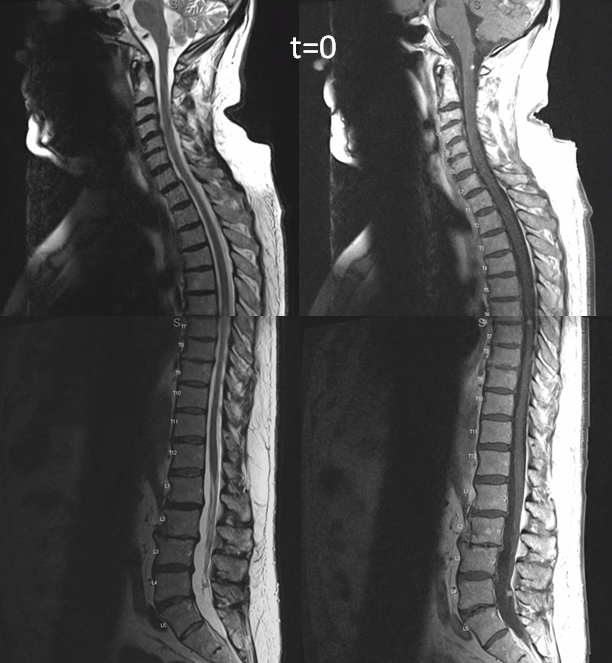
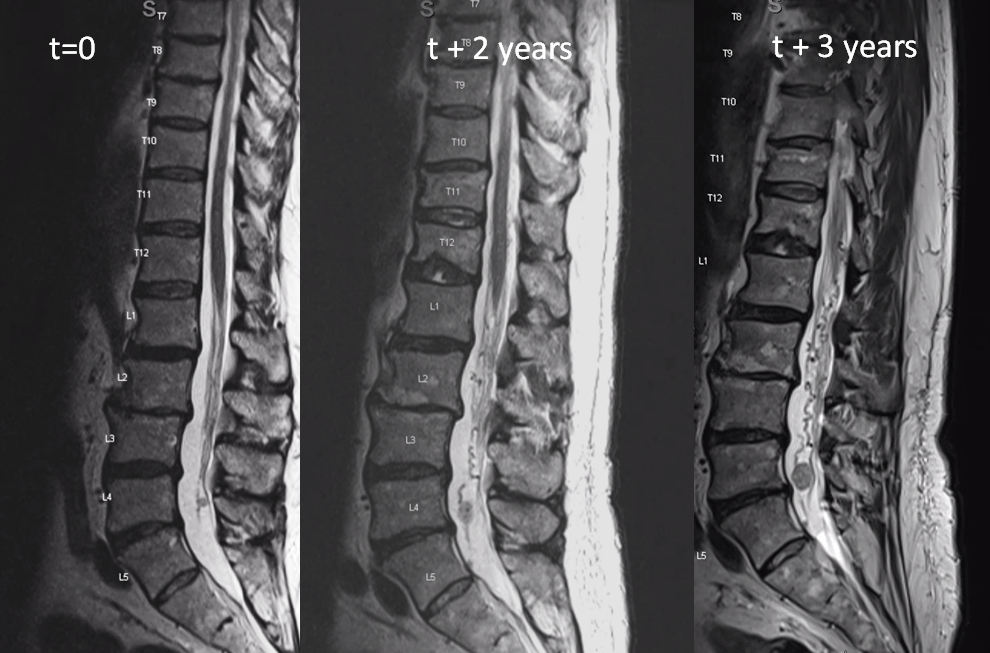
This is very unusual case of spinal vascular congestion. A patient with multiple spinal hemangioblastoma drop metastases, some previously treated with radiosurgery.
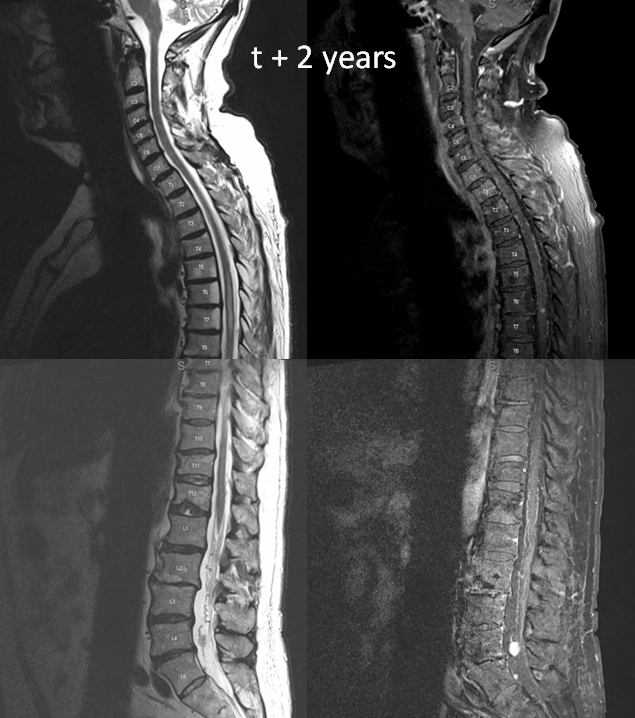
two years later, the L4/5 met is significantly larger and there are now some prominent flow voids/vessels cranial to it
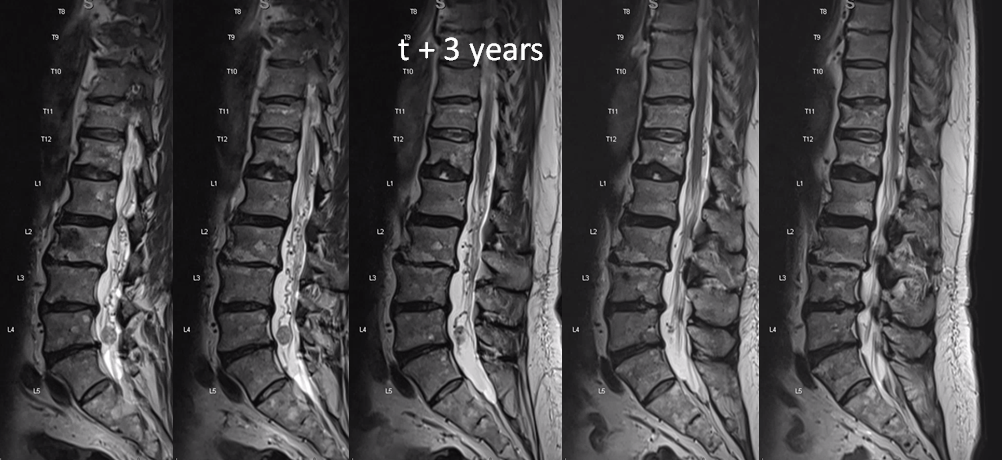
