VENOUS ANATOMY OF THE SPINE
The man behind the scene, par excellence! The venous system is, in many ways, more important in understanding pathophysiology of spinal vascular disease than the arterial one. Arterial strokes of the cord are rare, and their morbid anatomy relatively straightforward. Far more important are the varied and imperfectly understood injuries to the cord inflicted by dysfunction of the venous apparatus — venous hypertension, high-flow venopathy, and outflow obstruction, for example. The prototypical vascular condition of the cord — dural AV fistula — manifests its clinical and anatomical consequences as a function of progressive venous failure.
Our knowledge of the cord venous system remains limited. The chief problem is that we do not have reliable noninvasive method of visualizing intradural veins — intrinsic and surface cord, radicular, and bridging types. Invasive (angiographic) methods are quite imperfect. Cone beam CT (CBCT) and 2D-DSA are probably the best complementary methods of acquiring spatial and temporal venous information. More on that below.
COMPARTMENTAL ORGANIZATION OF SPINAL VENOUS SYSTEM
Broadly speaking, the spinal venous system can be divided into four systems/components:
Intrinsic (intramedullary / intrinsic cord veins)
Extrinsic (cord surface veins and the radicular / bridging veins which allow venous outflow to traverse the dura)
Extradural (epidural plexi and drainage into the bony spinal canal outside dura)
External (osseous, paravertebral, etc — anything outside the epidural space).
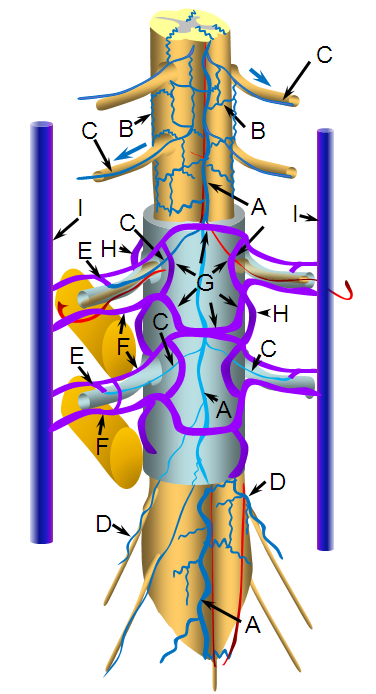
Classically, the names are anterior external, anterior internal, posterior internal, and posterior external venous plexuses, corresponding to intradural and extradural networks. The functional barrier between these systems is the dura.
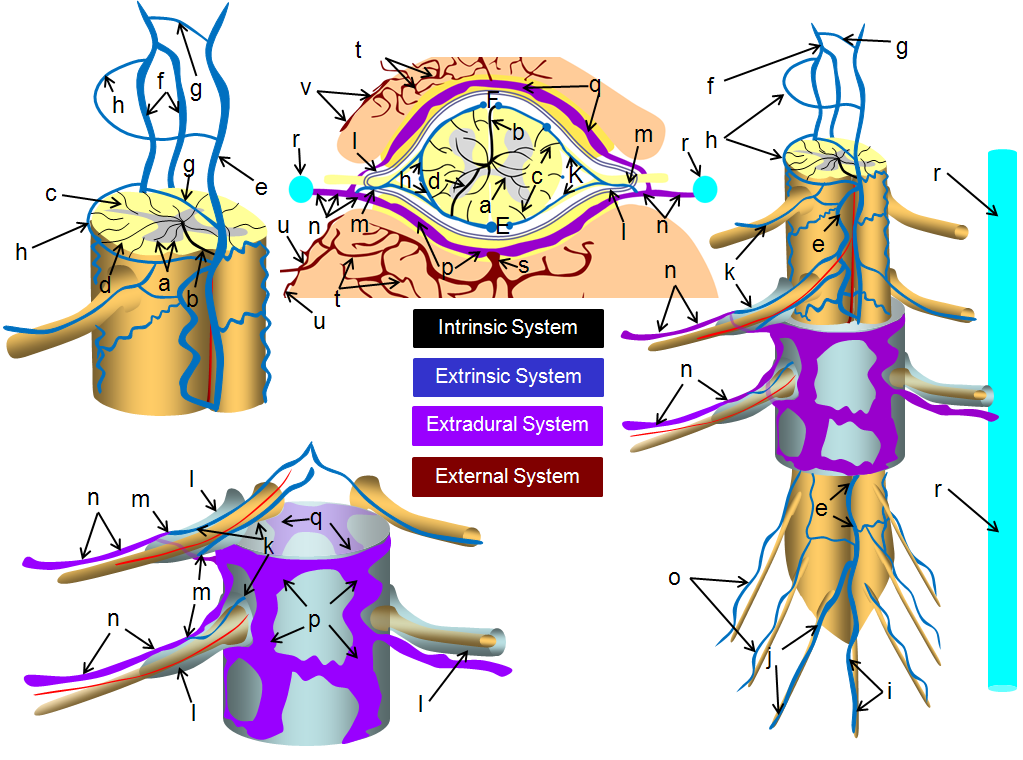
a –centripetal network of veins, predominantly draining the gray matter into: b – central (sulcal) veins of the intrinsic system; c – peripheral (radial, a.k.a. marginal) centrifugal veins of the intrinsic system; d – venous anastomosis between the centripetal and centrifugal systems; e – anterior (ventral) median vein; f – posterior (dorsal) median vein; g – transmedullary anastomosis between dorsal and ventral median venous systems; h – extrinsic surface anastomosis between dorsal and ventral median veins; i – vein of filum terminale; j – dominant radicular vein of the cauda equina; k – radicular vein (this is the weak link between the cord venous system and the extradural space); l – nerve root sleeve; m – shallow angle of radicular vein piercing the dura of the nerve root sleeve; n – intervertebral vein; o – radicular veins of the cauda equina; p – anterior epidural (a.k.a. ventral intrinsic) venous plexus; q – posterior epidural (a.k.a. dorsal intrinsic) venous plexus; r – ascending spinal (lumbar) vein; s – basivertebral vein, draining the intravertebral body venous plexus (t); u – anterior extrinsic venous plexus surrounding the surface of the vertebral body; v – posterior extrinsic venous plexus on the surface of the lamina /posterior elements, also participating in drainage of the paraspinal muscles
The veins are a capacitance network — about 75% of intracranial blood pool at any given time is situated in the veins. The same probably goes for the spinal cord. The intradural (intrinsic and extrinsic cord) and extradural (epidural plexus, paravertebral) systems are highly redundant and therefore fail only under extreme circumstances. The weak link, and threfore site of pathology, are the transdural connections — radiculomedullary and bridging veins. These veins are relatively limited, and have no effective alternative pathway for venous egress through the dural cover. Their paucity and failure is as much responsible for symptoms of dural arteriovenous fistula as the shunt-related pathologic inflow.
IMAGING OF SPINAL VENOUS SYSTEM
How do we visualize the above normal anatomy of the venous system? The answer is: with difficulty and inconsistency. Let’s go by compartments:
Intrinsic System: Commercially available noninvasive imaging has essentially no role. The intrinsic cord veins are below MRI resolution. Catheter angio — combination of 2D-DSA and CBCT are the best we have. We only see veins of the arterial territory we inject. More on this below
Extrinsic System: Cord Surface Veins (anterior/ventral and posterior/dorsal) veins are inconsistently seen on MRI. Sometimes too well, raising possibility of a fistula. Mostly not well enough. Well seen when injecting dominant artery (Adamkiewicz) by angio and CBCT. Bridging and radicular veins can be seen surprisingly well on high-res T2. Well seen on CBCT and angio if large enough artery is injected.
