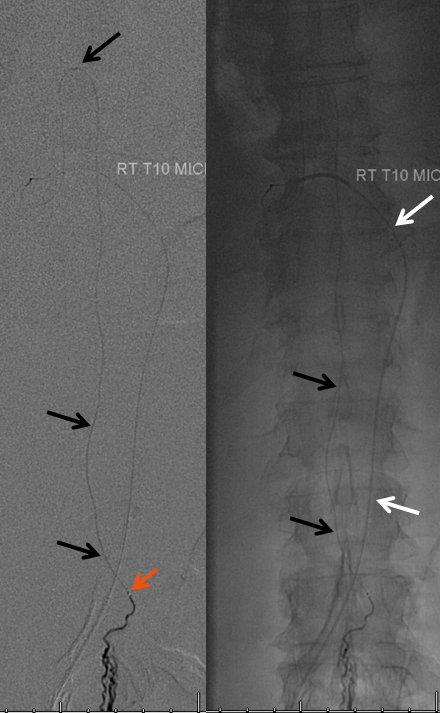
Same picture with bone landmarks.
The anterior spinal artery axis should be defined above and below the level of the lesion, should compromise of the immediately adjacent radiculomedullary artery occur.
Complex Cervical Spinal Fistula (Macro-AVF, IV-C)
This child presented with neck pain and vomiting. No hemorrhage was seen on MRI nor found on lumbar puncture. MRI shows a tangle of intradural vessels (red arrows)
Angiography defines the anterior spinal axis (pink), supplied from the right vertebal artery via a well-seen radiculomedullary artery (yellow). Notice reflux into a somewhat lower radiculomedullary artery on the left (green arrow), implying that another contributor must be present. This turns out to be the left deep cervical artery, injection of which, in turn, allows for reflux into the radiculomedullary artery of the right vert (yellow arrows). Notice also exquisitely demonstrated multiple segmental muscular anastomoses between the deep cervical and the left vert (brown arrows).
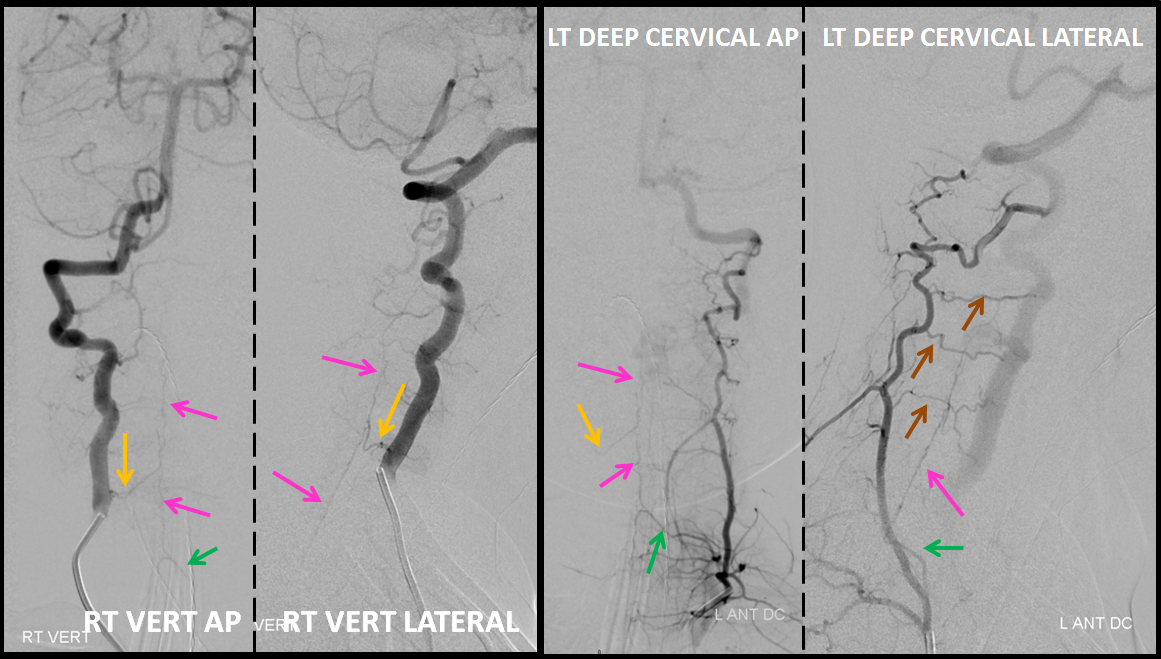
It is critical to define the anterior spinal axis in all spinal vascular cases. In this case, it is conclusively established that the ASA does not participate in supply of the AV shunt, which makes the diagnosis of an AVM very unlikely and, in any case, reduces the risk of embolization.