One year later. Flow voids are much larger. At this point there is low back pain, lower sensorimotor and other deficits — a clinical picture resembling spinal dural fistula

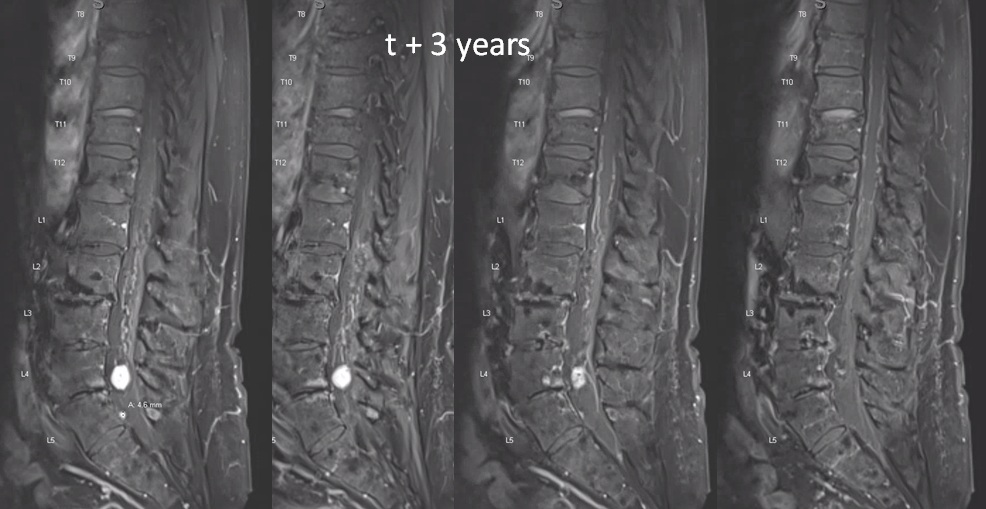
t1 post
The obvious solution is surgery. However, the patient does not want more operations. What to do? Standalone / primary embolization, if effective, can significantly necrose the tumor. Hemangioblastoma experience is nonexistent, however it does work with meningiomas. The Valavanis group probably has the most experience with this.