Extradural System: Can be seen well enough on MR/CT, especially when congested. Phlebograms are excellent, and more often done now for CSF-venous fistulas.
External System: MR/CT can be good. Catheter angio may be limited by unopacified inflow.
BACKGROUND ANATOMICAL / EX VIVO MATERIAL
The best anatomical demonstrations come from post-mortem studies, which produce exquisite detail but offer no direct hemodynamic information and are subject to multiple technical artifacts. Perhaps most importantly, basic research in this area is painfully rare. The last comprehensive treatment on the subject of venous anatomy was provided by Armin Thron, in his brilliant work “Vascular Anatomy of the Spinal Cord“, published in 1988. It was soon thereafter heavily referenced in the Lasjaunias and Berenstein’s “Surgical Neuroangiography”, first released in 1990. What we do know comes from a synthesis of existing anatomical works and a far less perfect understanding of the functional anatomy. The latter is based principally on pathophysiologic material, and therefore may be subject to erroneous inferences.
General 2D-DSA Spinal Cord Angiography
Injection of major cord arteries (sizable anterior and/or posterior spinals) should be followed, 4-8 seconds later, by appearance of cord veins. Failure to see these, provided good technique (breathold, apnea, pharmacologic paralysis) should be taken as evidence of venous congestion, requiring explanation.
Below is an old, typical cervical spine 2D-DSA imaging sequence. Note faint visualization of the sulco-comissural arteries (yellow). In the cervical spine, the posterior / lateral spinal arteries are often prominent — which means that when the posterior spinal veins (dark blue) are not well seen (as in this example), it may be because their arterial territory was not injected, and not because they are intrinsically small.
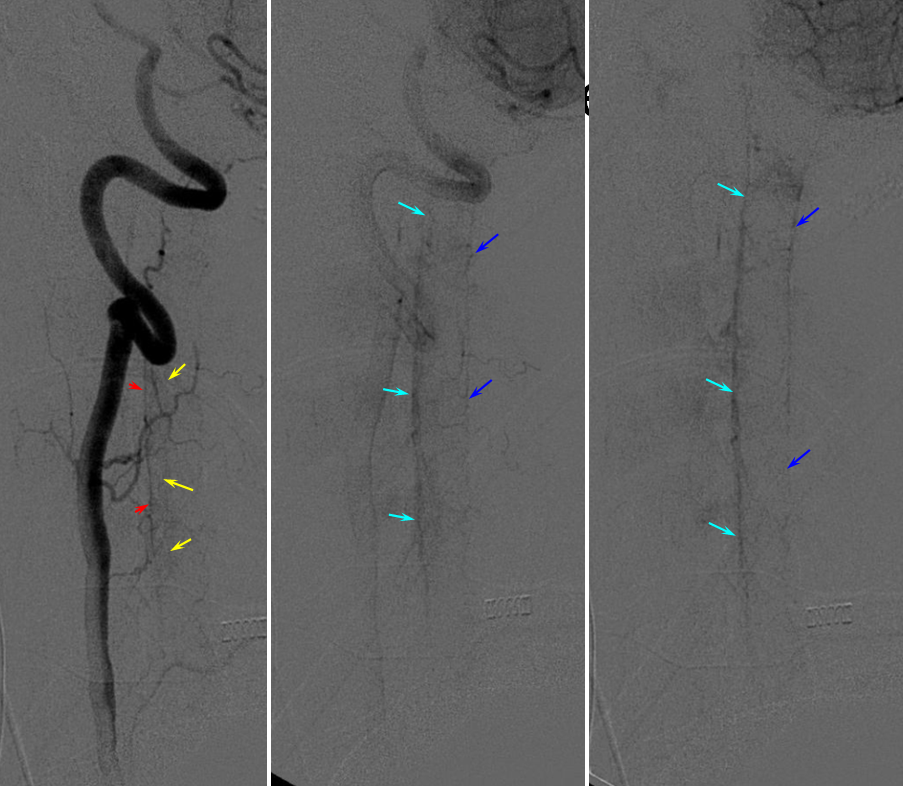
Another, more modern 2D-DSA sequence of vertebral injection. Lateral views show extremely well the posterior spinal axis (white dashed arrows), the position of spinal cord in the osseous spinal canal (leftmost image), a beautifully seen faint line of sulco-comissural vessels terminating in the depth of the ventral median sulcus (black dashed arrows), and circumferential / transmedullary spinal veins (black arrowheads)