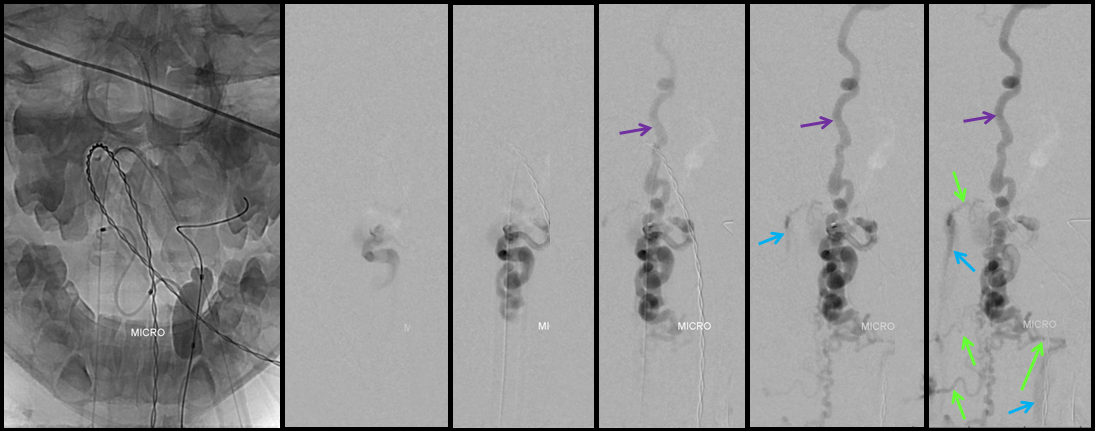
A series of frontal and lateral projection digital subtraction angiographic views of the left vertebral artery demonstrates the arteriovenous shunt (red), supplied by a single radiculopial pedicle (yellow). Venous drainage is conducted cranially into an enlarged anterior spinal / anterior medullary vein (purple), and subsequently via the lateral mesencephalic vein (black) into the superior petrosal sinus (white) and jugular vein (dark blue). Inferiorely, there is exquisite demonstration of several bridging (radicular) veins (bright green) draining into the vertebral venous plexus surrounding the vertebral arteries (light blue), and via several (trans) foraminal veins (brown) out of the spinal canal into regional paravertebral veins. The busy appearane of tortuous veins (red) may be mistaken for an AVM nidus. This is the so-called complex / macro / Type IV-C fistula. However, lack of anterior spinal supply already makes AVM extremely unlikely.
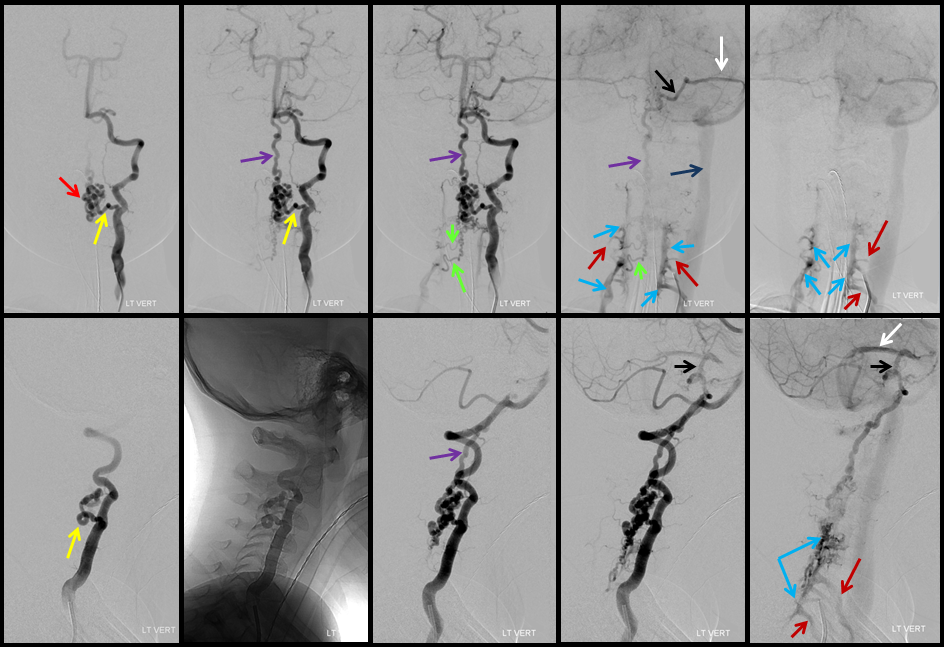
A microcatheter run with a balloon inflated in the vertebral artery across the feeding pedicle (to further guard against reflux of nBCA), shows better the fistulous nature of the lesion.
