Perhaps there is an even more obscure topic out there. But this is not grade school math — we are not searching for the lowest denominator. Venous embryology, a topic of far less exposure than even its arterial counterpart — is just as clinically relevant for a given set of pathologic and physiologic conditions. Serious students of neurointerventional radiology should have a level of understanding here — hence the purpose of this page is to provide one with basic knowledge and some clinical correlations. The occasional reader looking for more will be well served by reading the sources mentioned below.
This work is heavily based on seminal investigations carried out by Dr. George Linius Streeter, Dorcas Hager Padget, and later by Dr. Yun Peng Huang and his colleagues; this page is essentially a condensed and thus imperfect summary of their work, augmented by modern angiographic and non-invasive cross-sectional imaging examples. Clicking on images below will take the reader to sites with additional information on the aforementioned pioneers. The original idea was to present this topic separately from a standalone Yun Peng Huang venous embryology page. However, I later decided to place the entire page under the Yun Peng Huang Collection umbrella, both to highlight the contribution of Dr. Huang and his colleagues to the small body of venous embryologic knowledge, and to place their work in appropriate and clinically-relevant context.
Yun Peng Huang, MD
Summary: Though arterial development receives considerably more attention, venous neuroembryology is critical in appreciating the full spectrum of venous physiology and pathophysiology, including a vast array of “normal” variants, predominantly nonpathologic states such as Developmental Venous Anomalies, and pathologic processes such as “Vein of Galen” aneurysms, Sturge-Weber Syndrome (encephalotrigeminal angiomatosis), and others.
By the time the neural tube closes, simple diffusion cannot sustain the demands of growing tissue. An early arteriovenous plexus, known as meninx primitiva, envelops the tube on the outside. Invagination of the same meninx into the tube gives rise to the choroid plexus, which is the most vascular structure related to the central nervous system at this stage. Drainage of the thin neural tissue is therefore centrifugal (inside to outside), while that of the internal choroid plexus is centripetal and has little to do with neural tissue. With continued growth, coalescence of plexiform menix primitiva into future dural sinuses and early surface venous channels takes place; some of these will regress due to continued development of the brain and skull. Maturation of transverse and sigmoid sinuses is dictated early on by development of brainstem structures, rather than the comparatively insignificant neocortex which grows subsequently. Venous (and arterial) channels which form later in development (torcular and basal vein, for example) are prone to more variations than those established early on. Development of basal ganglia and other deep structures leads to emergence of the paired internal cerebral venous system draining in centripetal fashion towards the primitive Vein of Markowski, of which the terminal part remains as the Vein of Galen. Tremendous growth of the neopallial cortex leads to emergence of robust surface venous channels such as Rolando, Trolard, Labbe, and superficial Sylvian veins. Balance between superficial and deep venous systems, with a “watershed” in the deep white matter, is exemplified by frequent persistence of venous arrangements favoring either superficial or deep drainage, known as “Developmental Venous Anomalies” or “Medullary Venous Malformations“, and by extreme variants such as absence of effective superficial drainage in Encephalotrigeminal Angiomatosis, aka Sturge-Weber. Venous development continues beyond birth, for example with maturation of connections between the Sylvian veins and Cavernous Sinus (which is developmentally an extra-dural structure) and dwindling of the occipital sinus. These post-natal developments are important in management of conditions such as some Vein of Galen aneurysms.
Developmental Stages
At ~5 mm Crown-Rump length, the meninx primitiva which envelops the developing brain coalesces into anterior, middle, and posterior plexi, corresponding to the three brain vesicles. The brain is drained by paired primary head sinuses. Note relatively small size of the prosencephalon at this stage. There is no deep venous drainage yet.
At 10 mm, there is a primitive marginal sinus. The telencephalon has not developed enough yet for a more extensive sinus system, however there is a primitive telencephalic vein now. The primitive maxillary vein will ultimately develop into the cavernous sinus. The entire drainage is directed into the primary head sinus, which is located ventral to the otic vesicle (which is important)
At 14 mm stage, there is noticeable growth of the otic vesicle. The earliest sinus of the telencephalon is the marginal sinus. A developing telencephalic vein is seen (black marker) — notice that it is draining posteriorly, and not into the future cavernous sinus. There are no midline sinus structures and the telencephalon is very small compared with the other two vesicles.
At 18 mm stage, many important things are taking place. According to Padget, growth of the otic vesicle forms a kind of barrier between the primary head sinus/ stem of middle dural plexus (red), and the internal jugular vein. As a result, the sigmoid sinus (orange) emerges as a secondary anastomosis between the marginal and developing transverse sinus and the jugular vein. The adult inferior petrosal sinus however is located ventral to the otic vesicle — I am not sure that I fully buy the explanation, but the fact is that sigmoid sinus appears to be a “secondary” structure of sorts.
Also developing is a midline venous structure from the heretofore dominant paired primitive mariginal sinuses (green) — the sagittal plexus (pink). The tentorial sinus is also well-visible in these embryo images, and drains posteriorly.
The developing choroidal plexus leads to emergence of a deep venous system, which is only shown schematically here, as a primitive straight sinus.
24 mm stage
The 24 mm stage shows continued development is marked by growth of the choroid plexus (red), with its blood supply and drainage, as seen from MRI images below:
An emerging vein connecting the plexus to the developing superior sagittal sinus is labeled by Padget as “internal cerebral vein”. However, most consider it to be the somewhat cult-classically famous “Primitive Vein of Markowski (VoM)”. This is a single, unpaired vein throughout its length (pink arrows, “A” below). According to the presently accepted theory, the true internal cerebral veins (purple arrows, “B” below) develop somewhat later, with the growth of the now yet insignificant basal ganglia and periventricular tissues. These future paired internal cerebral veins will drain into the Vein of Markowski, such that the posterior part of the VoM, beyond the confluence of the internal cerebral veins, persists as the Great vein of Galen (pink arrow, “B” below). The anterior or ventral part either remains a tiny channel somewhere in the roof of the third ventricle (white arrows, “B” below), or maybe disappears altogether (less likely). However, if a high flow state in the VoM persists, such as due to a developing “vein of Galen” aneurysmal malformation (black arrow, “C” below, the arteries feeding the shunt are marked with red arrows), the VoM will persist.
Another source of drainage of the choroid plexus are the dorsal and ventral diencephalic veins, which drain the choroid plexus into the marginal and tentorial sinuses respectively. Notice absence of basal vein. In contrast to superficial lateral veins of the future cerebral hemispheres (such as the Trolard or Labbe veins), the basal vein is embryonically more related to drainage of the choroid plexus than the cerebral mantle. It does not form until the ~80 mm stage, and even so often remains discontinuous. It is a general rule of embryology that structures which form later in development show more variation than earlier ones.
Notice also lack of connection between the developing superficial middle cerebral vein and the cavernous sinus — the latter drains a prominent supraorbital (a.k.a. superior ophthalmic) vein only.