Below is an example from Adamkiewicz (red) injection. You can faintly see tiny dots to the left of the ASA in the middle image — those are sulco-comissural arteries, seen end-on. In venous phase one can see a single (usually dorsal) longitudinal vein (darr blue) continuing down as probably vein of the filum terminale, and multiple radicular veins (light blue) on surfaces of the cauda roots.
Normally, intrinsic arteries of the spinal cord are angiographically invisible, even under optimal conditions of general anesthesia and paralysis (which is how we do spinal angiography). In this young, very thin patient, injection of the Adamkiewicz beautifully visualized multiple end-on sulco-commissural vessels, seen as dots (purple) next the anterior spinal artery (red). Notice several vasocorona surface vessels (green) which opacify the posterior spinal arteries (yellow), which are usually contiguous at the conus (rami cruciantes). Venous phase image (right) shows a slanting direction radicular vein (dark blue), as is often the case, draining the basket, and additional left radicular veins higher up (not labeled). A hint of radiculomedullary veins is present also (light blue)
Pathologic Correlation — Dural Fistula and Cord Venous Congestion
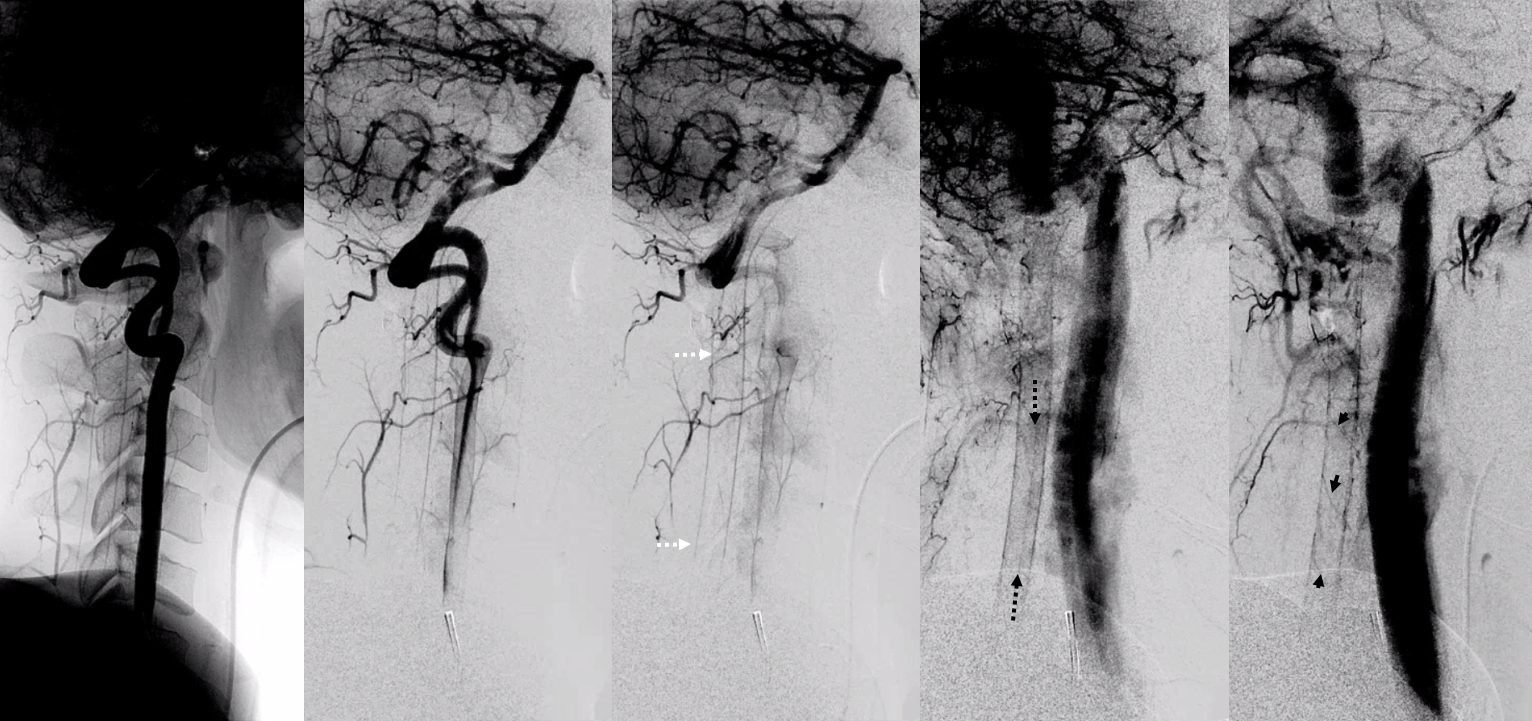