Side by side progression
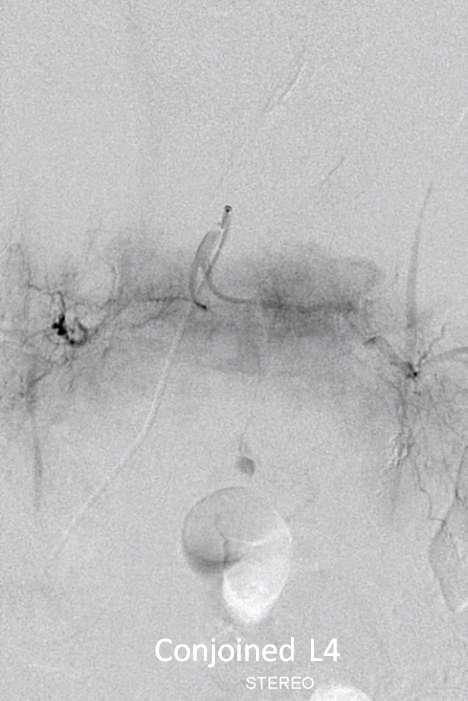
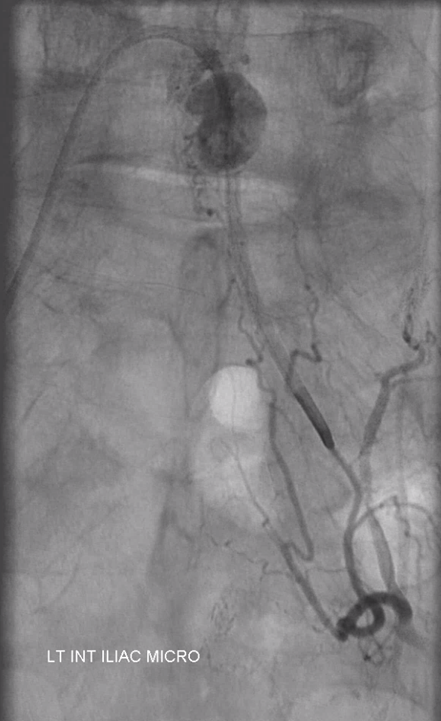
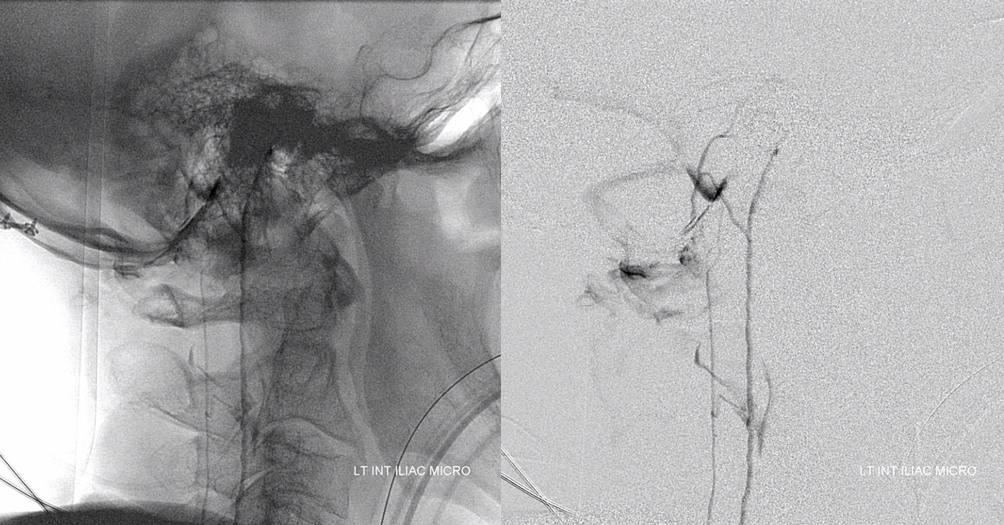
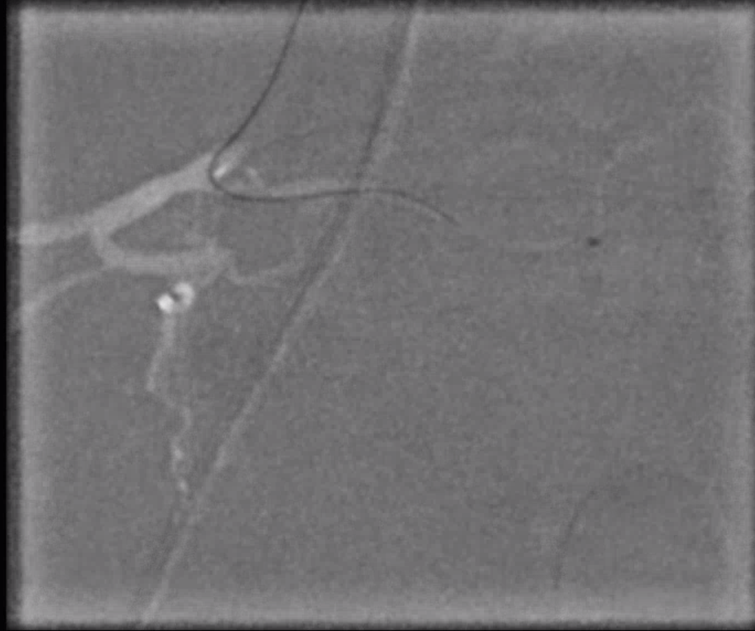
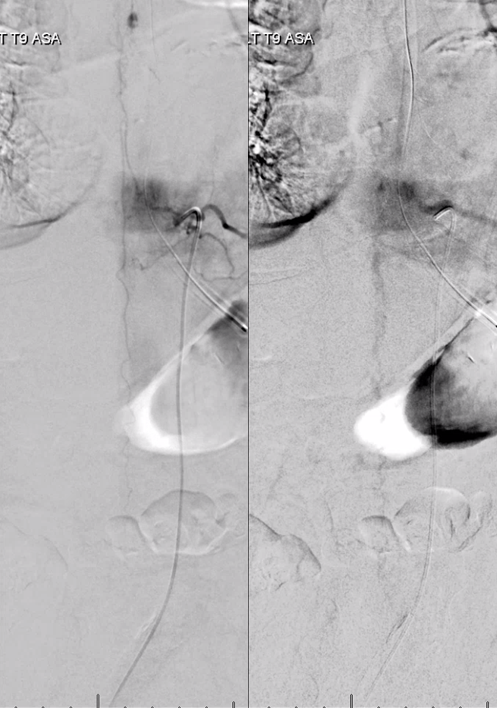
Angiogram shows tumor supply from L4 level — small bit
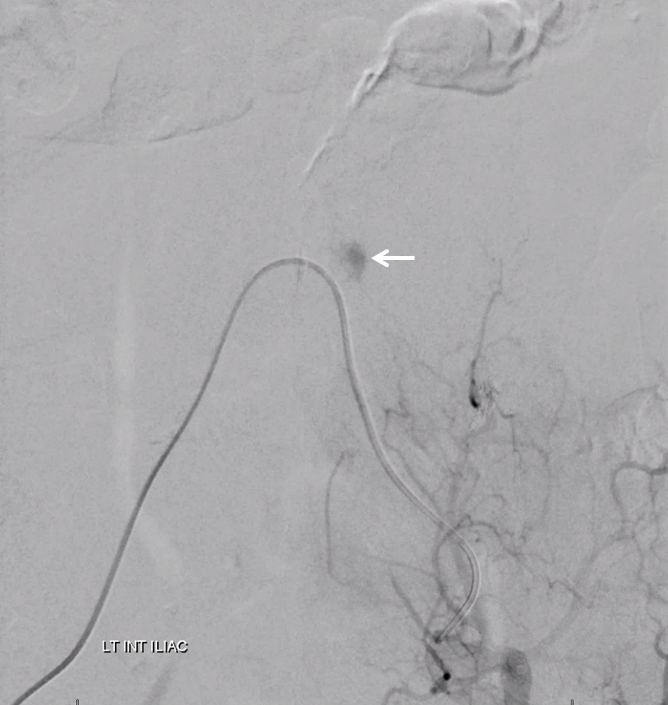
The bulk comes from L5 radicular artery, which comes off the internal iliac
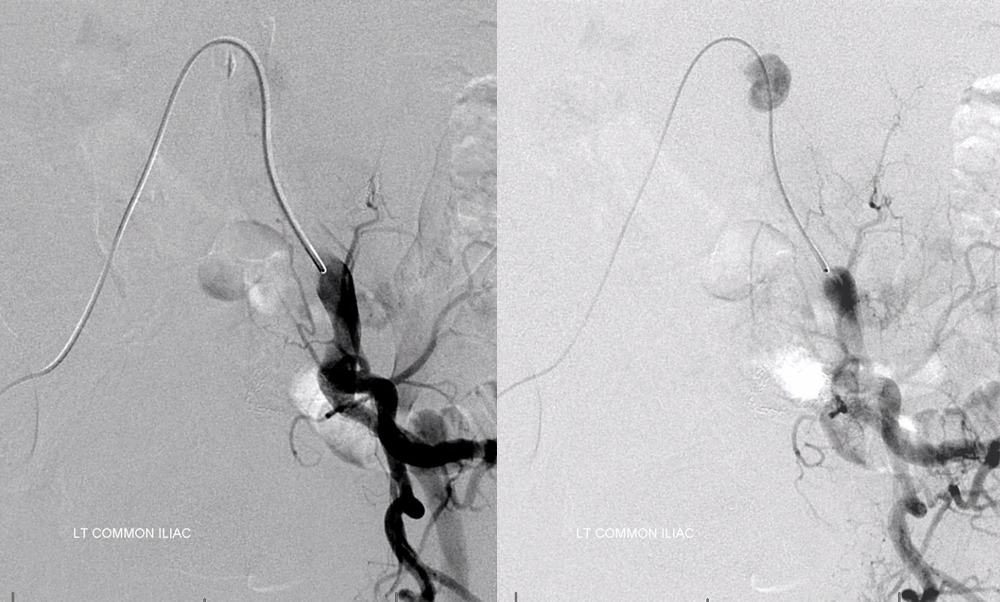