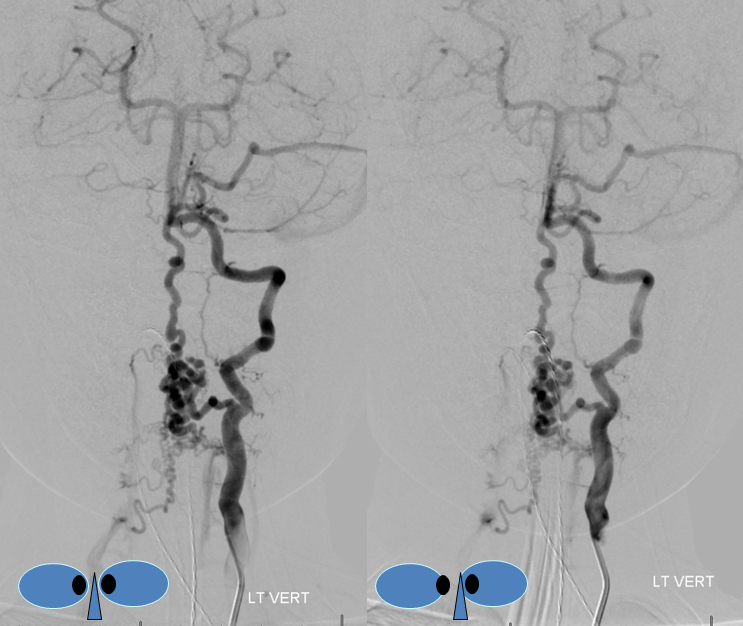
A nice stereo view of right vertebral injection, for pure visual pleasure.
Conus intradural fistula
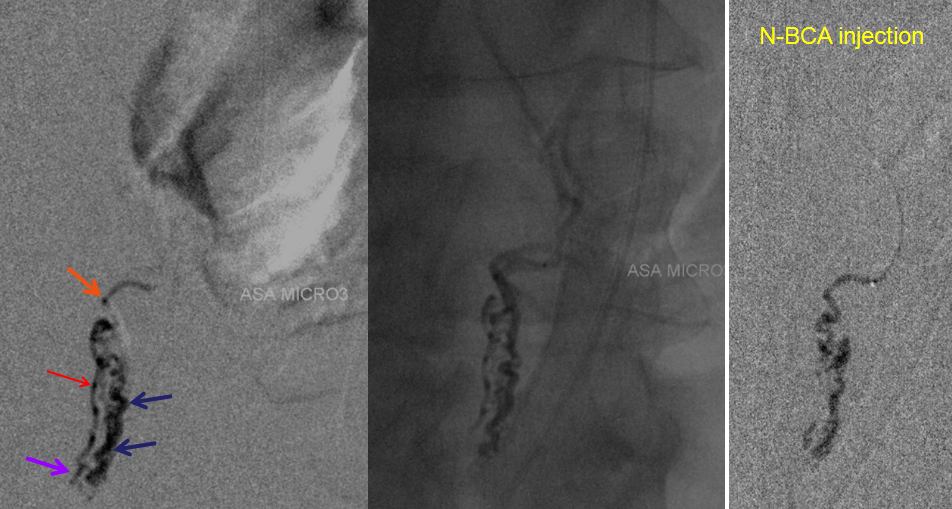
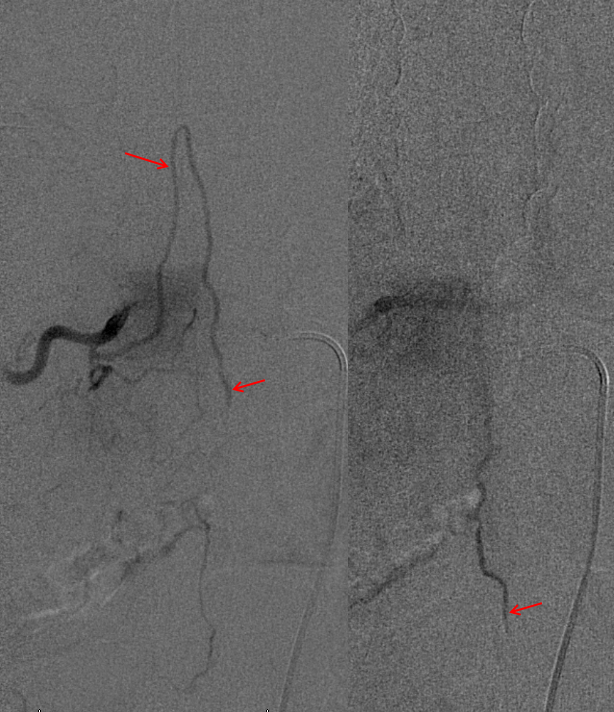
Further reading
For unsurpassed discussion of spinal vascular malformations, nothing beats Lasjaunias & Berenstein 1st edition, volume 5, dated 1990, and Lasjaunias, Berenstein, and Ter Brugge 2nd edition Chapter 11 — still the best. Note that academic institutions and other organizations may have access to full book PDF version through SpringerLink.
An excellent review by the heavyweights — Krings, Lasjaunias, Thron — published in Neurosurgical Review, is available free of charge here: http://campus.neurochirurgie.fr/IMG/pdf/JJM3.pdf or by clicking here.