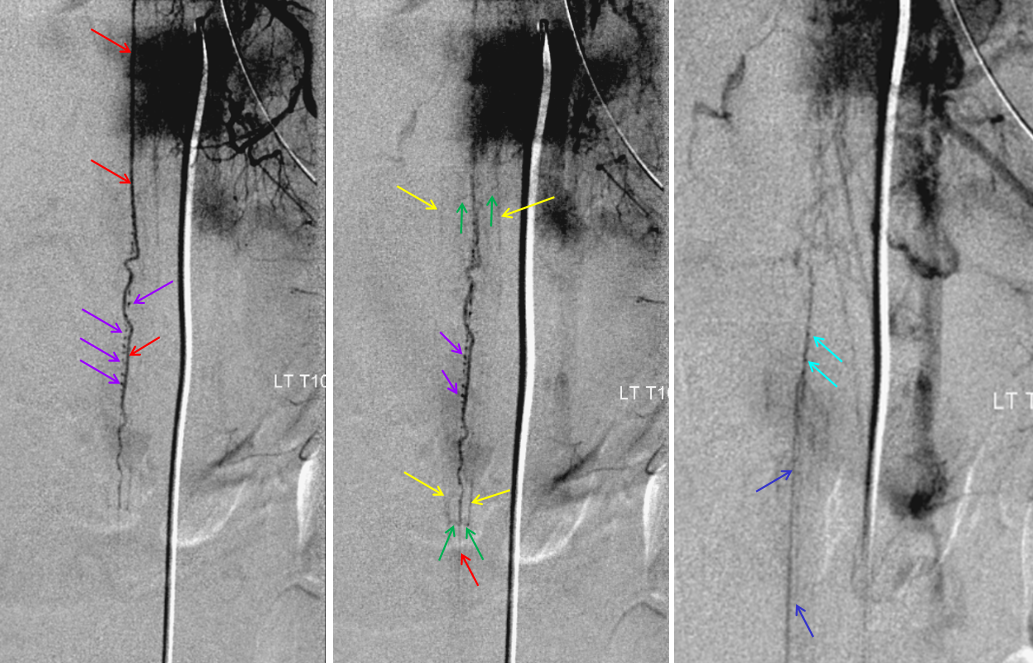
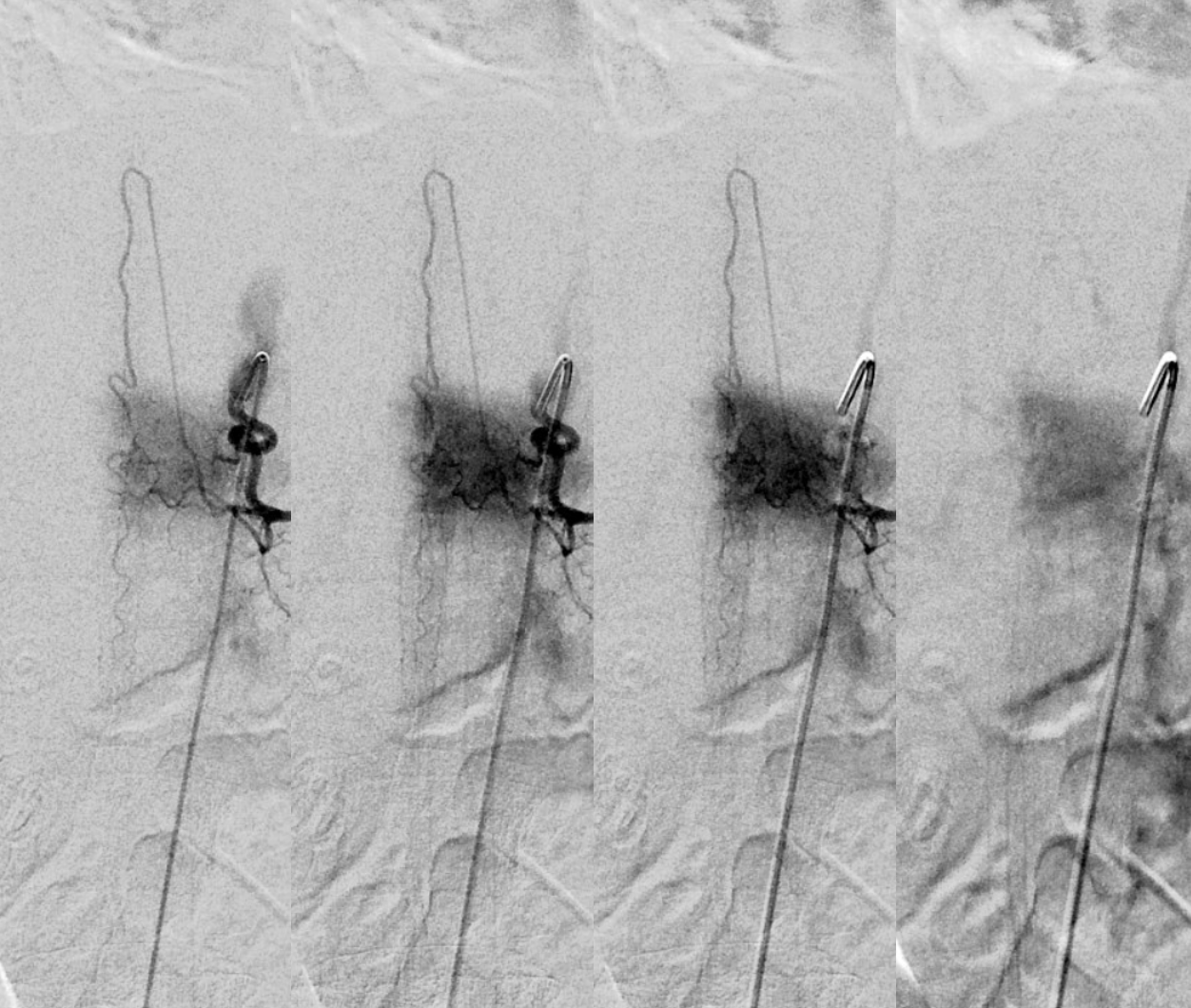
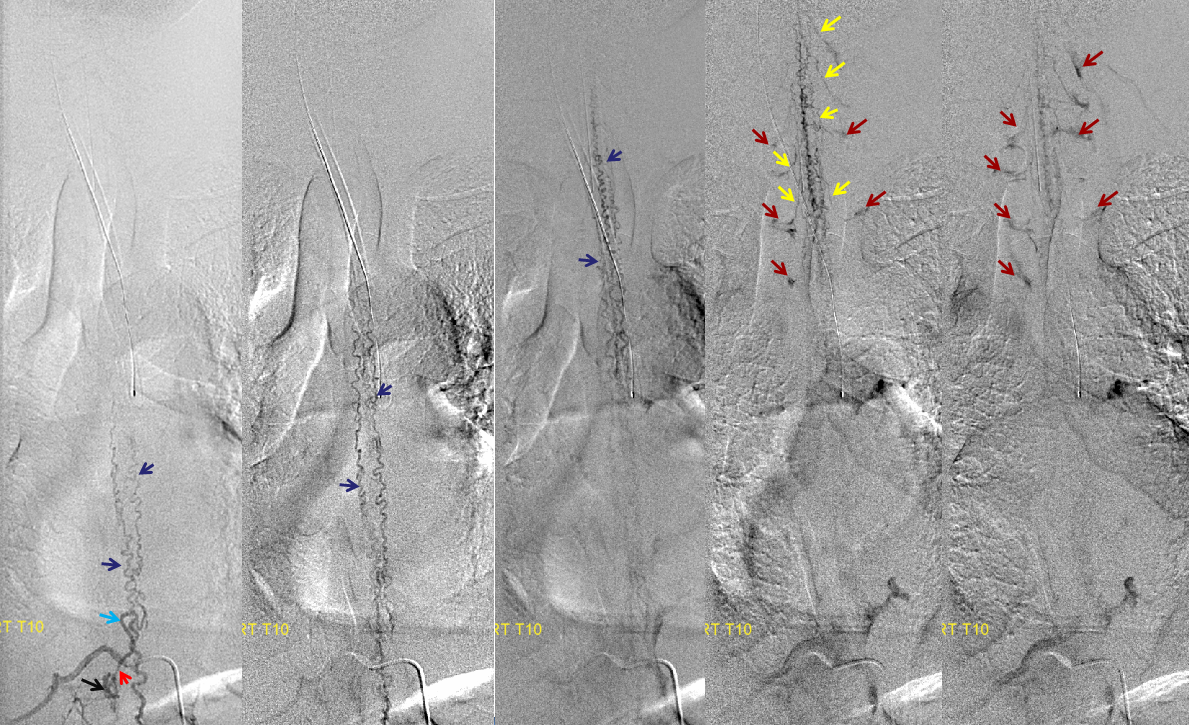
Spinal dural fistula (see lots of cases in Case Archives) is a prime example of spinal venous congestion pathology. Two factors determine how bad the clinical status is. One is competing inflow from the fistula. The other, just as important, is lack of venous outflow via radicular or bridging veins (later on that). The end result is cord venous congestion. How does that manifest angiographically? By not seeing cord veins in their expected venous phase. Below is an example of Right TT origin Adamkiewicz injection. Center and right images show cord blush, but no radicular veins — congestion….
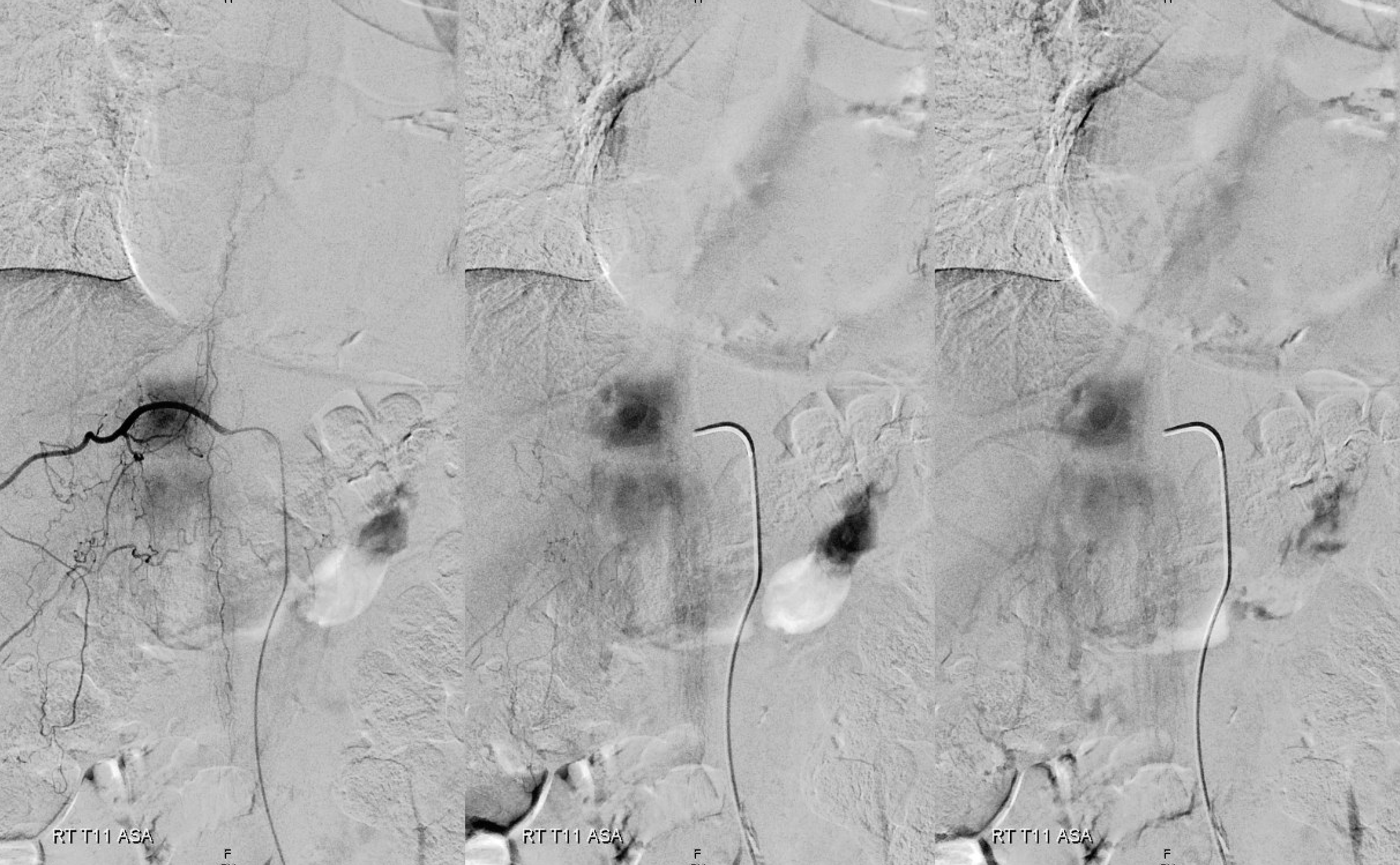
Fistula is at left T6. Note lack of radicular outflow, which is the main problem in highly symptomatic fistulas like this one
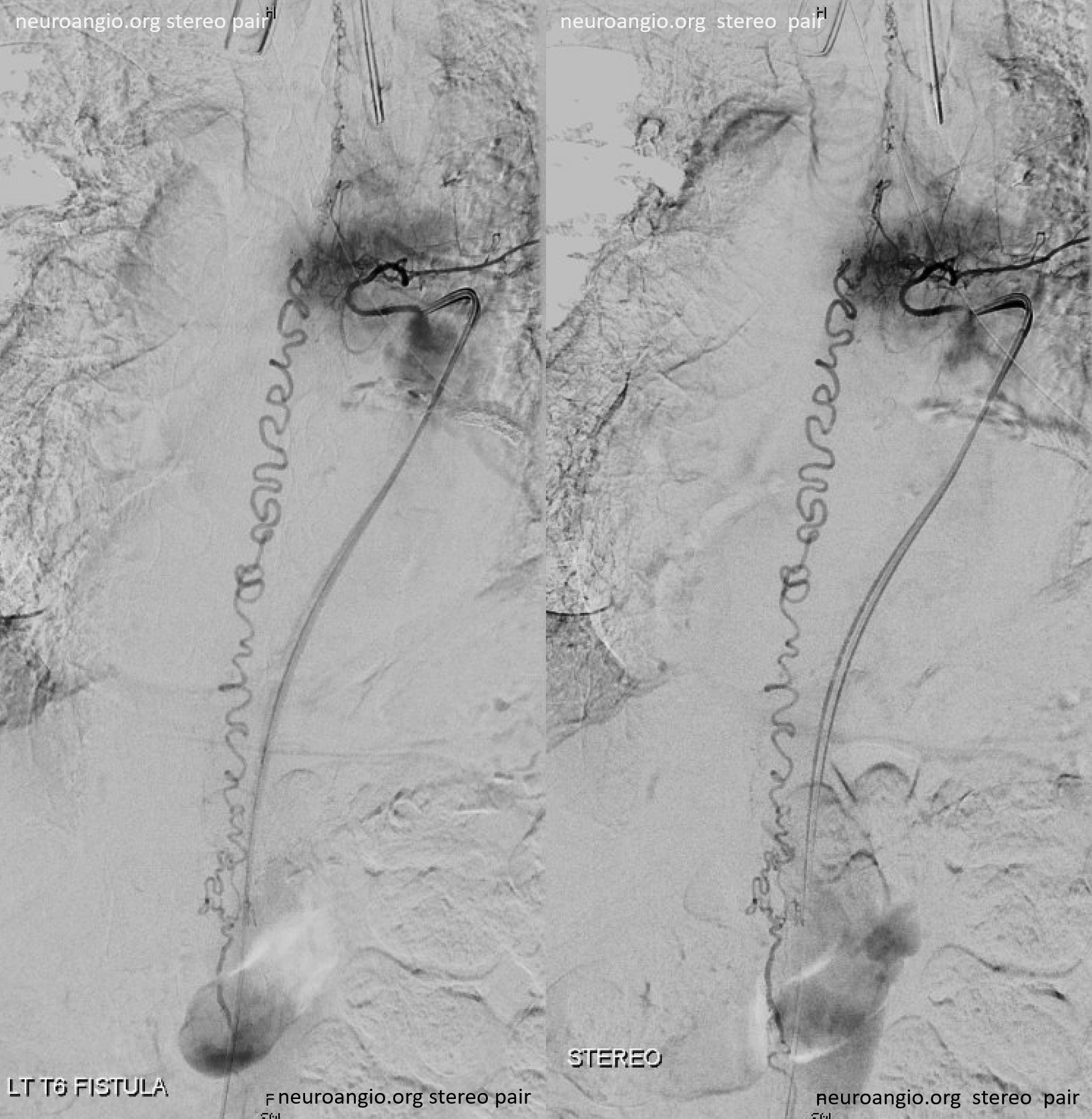
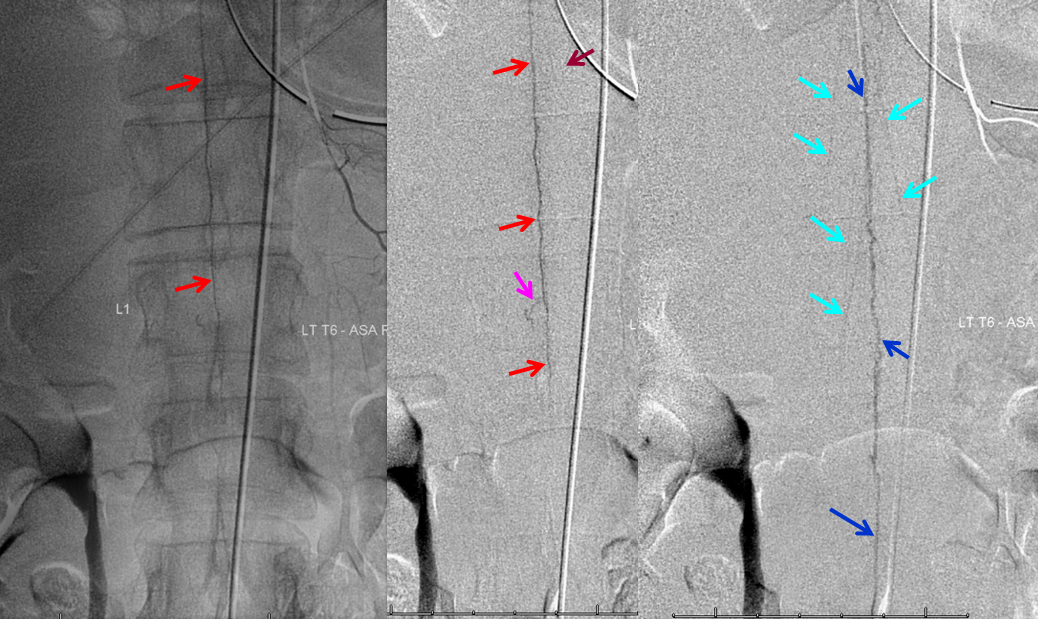
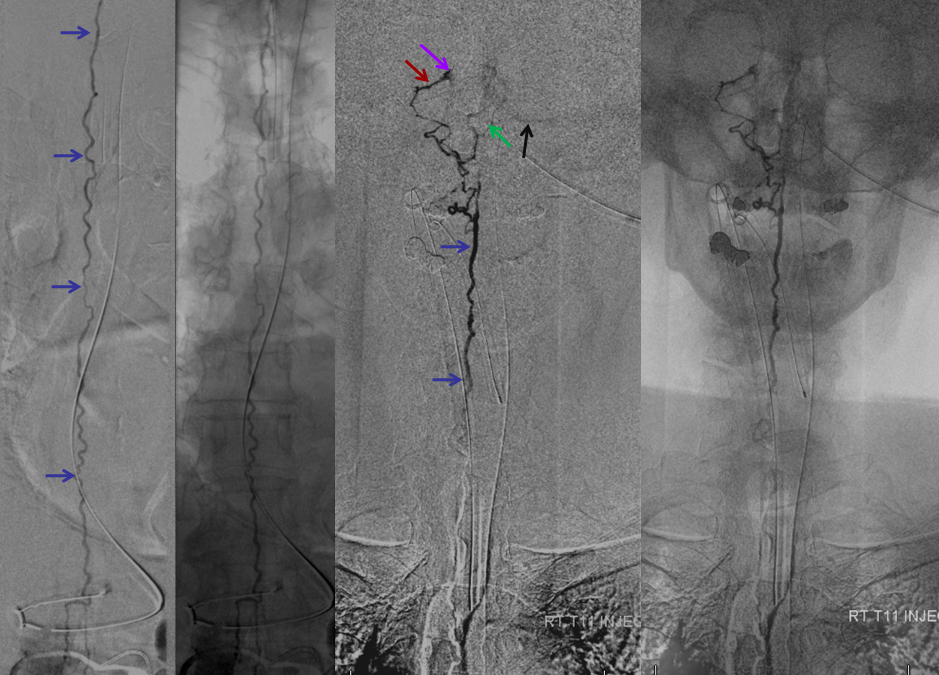
Following endovascular occlusion on the fistula (full case here), injection of the Adamkiewicz now visualizes the spinal veins previously parasitized by the fistula and now “returned” to the cord