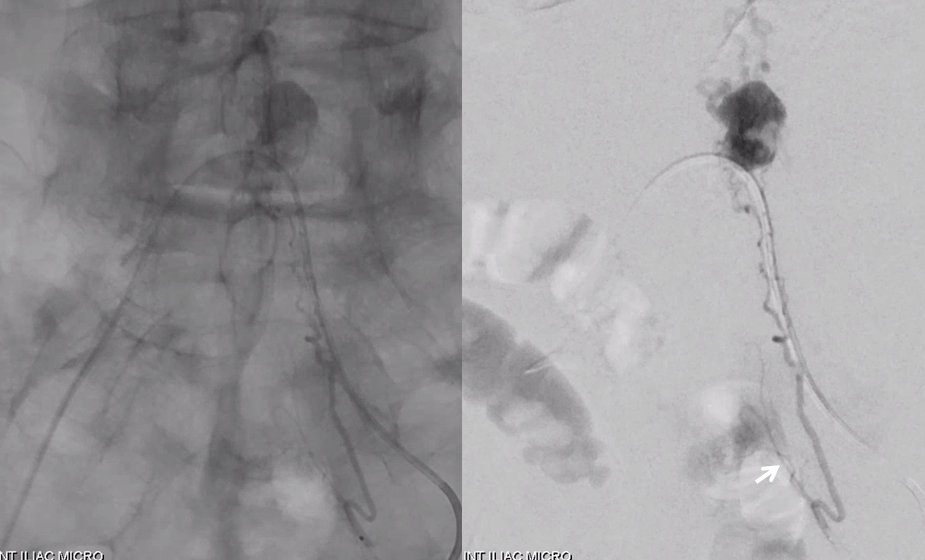
Don’t forget to inject those iliacs when looking for a dural fistula. Sequential microcatheter injections
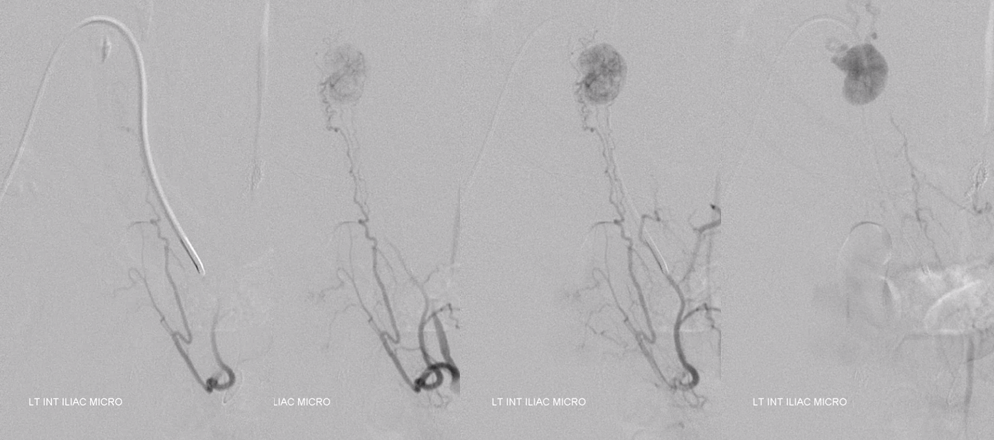
Native view
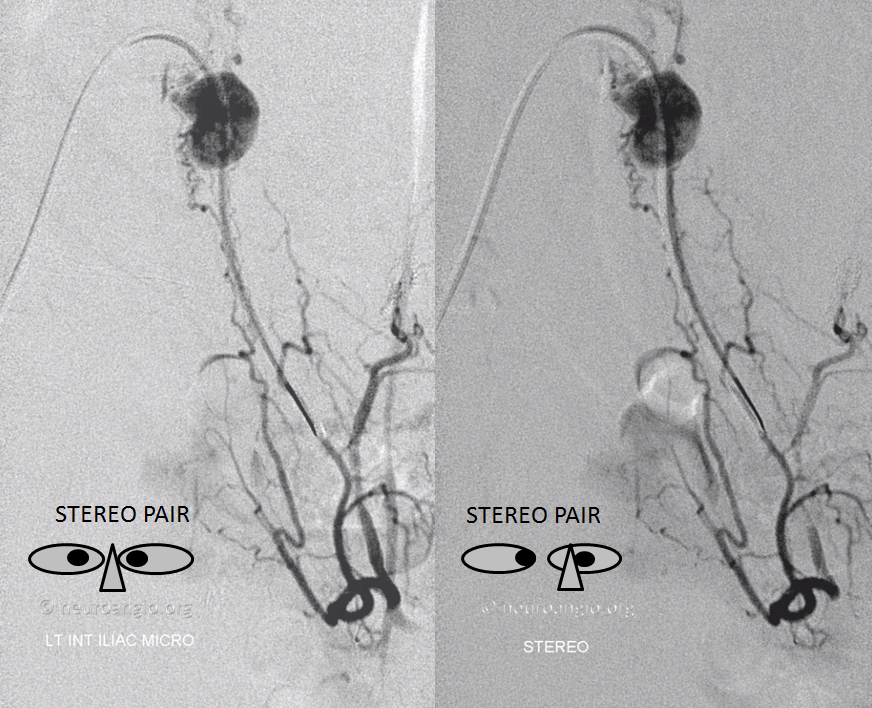
Stereo pair
Now for the real proof. Look at this venous congestion. Arrow points to one still existing radicular outflow artery. See spinal venous anatomy and spinal dural fistula pages for more about venous congestion.