Carnegie Stage 23
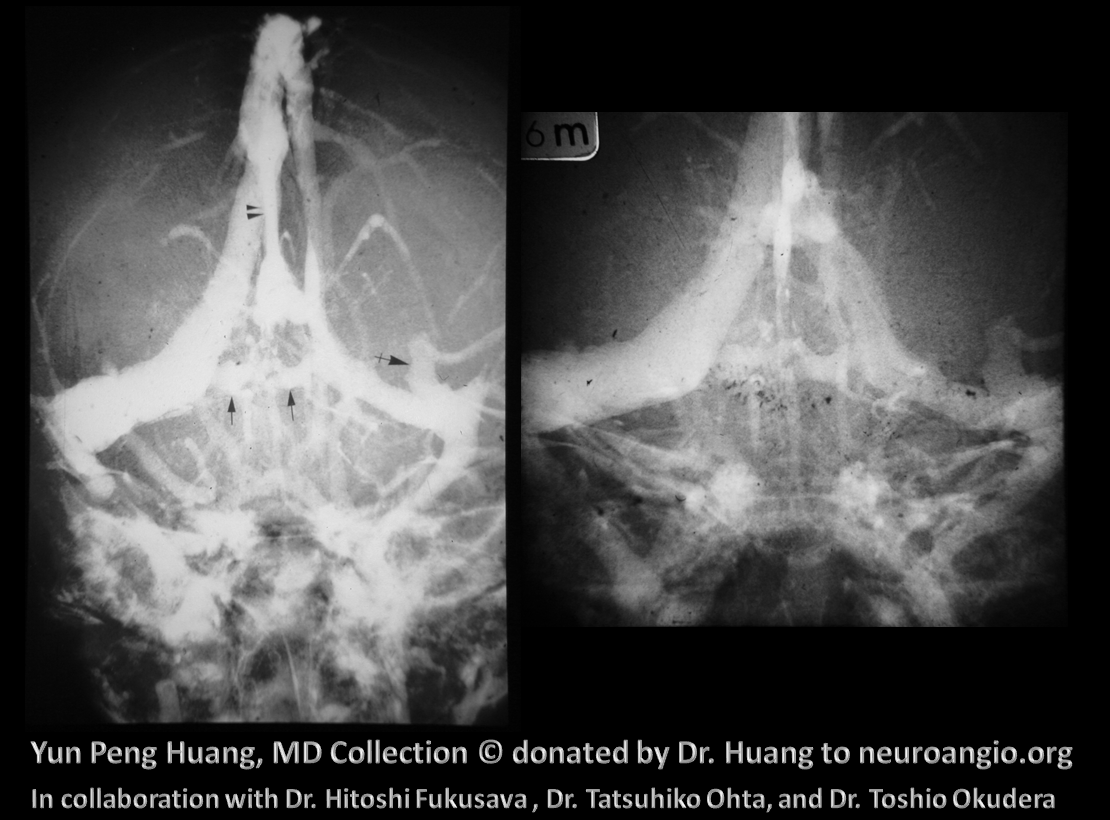
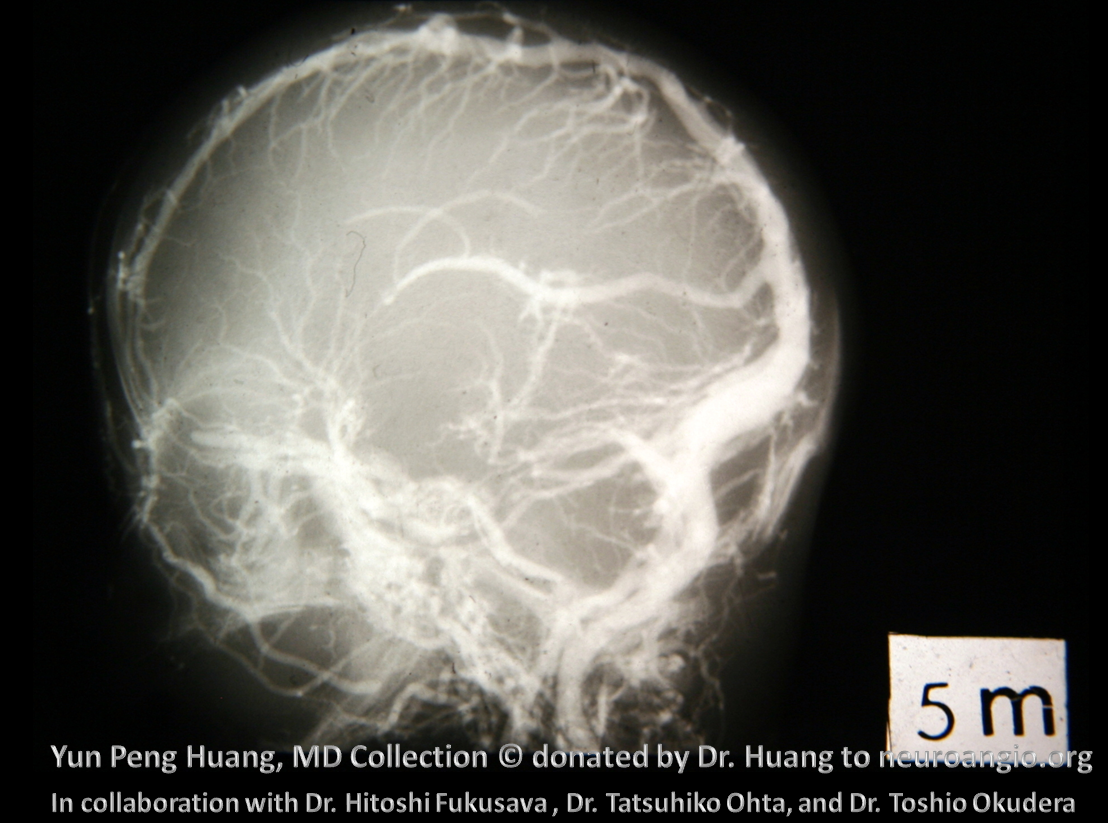
The anterior venous plexus persists until pretty late; dominant venous channels arising from it will eventually form the torcular. Because the process is late to occur, many variations in torcular morphology will result, including frequent presence of multiple superior sagittal sinus-like channels leading up to the torcular, multiple channels, shift of distal SSS away from midline, etc — all attesting to its late plexiform nature. This is important for surgeons operating in the area. Below is an example of plexiform sagittal sinus variation in two 6 month old embryos from the Yun Peng Huang Collection. The right and left limbs of the sinus are particularly well seen on the right image. This makes the “torcular” somewhat of a moving target
Extreme variation of sagittal sinus plexiform nature in an adult with a GBM
Also, for various to me still unproven reasons, the right jugular outflow and right-sided transverse and sigmoid sinuses are usually larger than the left ones. Most explanations have to do with the right atrium being on the right side, somehow. Some even suggest that a strongly left-dominant jugular is more likely to be seen in patients with developmental cardiovascular anomalies or may even be pathologic in itself.
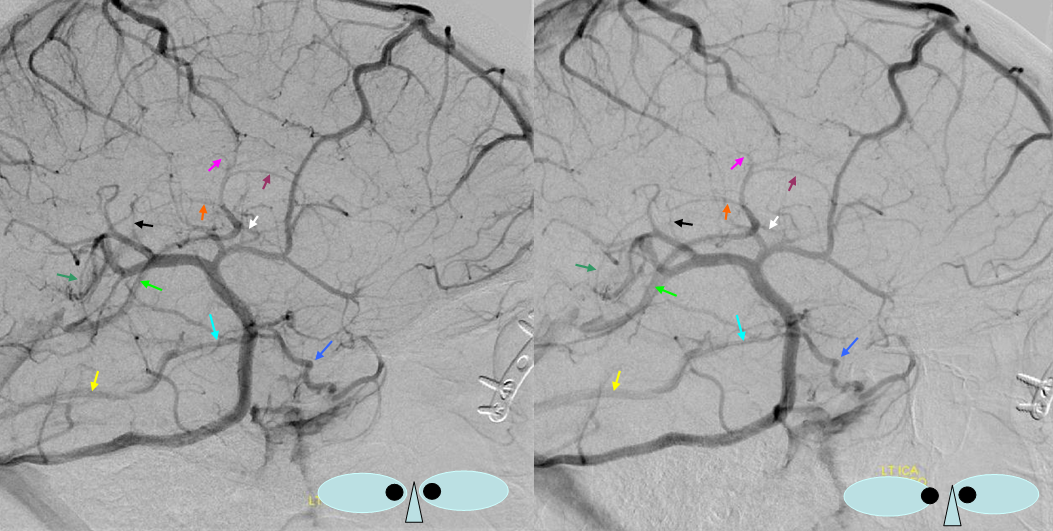
The middle cerebral veins continue to drain via the tentorial sinus into the marginal/transverse sinus systems. According to the classical dogma, this sinus will dwindle, except for its proximal part, which will persist as the sphenoparietal sinus — this explanation at least clarifies the otherwise inappropriate “parietal” portion of the name. This sphenoparietal sinus, which also receives the somewhat obscure middle meningeal veins, will later join the cavernous sinus — which at this point remains a structure draining tissues outside of the cranial vault — orbit, face, etc. with an inferior petrosal sinus connection.