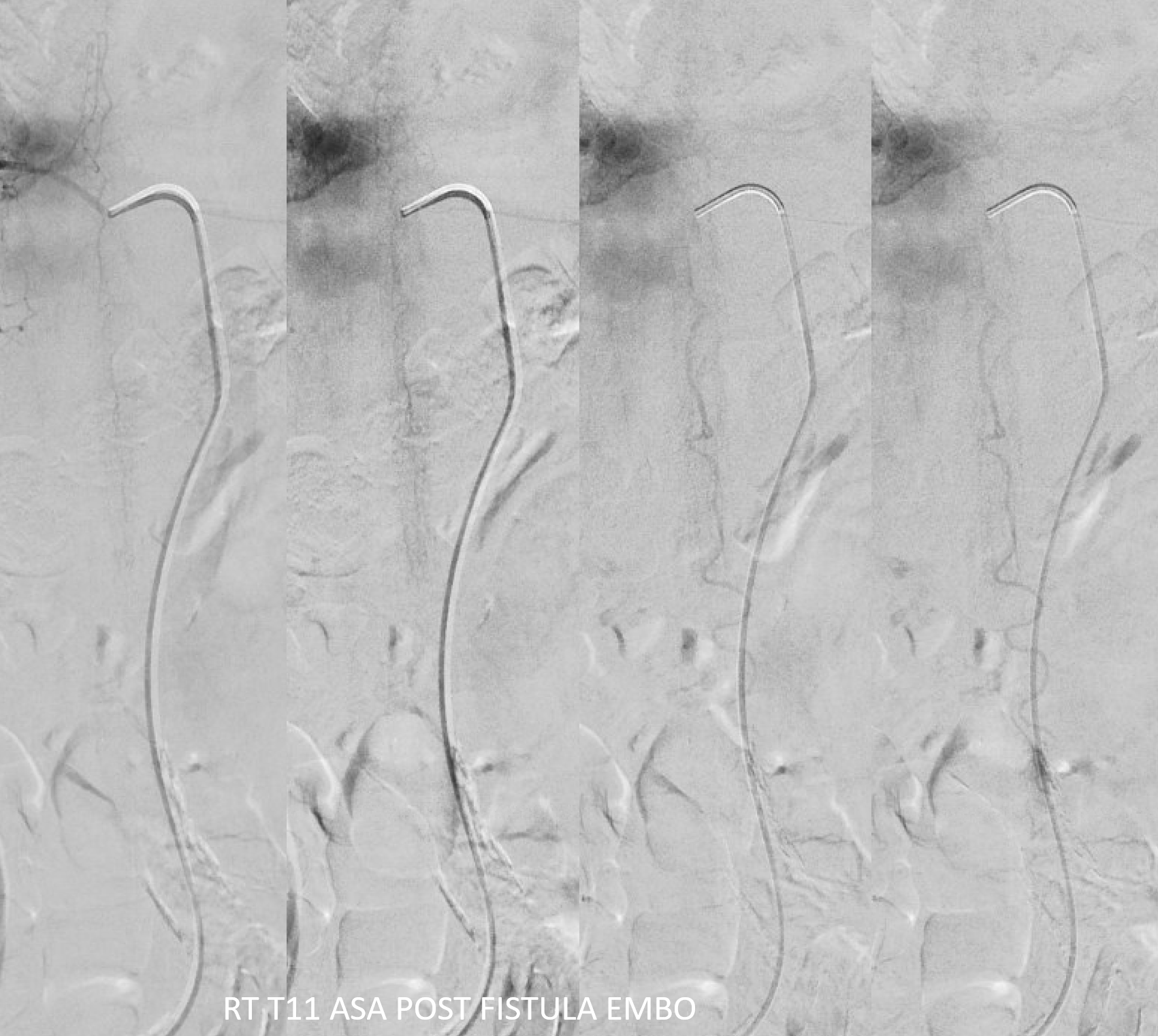
The venous system remains abnormal, with paucity of radicular drainage. This can contribute to limitations in recovery, along with any “permanent” congestion-related cord damage. This, in a nutshell, is Dural Fistula 101 Primer.
ADVANCED SPINAL ANGIOGRAPHY — CONE BEAM CT ANGIOGRAPHY (CBCTA)
Our group champions use of CBCTA. Details of protocols are found here and examples of breathtaking images here. The basic idea is to choose the protocol with highest spatial resolution, zoom in as much as desirable, and use some delay (up to 6-10 seconds) to pre-fill the vasculature. Present-day CBCTA acquisition times do not allow for reliable separation between arteries and veins yet. 6 seconds is a start — may be able to see arteries with little venous contamination. To see only veins, though, one has to complete the spin fast enough so that the arteries are no longer visible, but the veins have not yet cleared. That’s like 3-4 seconds in the cord. So a 6 second CBCTA is maybe good enough. Mostly, we just do the best spatial resolution acquisition and choose a long xray delay (6 seconds) to have a fully mixed arteriovenous phase. Below are examples.