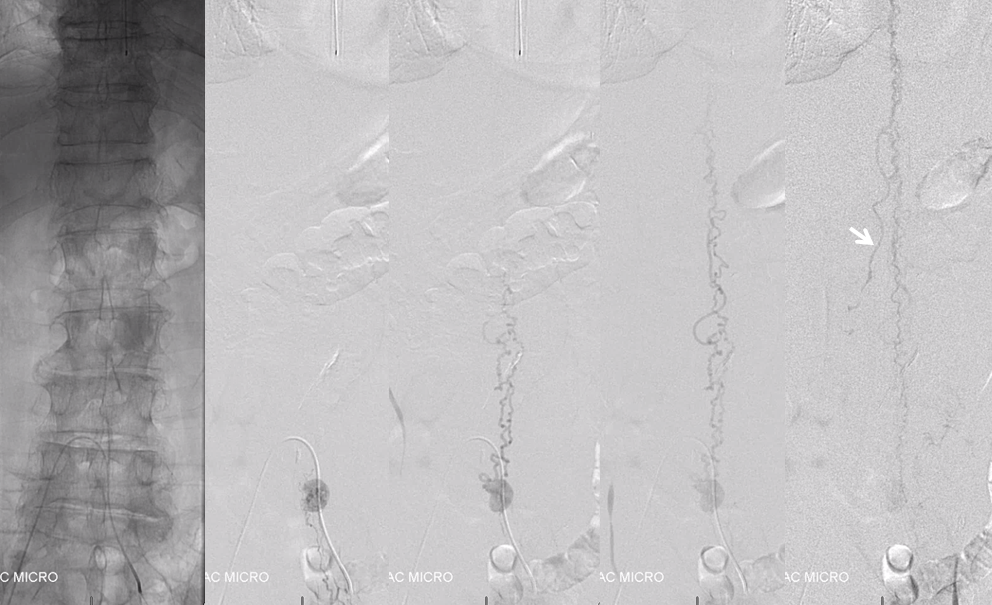
The problem is that there is no nearby venous outflow. Look even higher
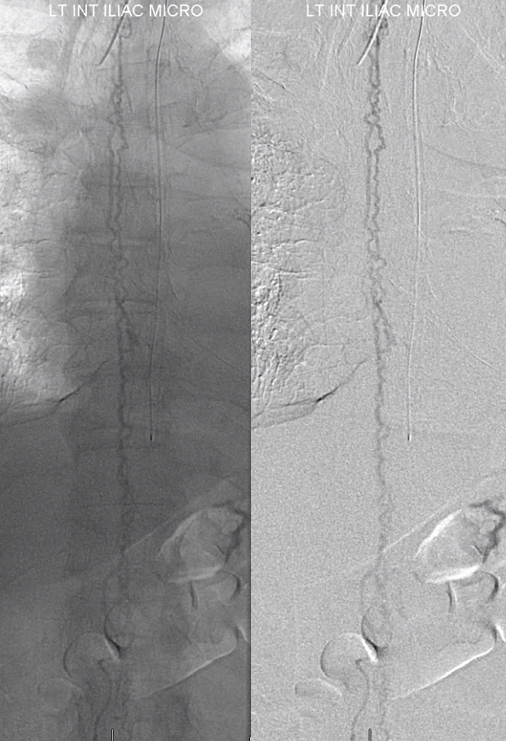
And higher
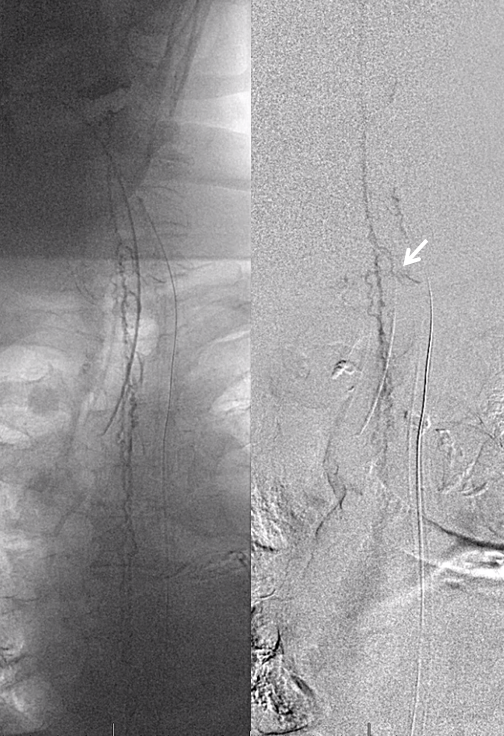
And higher still. Arrow points to the anterior pontomedullary vein
Lateral view
Look how much information angiography provides. We know the tumor is stuck to the left L5 root because it is supplied primarily by the left L5 radicular artery. We know that there is paucity of radicular veins which drain the cord and thus, combined with the tremendous hypervascularity of the tumor, produce amazing venous congestion.
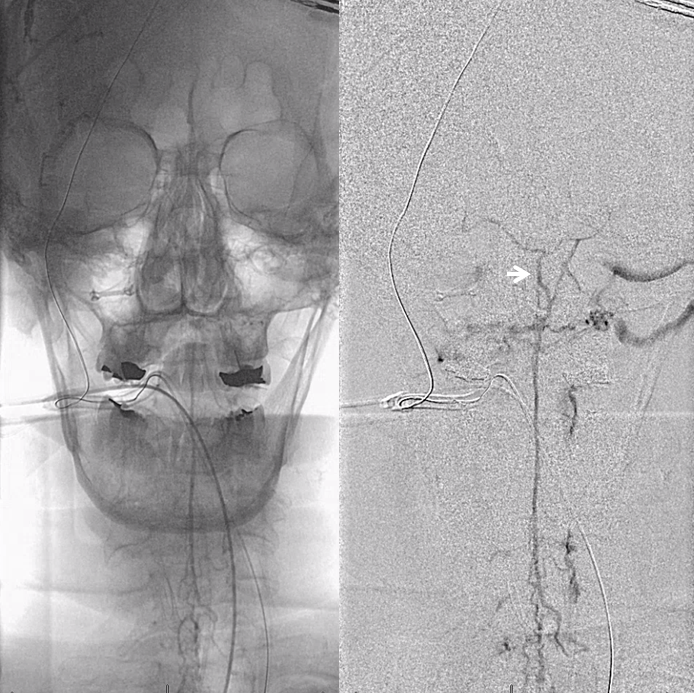
Another evidence for venous congestion comes from injection of the Adamkiewicz. Normally we should see spinal veins in the venous phase of this injection. Here we dont — because they are taken over by the hemangioblastoma.
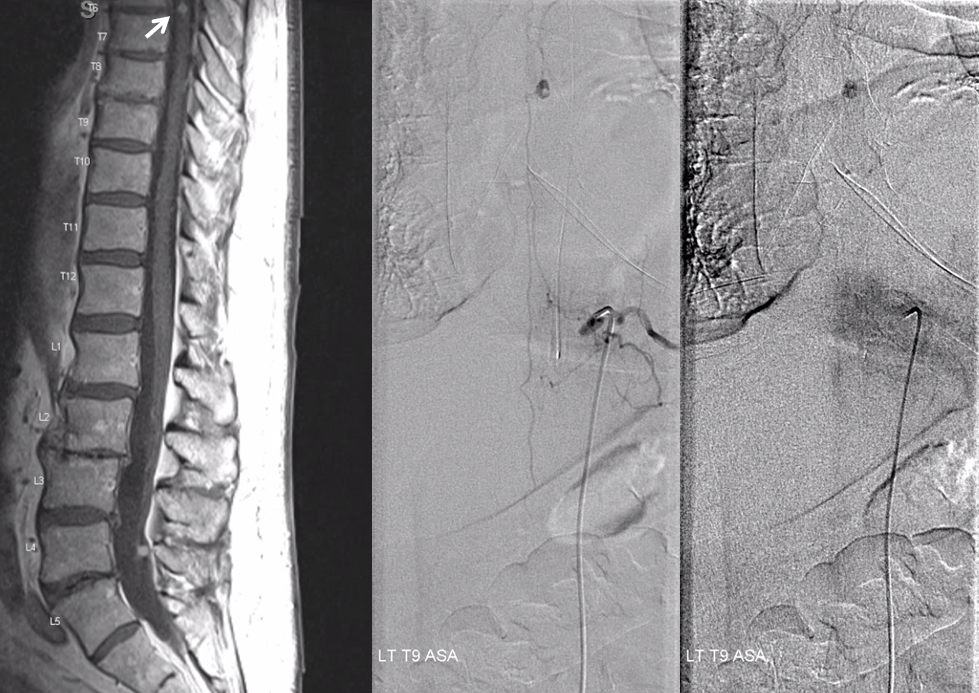
Another useful point is another hemangioblastoma we see here. Supply comes from a small vasacorona vessel off the anterior spinal artery. Thus, we know the tumor is on the surface of the cord and not on the nerve root. It was previously radiated.