There are several problems with this theory, in my opinion. For one, i am not sure why we do not more often see persistence of the full tentorial sinus in the adult (Padget draws a separate superior petrosal sinus). Second, some believe that superficial sylvian veins drain directly into the cavernous sinus and the sphenoparietal sinus is a separate structure (see Cavernous Sinus page). Third, the idea that Sylvian veins do not connect with the cavernous sinus until postnatal period is i think not supported by more modern observations facilitated by use of MR and angio in newborns and in embryos of Huang et al, as seen below in several of Dr. Huang’s embryos where sylvian vein drainage seems to be cavernous-bound
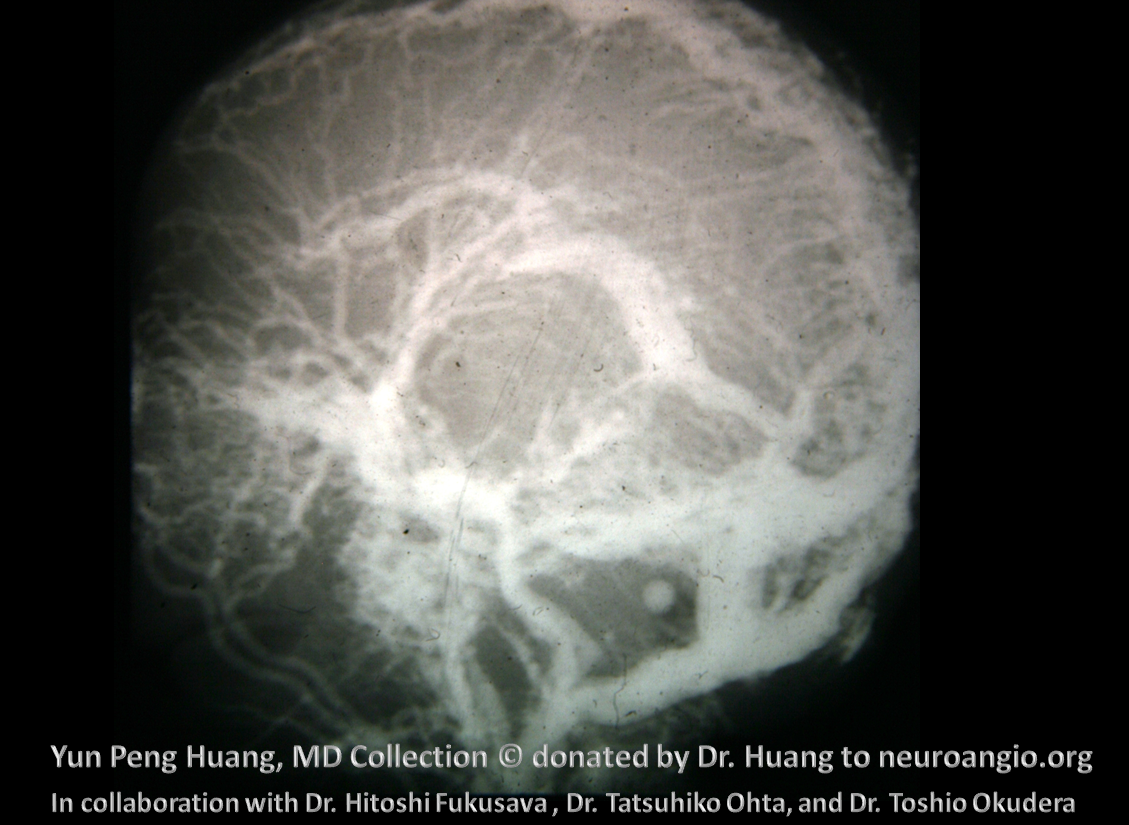
Another example
Whatever the case, you now know the dogma and its critique, to some extent.
The basal vein area is drained by separate venous channels — such as dorsal and ventral diencephalic veins. It is late to form and shows multiple variations in the adult (see below as well as deep venous system page)
End of First Trimester
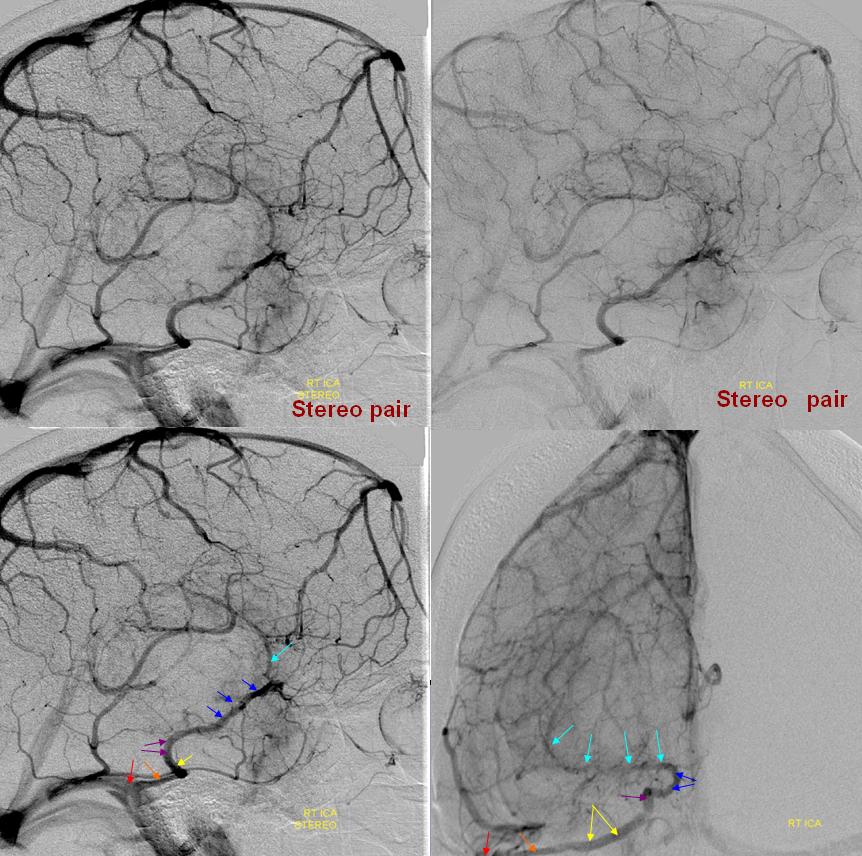
Broadly speaking, end of embryogenesis corresponds to establishment of a near-developed venous pattern. The torcular is pretty much established. So is the internal cerebral venous system blueprint. The delinquent basal vein, still in formation, is a network of telencephalic, diencephalic, and mesencephalic components. Notice that it development represents an illustration of the recurring theme when longitudinal anastomoses emerge between initially disconnected transverse venous channels — in this case between the inferior choroidal, dorsal, and ventral diencephalic veins, and the mesencephalic vein. All of these will represent, in the adult, the many variations in drainage of the basal vein. In its full expression, basal vein is an unbroken conduit between the cavernous sinus and the Galen. Any of the three parts of the Rosenthal can be hypoplastic, however, and alternative drainage of the basal vein into the lateral mesencephalic vein attests to its developmental role as a longtitudinal connector.