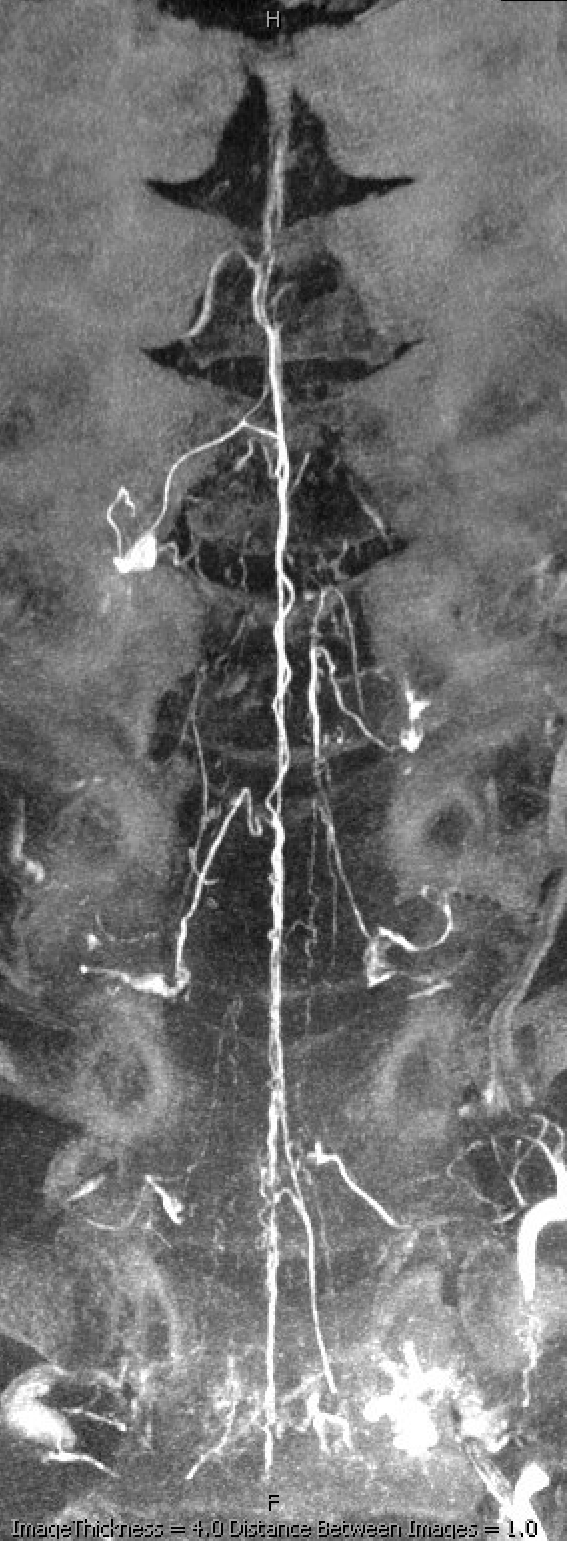
Mixed arteriovenous phase CBCTA. Beautiful intrinsic vessels of the cord. Mixed arteries and veins — which means that most are veins. In contrast to arteries, veins do not all go through the ventral median sulcus, the way sulco-commissural arteries do. Some are seen draining to the back at 4 and 8 o’clock positions. There is a large transmedullary vein in middle right image. A lot of radicular veins are seen too (more on that below)