Embo starts at L4. Key to success of standalone embo is meticulous complete embolization with small particles. Stuff like onyx or glue do not work in this kind of setting. And every pedicle must be embolized or it will rescue the tumor from necrosis.
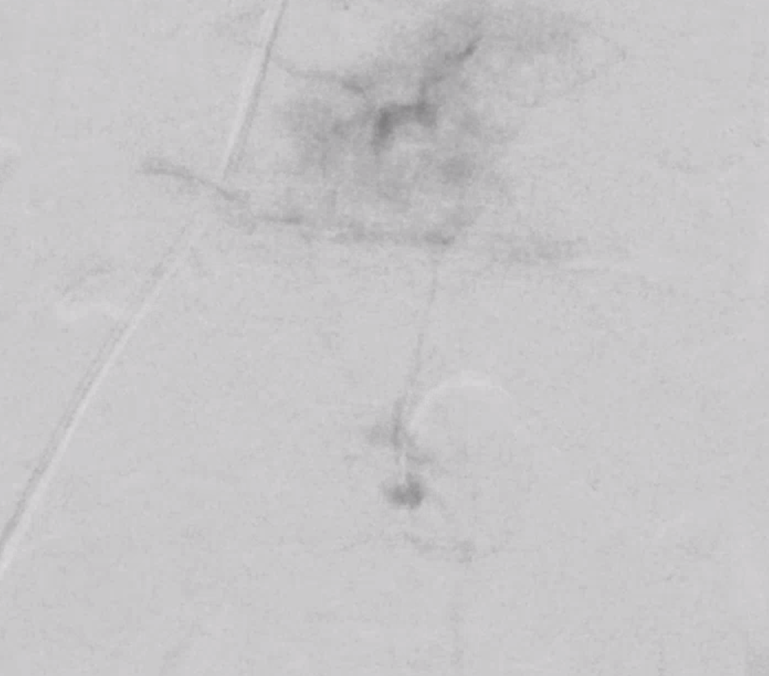
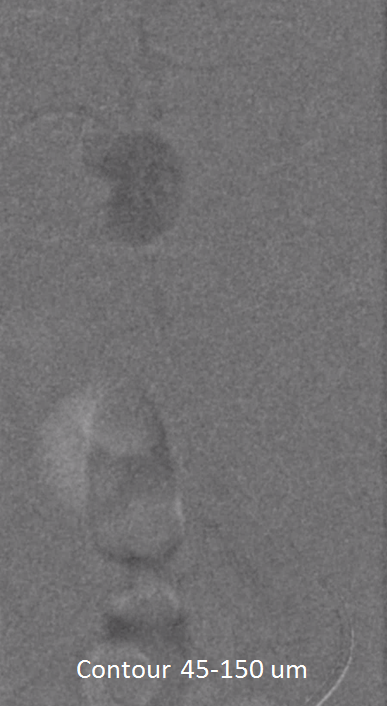
We almost always use 45-150 um Contour PVA particles. There are many other choices. Key is small particle size, which means deeper tumor penetration and more necosis

Post
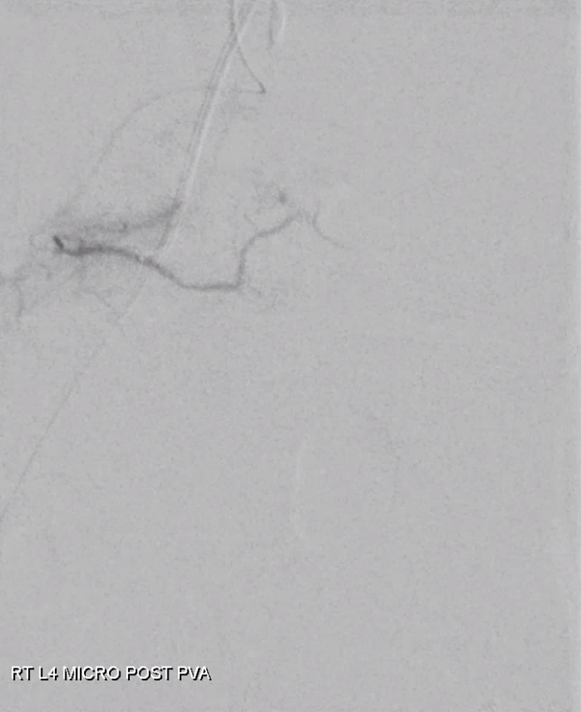
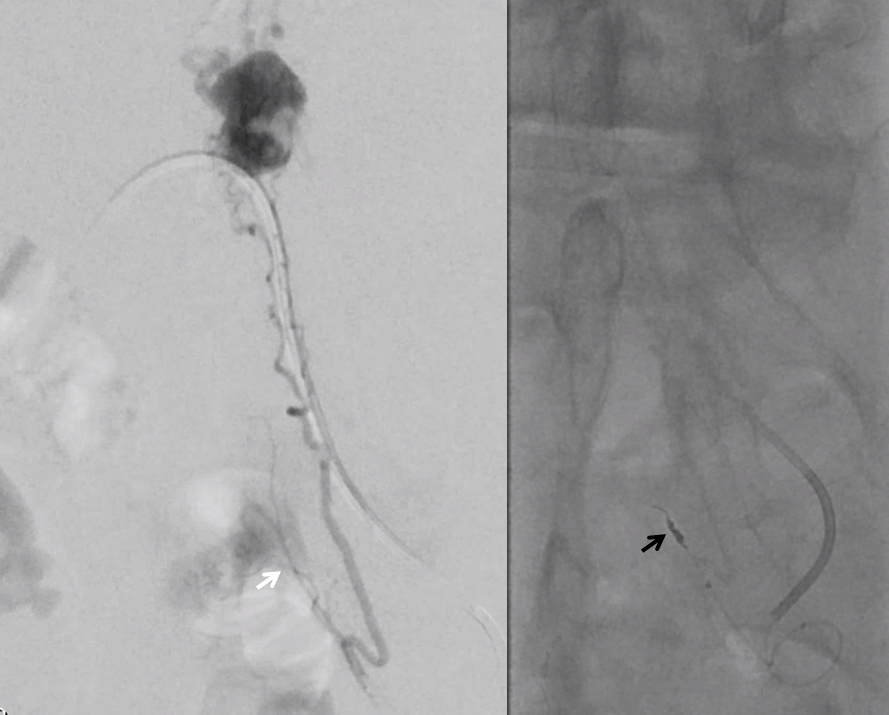
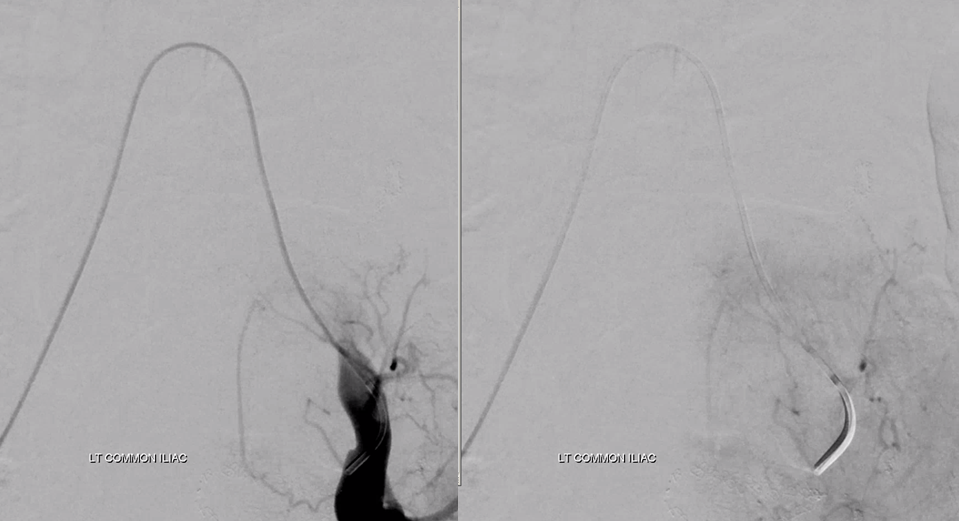
Moving on. See that S1 nerve root (white arrow)