Stereo pair of the fully developed basal vein (pink, black, purple) connecting the cavernous sinus to the Galen
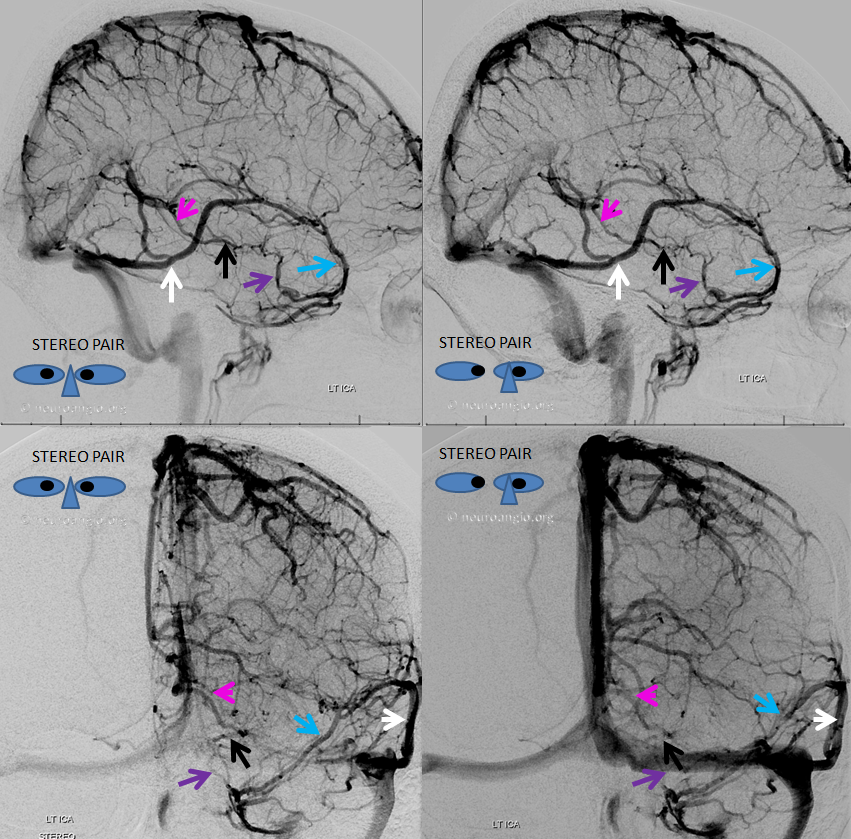
Here, basal vein drains via a short tentorial sinus (posterior purple arrow) directly into straight sinus, bypassing the Galen
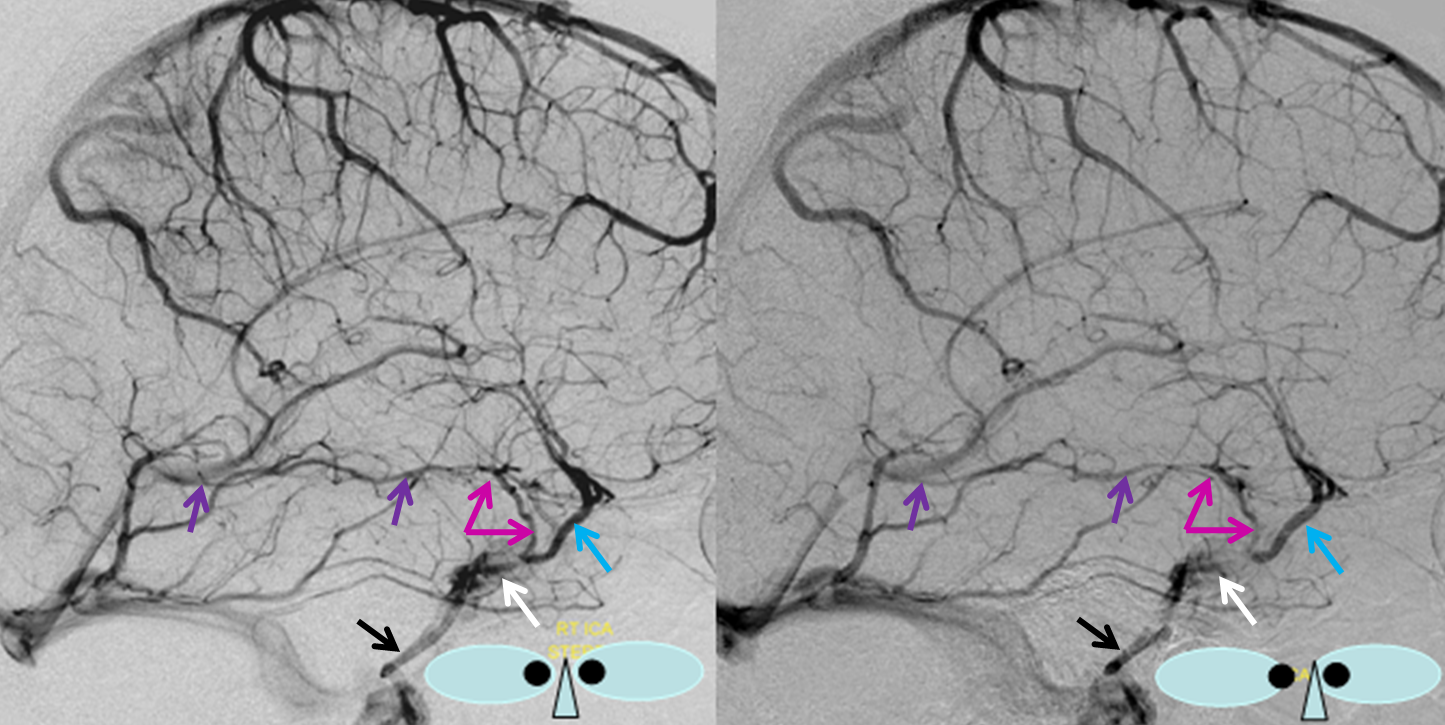
“Classic” appearance of a well-developed basal vein (purple, pink) draining exclusively towards the Galen, in stereo
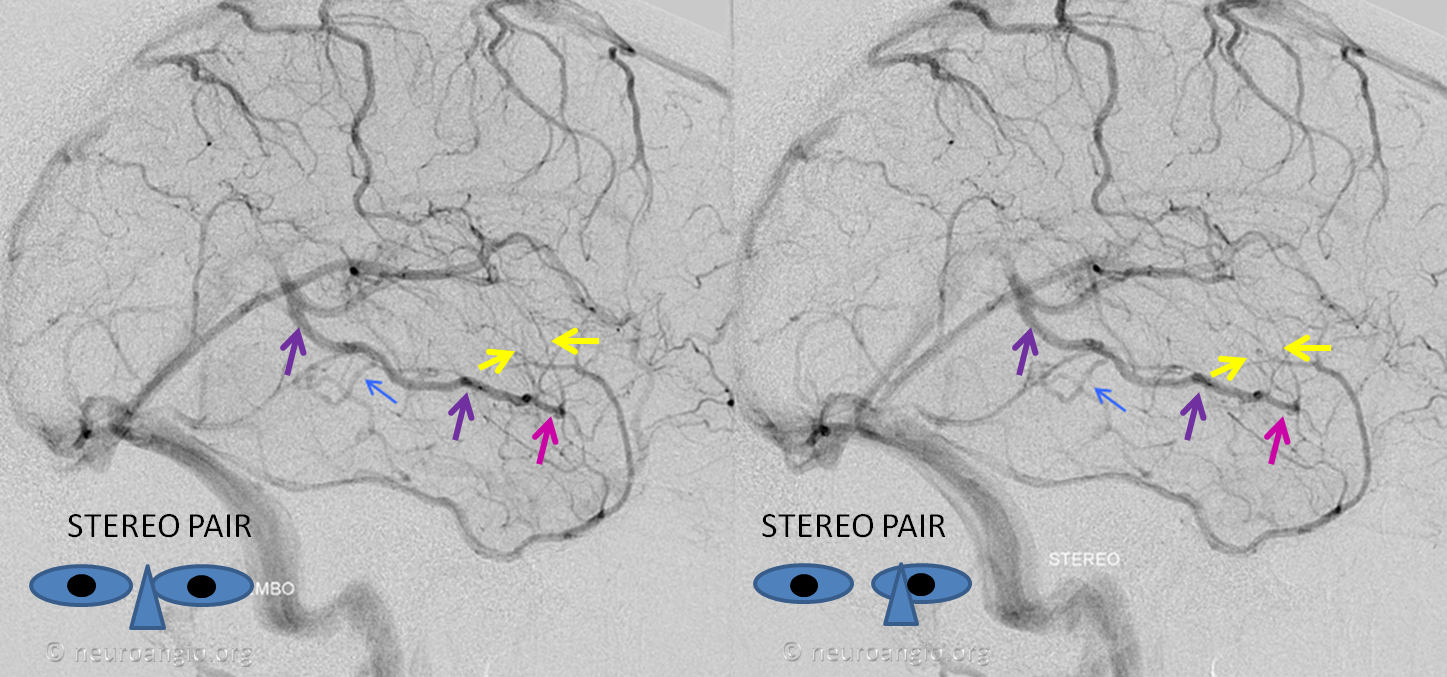
Basal vein hypoplasia
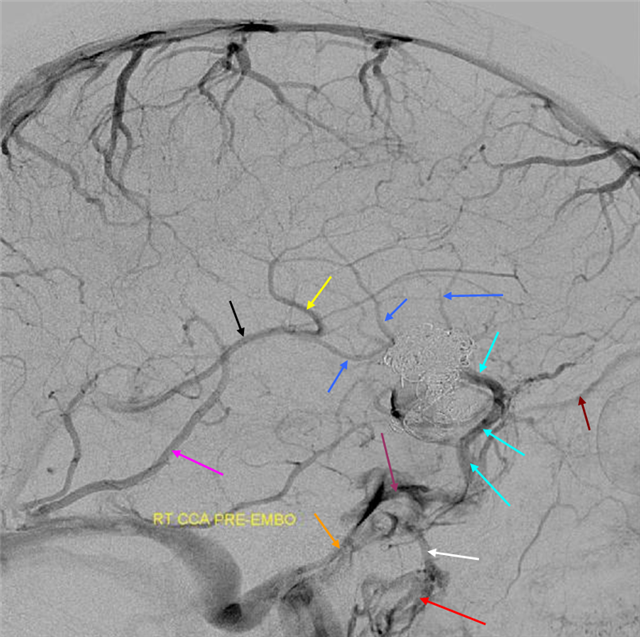
Below is an example of anterior (telencephalic and diencephalic) basal vein draining via the lateral mesencephalic vein into the superior petrosal sinus.
Basal vein (blue) draining into a tentorial sinus (yellow) — a reminder of the plexiform nature of dural sinuses which will not infrequently persist in the adult
The middle cerebral vein continues to be disconnected from the cavernous sinus, in Padget’s opinion, until after birth. The cavernous sinus is, emryologically, an extra-dural structure draining the face and orbit.
APPLICATIONS
Developmental Venous Anomaly, also known as Medullary Venous Malformation — see dedicated page for detailed discussion. Briefly, the DVA as we know it now (term coined by Lasjaunias), or MVM as Yun Peng Huang has called it, is a prominent vestige of the normally inconspicuous transcerebral venous network — thin veins connecting the supependymal network which drains into internal cerebral vein (or 4th ventricular veins such as the vein of the lateral recess) and the superficial venous system of the cortex or the cerebellum. These connections traverse the deep white matter and have the potential, over time, to partially compensate for constraint of either superficial or deep venous systems. For example, when the Galen is overwhelmed by a shunt such the falcotentorial dural fistula, the trans-cerebral network can help drain the internal cerebral vein, averting disaster. Alternatively, when the superficial system is dysfunctional, for example in cases of Encephalotrigeminal Angiomatosis, the trans-cerebral network of medullary veins is prominently recruited to attempt compensation. However, in most instances such life-saving measures are not necessary. Instead, a particular medullary vein can be found to be enlarged, ranging from slightly to very prominently, as a hypertrophied collector of the adjacent normal brain. This is what a DVA is — deep vein collecting ajdacent normal brain, and draining either towards the subependymal system (deep) or the cortex (superficial). The Developmental Venous Anomaly term emphasizes its developmental nature, and Medullary Venous Malformation name is more reflective of the white matter, medullary, location of the vein. MVM was used by Yun Peng Huang, who probably did more than anyone to understand this lesion and educate the world about its essentially non-pathologic nature. Nevertheless, the name DVA, proposed by Lasjaunias, won out in the end. As we say in the United States, such is life.
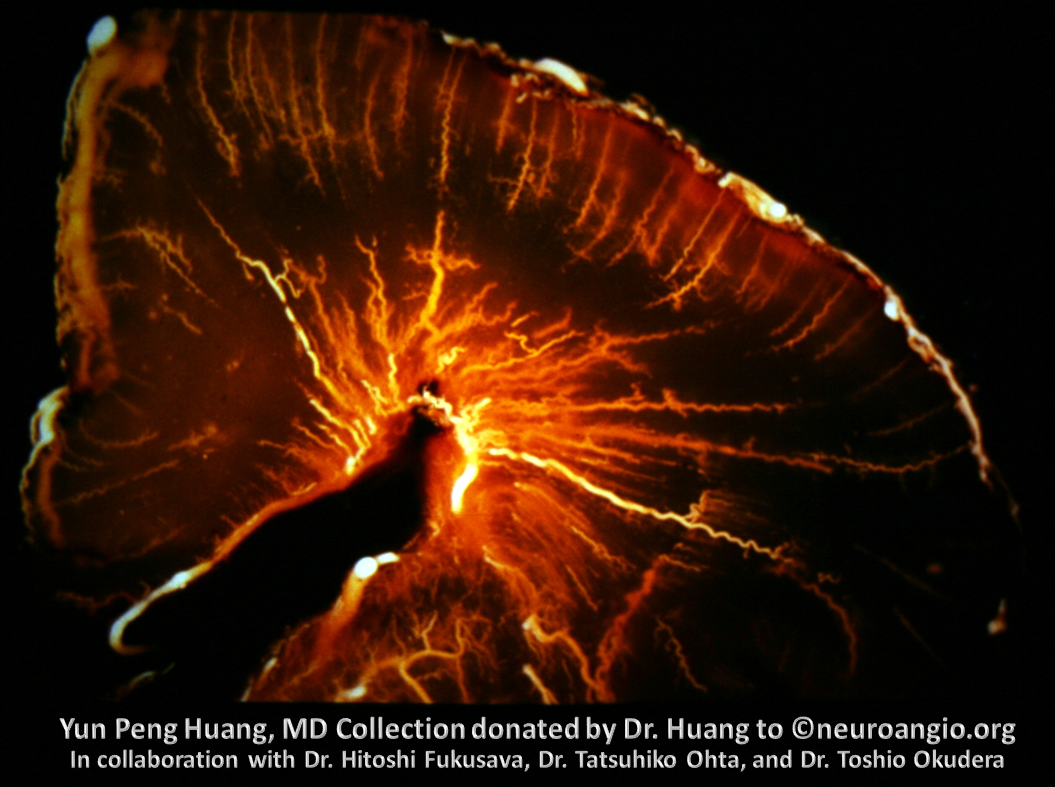
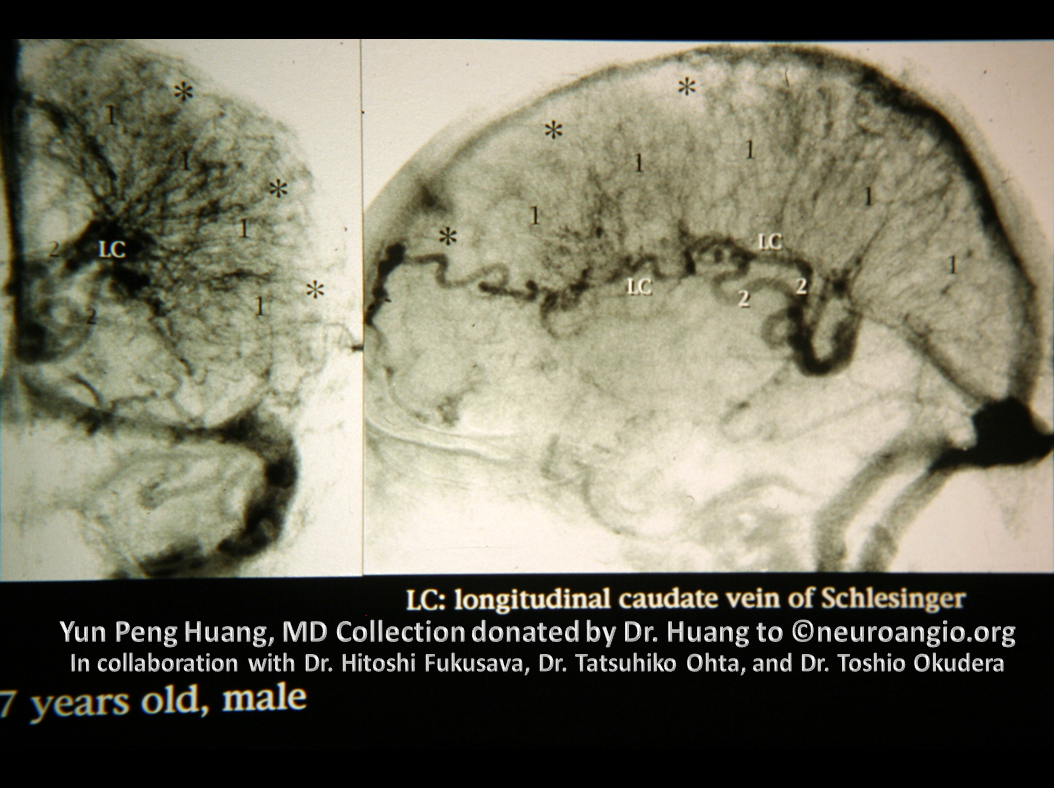
Here is an example of the transmedullary veins in the developing embryonic cortex
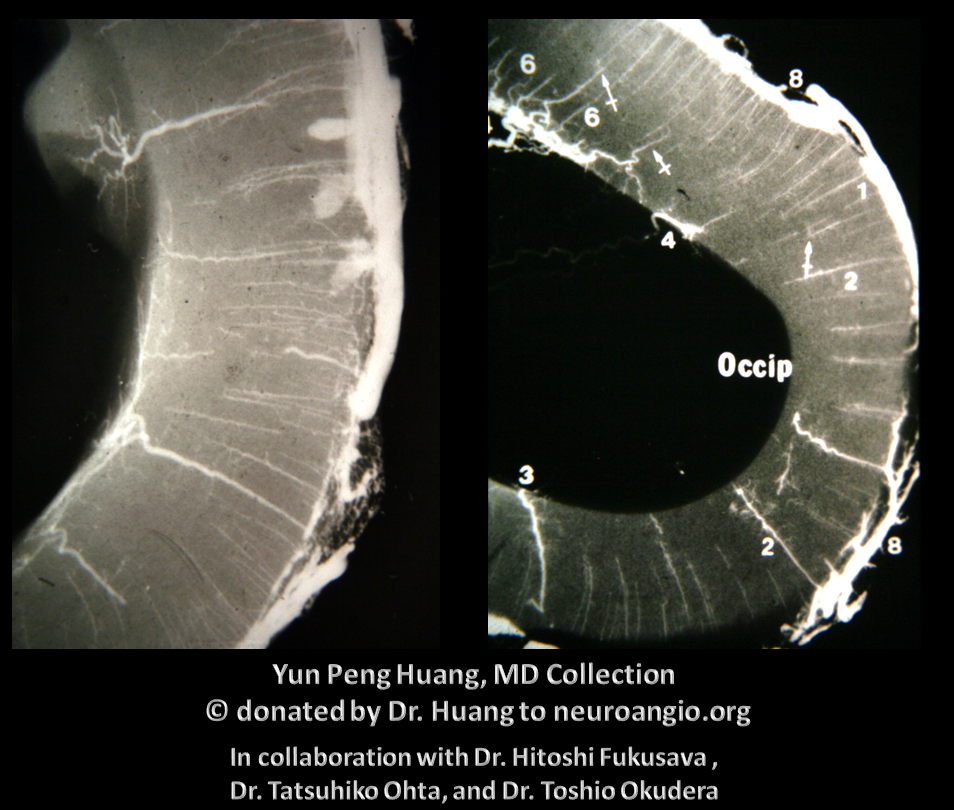
Another beautiful image
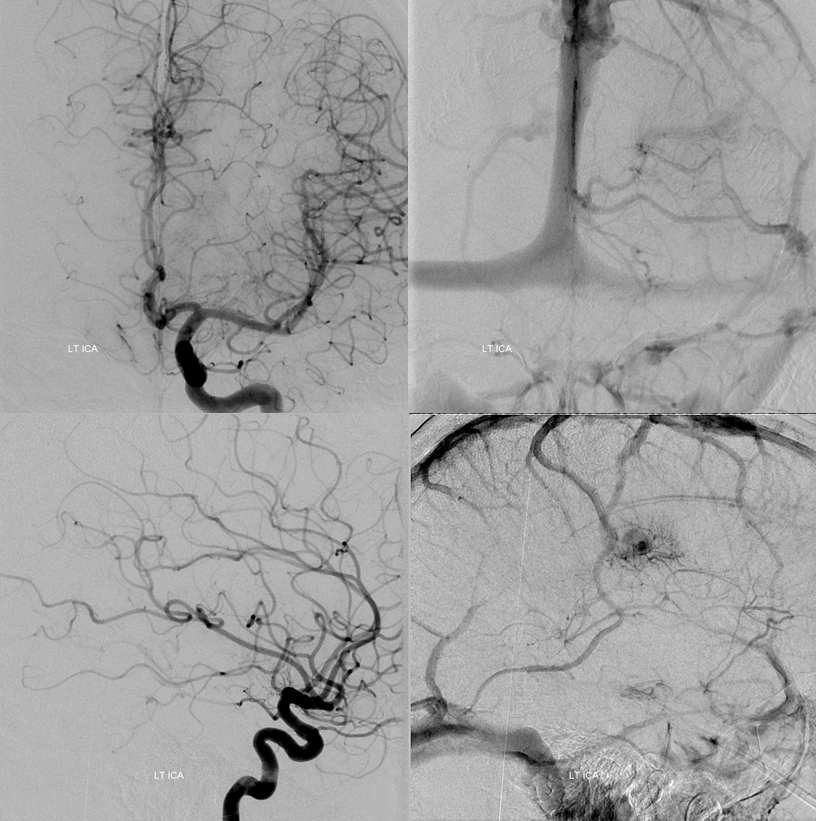
Adult brain, 7 Tesla MRI — see beautifully depicted deep veins
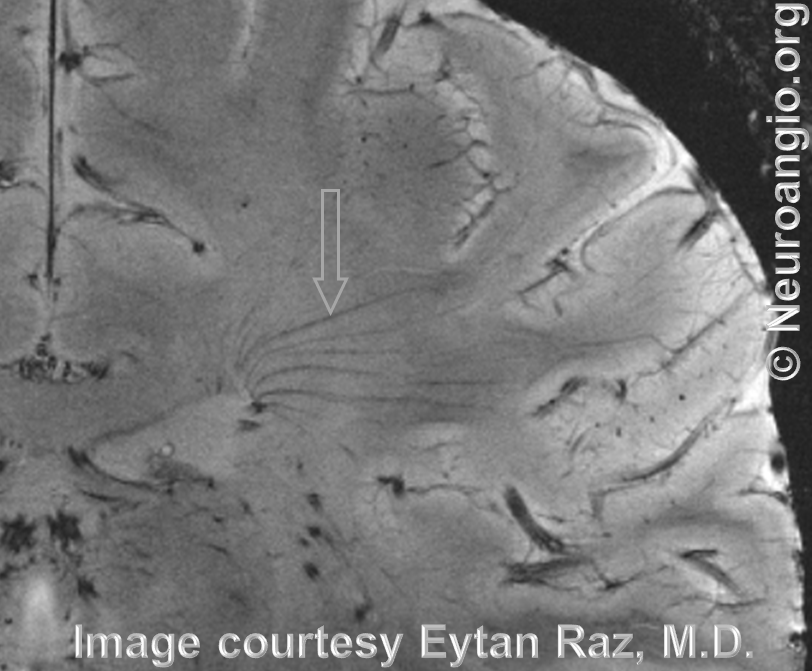
Schematic of DVA as a hypertrophied deep venous collector
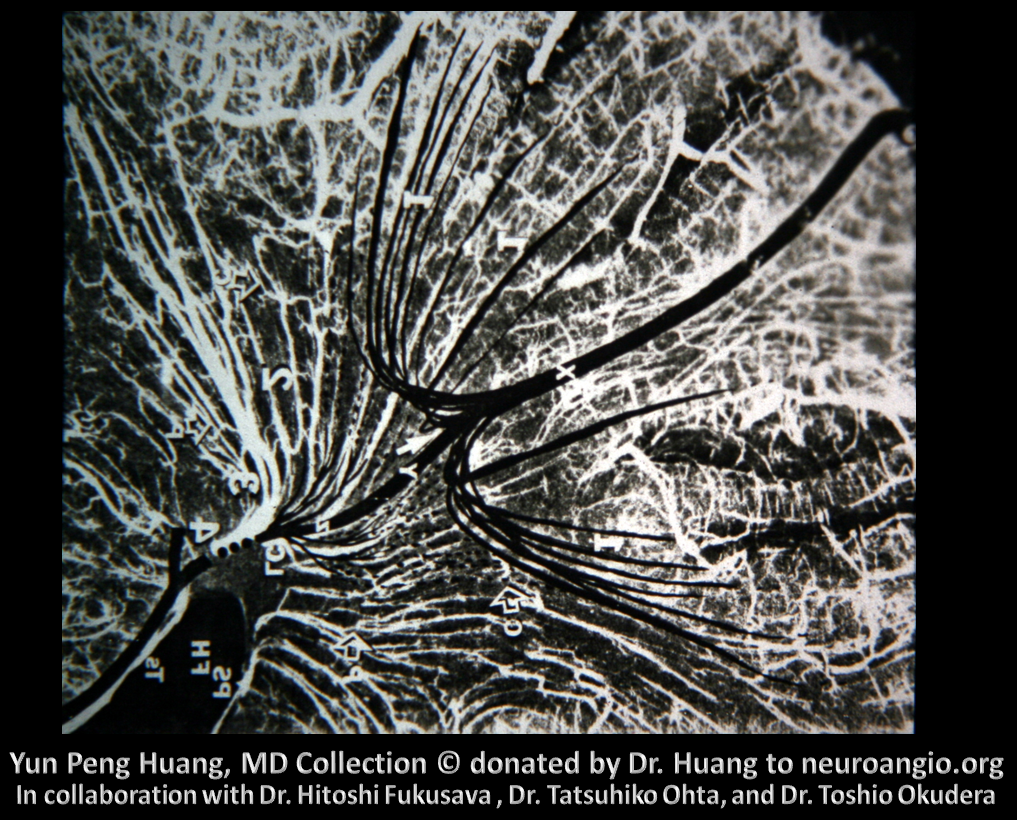
Early image from Yun Peng Huang Collection, demonstrating a DVA draining superficially
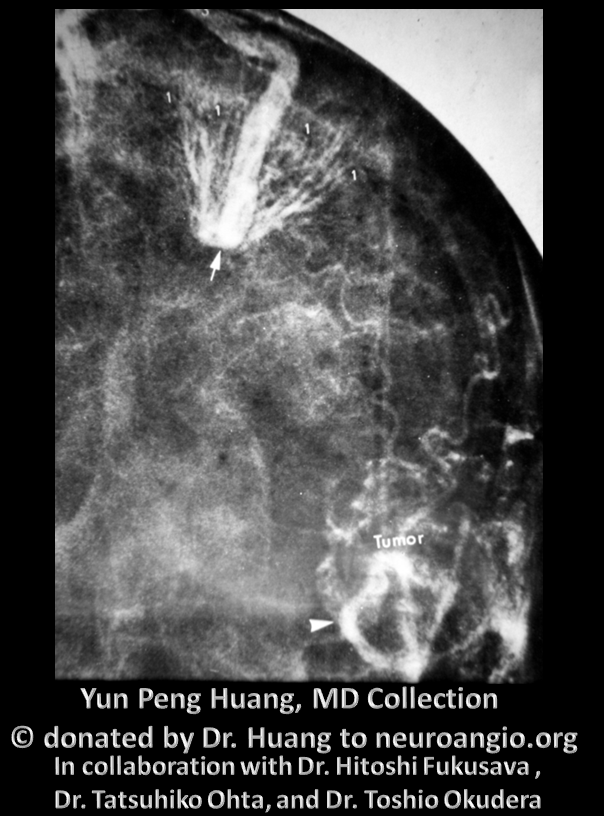
Deep draining DVA