Its a good idea to protect it from particles since more distal catheterization was not looking very likely without risk of vessel injury, which would completely ruin the embolization. So instead with block it with a small coil (black arrow). The nerve will refill from above and will be just fine
Embolization
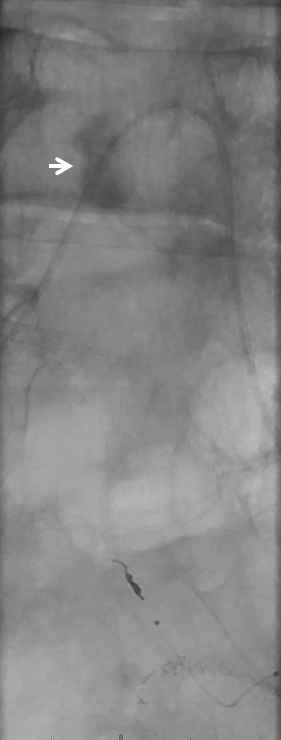
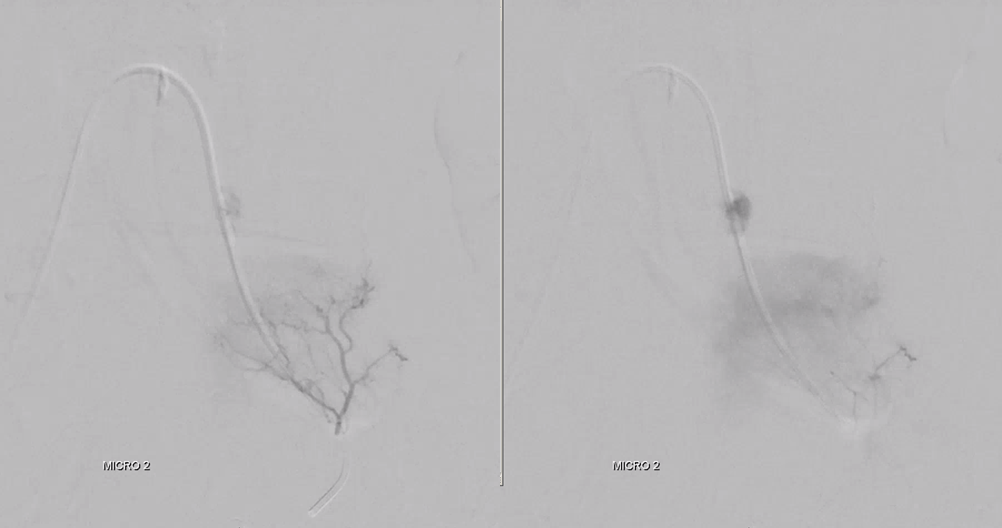
Look for prolonged contrast statis in the tumor (arrow) as an excellent sign of successful deep tumor parenchymal particle penetration. Proximal and other ineffective embolizations will not have that
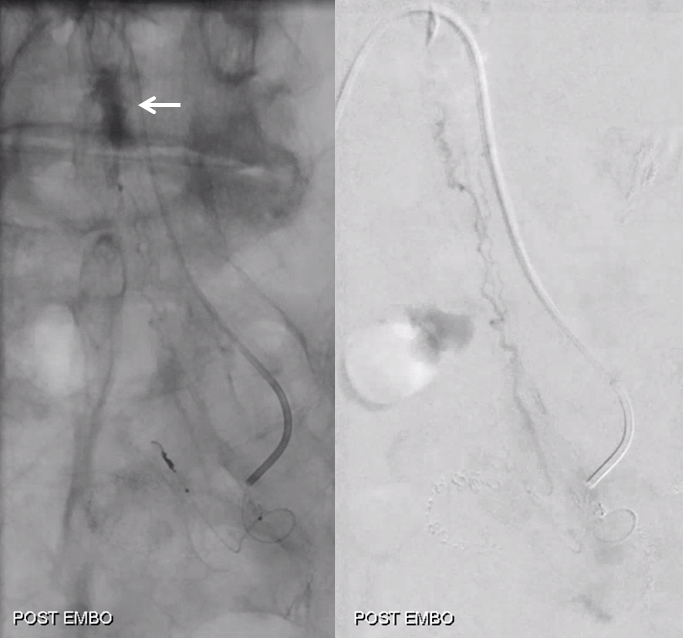
Post embo stasis and no visible tumor on DSA. Notice however that the lateral portion of the tumor has no contrast.