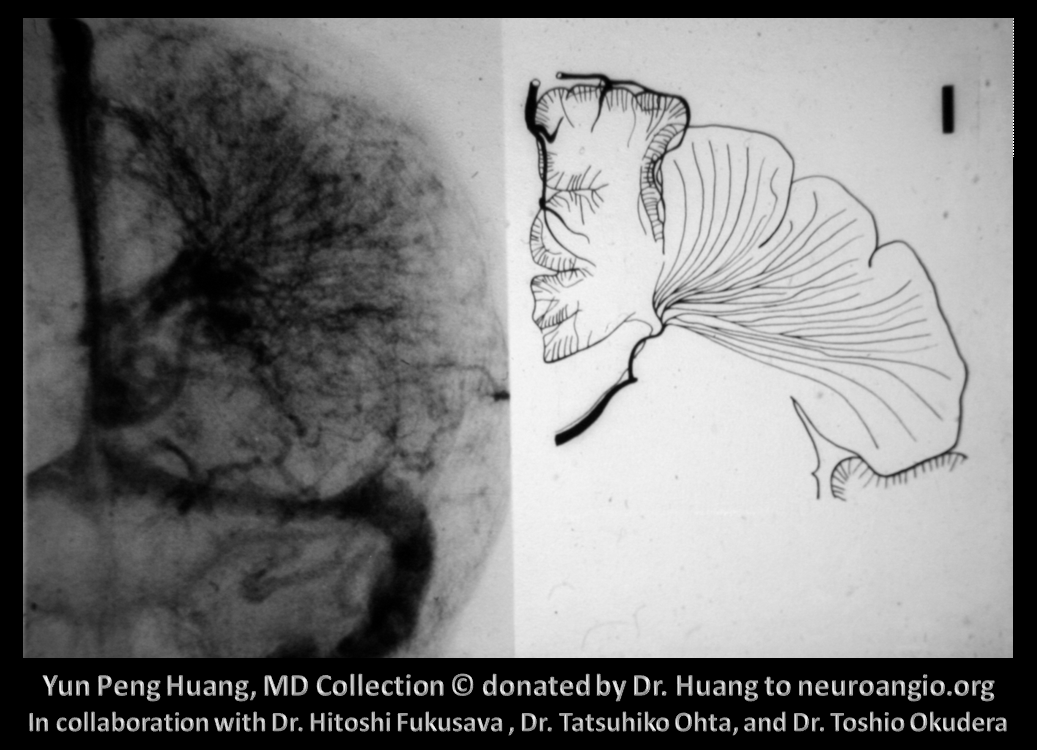
Extreme example in a patient with Sturge Weber (encephalotrigeminal angiomatosis, for the politically correct), characterized by lack of functional cortical venous network, with transmedullary drainage into the subependymal system. Another case, will full MR, CT, angio images, well worth looking at, is here
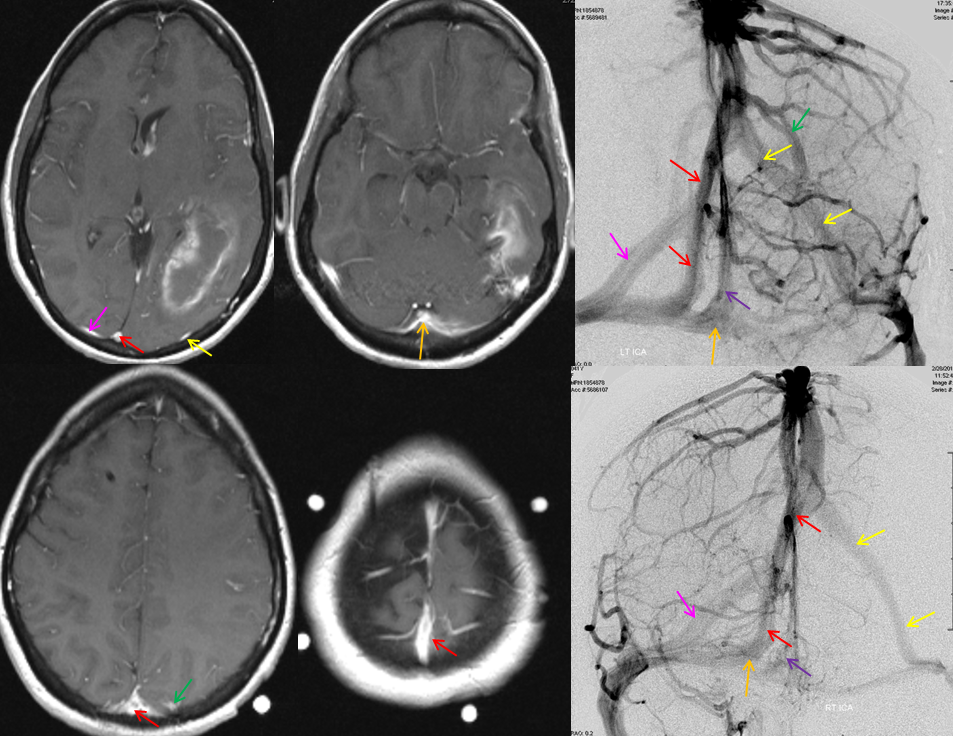
MRI of patient with falcotentorial dural fistula (see full case here) demonstrating a large transmedullary vein
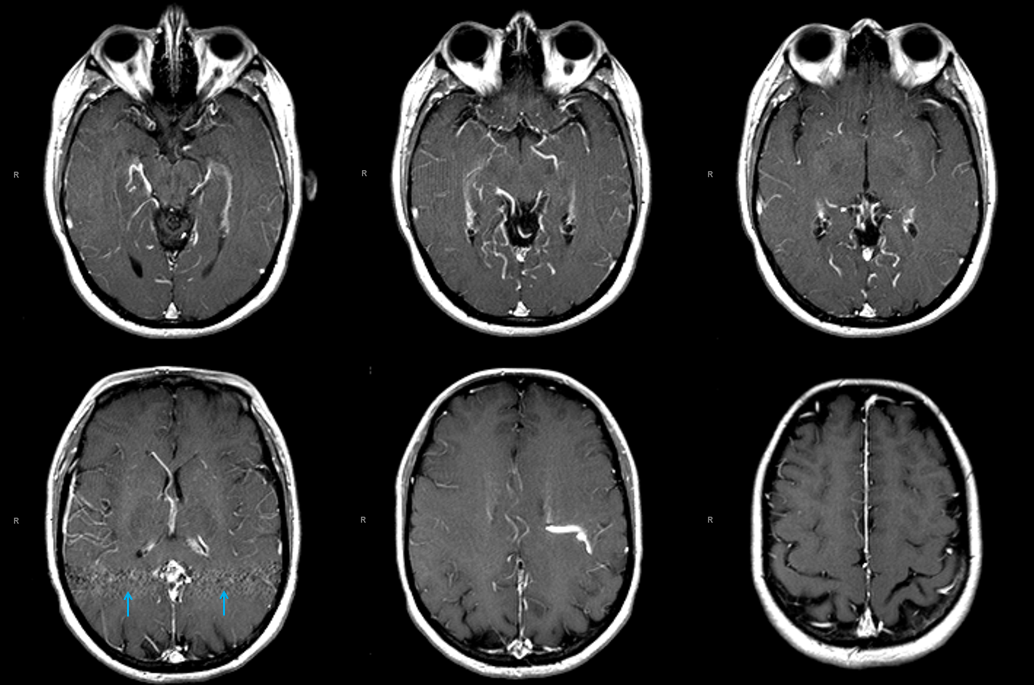
This vein helps drain internal cerebral territory, since the Galen is utilized exclusively by the fistula
Further reading
Streeter, L: https://embryology.med.unsw.edu.au/embryology/index.php/Book_-_Contributions_to_Embryology_Carnegie_Institution_No.24
Raybaud, C: https://www.ncbi.nlm.nih.gov/pubmed/20561492
Velut, S: https://www.ncbi.nlm.nih.gov/pubmed/3683704
MacDonald, L: http://thejns.org/doi/abs/10.3171/jns.1996.85.1.0001?journalCode=jns
Relevant neuroangio.org pages
Venous Brain Anatomy Section — all pages
Yun Peng Huang Collection — Medullary Venous Malformation — DVA
Encephalotrigeminal Angiomatosis — Sturge Weber
Questions/Comments/Complaints: Go here