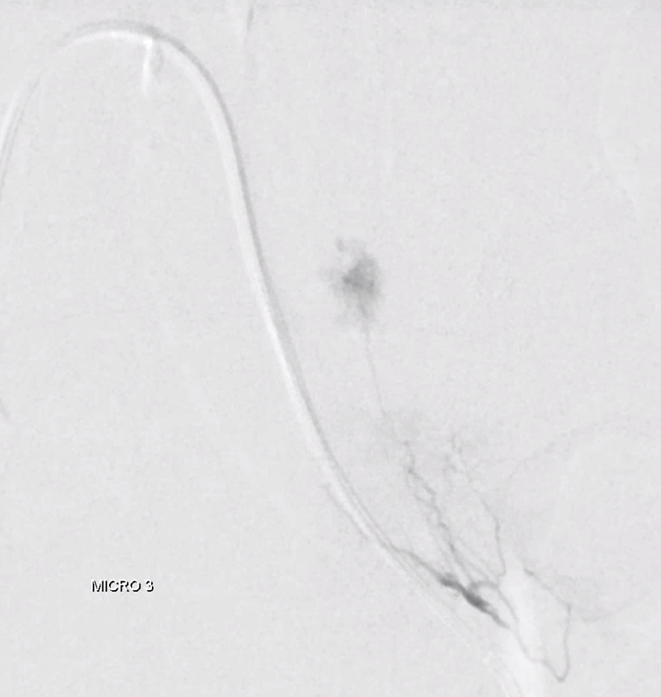
As suspected, the lateral part is supplied by another branch. Small, but important to get that.


Now we are done
Post embo Adamkiewicz injection now shows spinal veins back in use by the cord. A good secondary demonstration of embolization success
MRI 3 mo post. Reduced venous flow voids. Tumor is partially necrotic (T2 hyperintense part with a sharp T2 hypointense rim. The dead part corresponds to the main left L5 supply portion. The other two smaller pedicles were probably too small to allow for effective particle penetration.
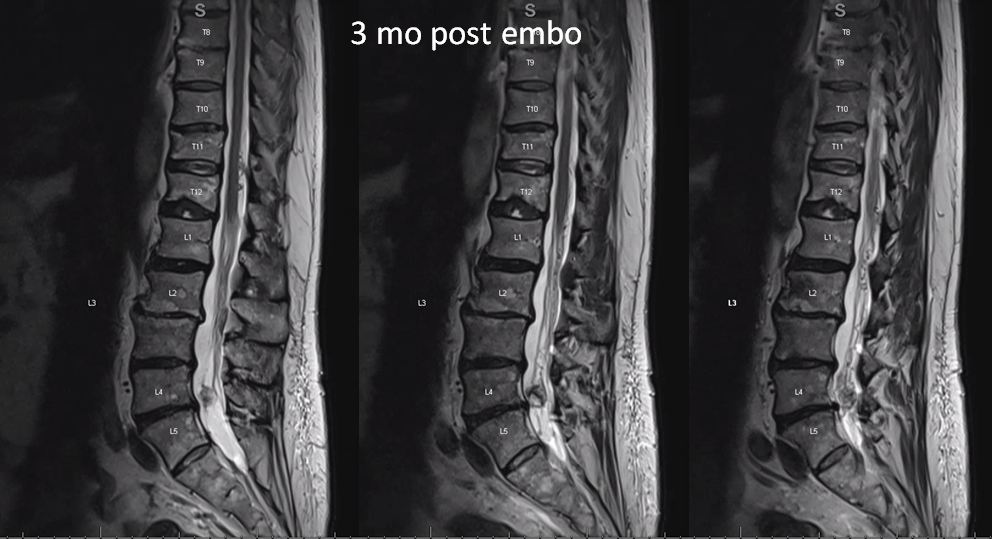
T1 post — same partial necrosis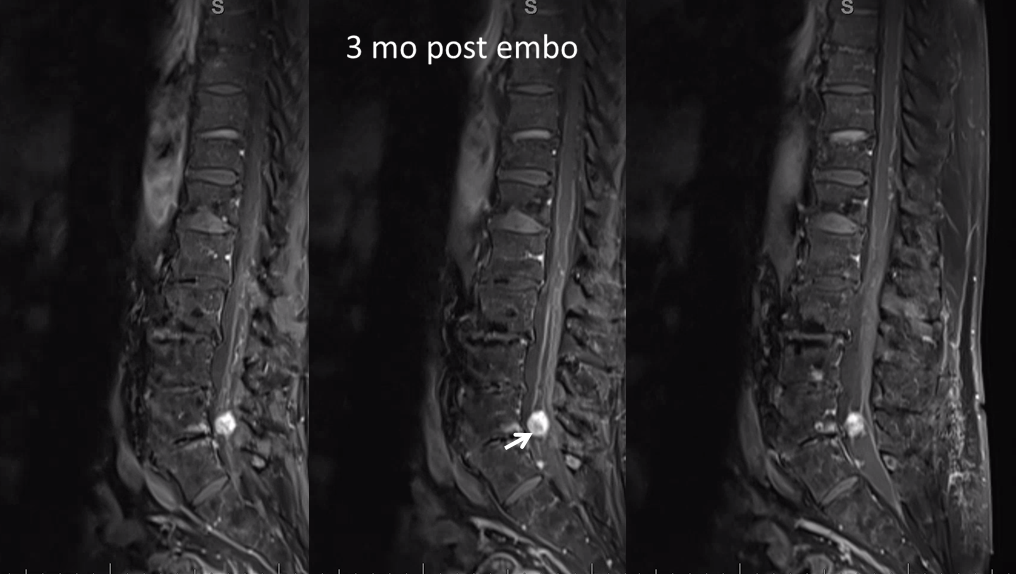
 So far symptoms have improved.
So far symptoms have improved.
Learning points: Dural fistula is the classic spinal venous congestion disease. Its clinical picture is perfectly explained by pathoanatomic changes of cord congestion. Occasionally, other conditions can also produce the same symptomatology by same final pathway of venous congestion. This is a unique example of such pathology, leading to a rational therapeutic plan
There is little interest and correspondingly little literature in this neck of the woods. Here are some. None has more than 10 citations. An underutilized technique for sure.
https://www.ncbi.nlm.nih.gov/pubmed/12802546
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3399720/
https://link.springer.com/chapter/10.1007/978-3-642-84434-8_5