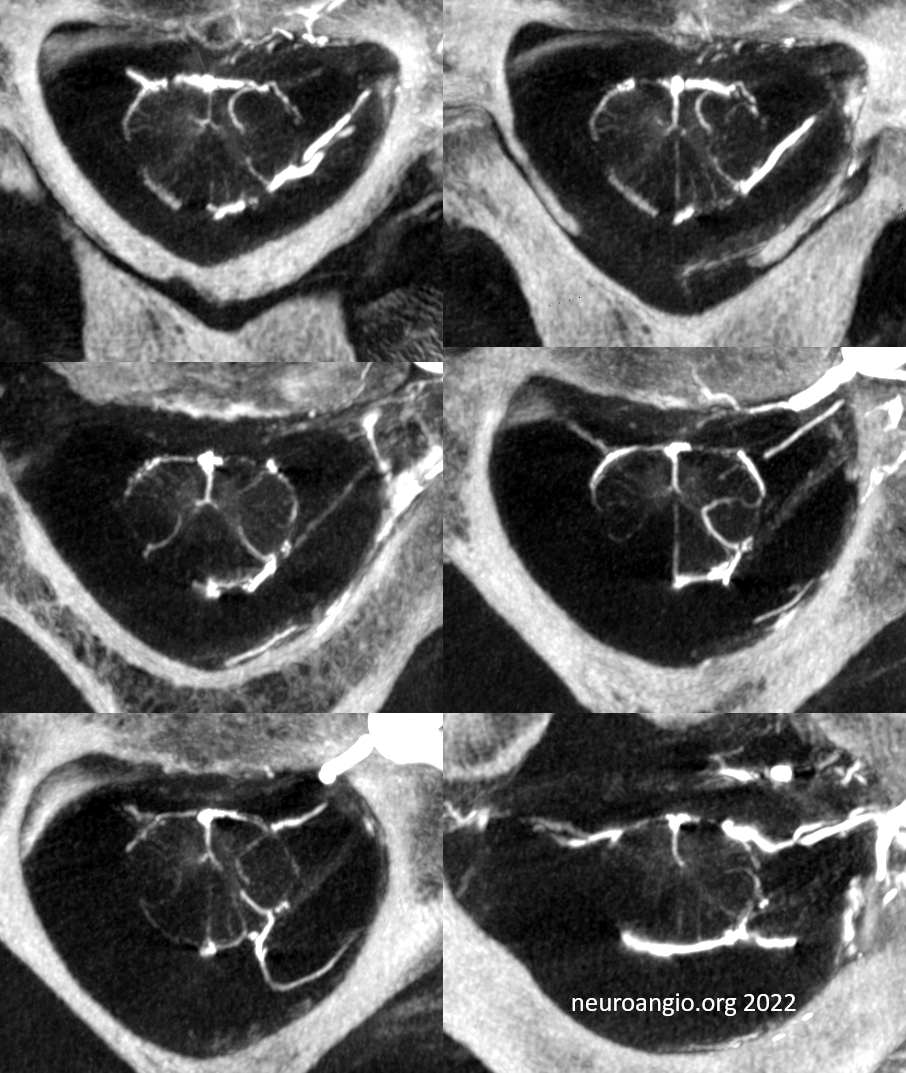
Video of the same
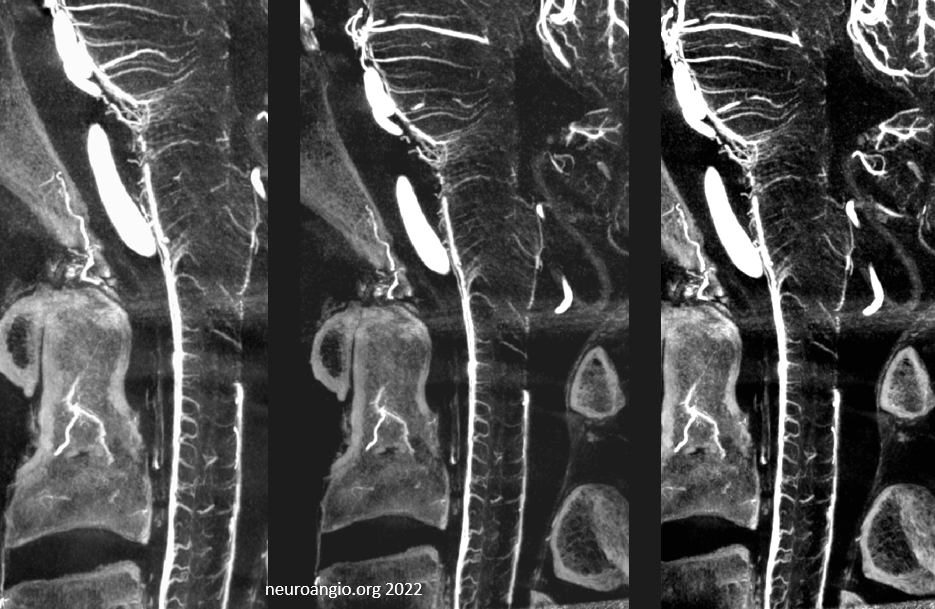
Sagittal MIPs — note striking homology between both arteries and veins of the cord and brainstem. There is nearly always present at least one prominent median brainstem vein extending to the floor of the 4th — often the collector for deep cerebellar DVAs
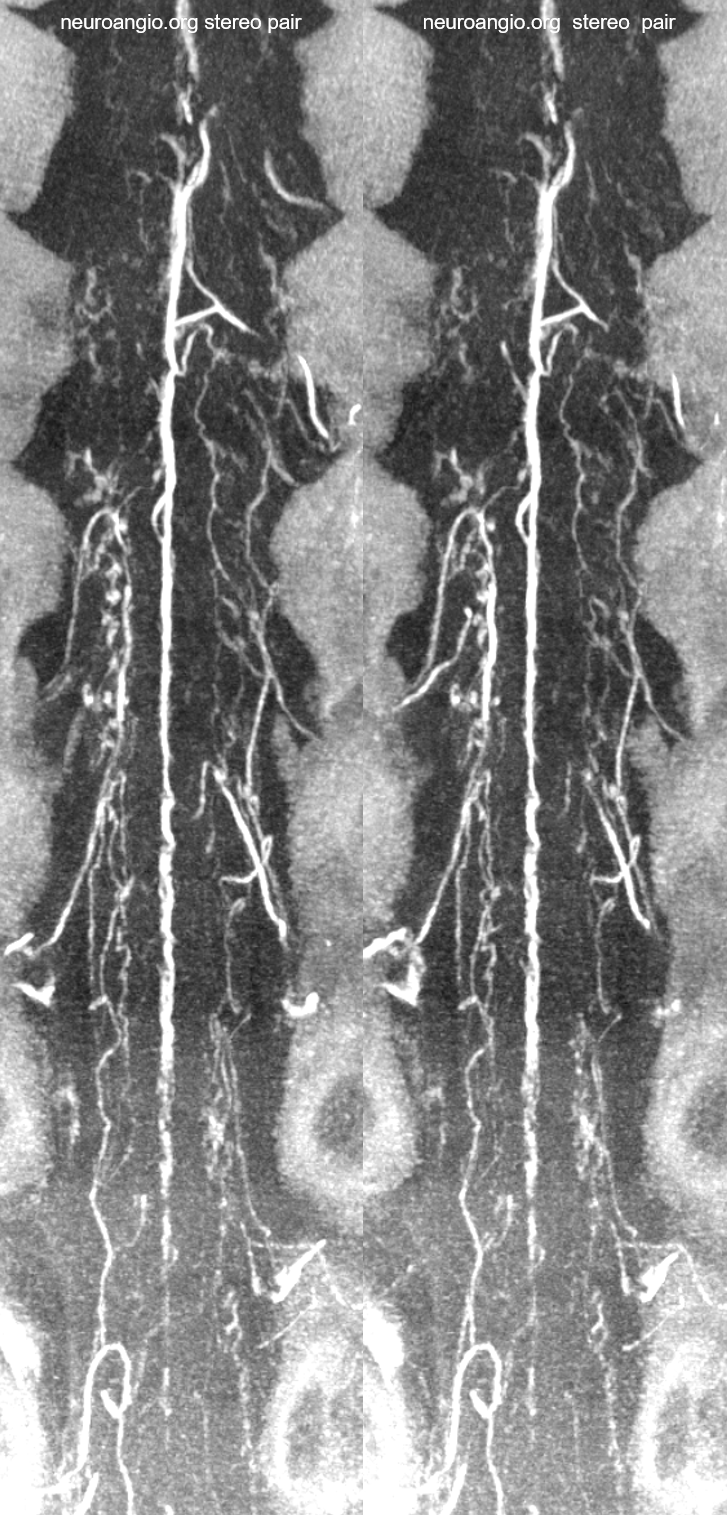
Cross-eye stereo pair
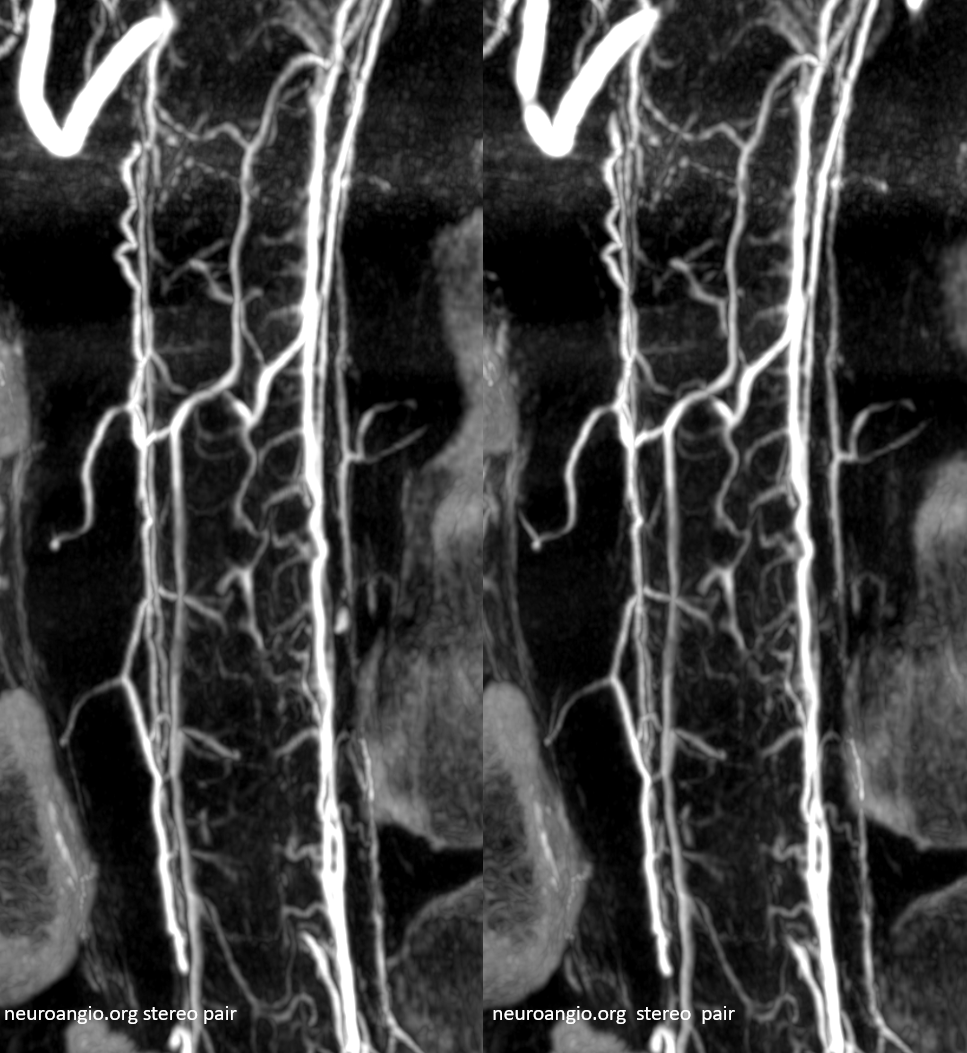
Cutaway stereo MIPs show dorsal and ventral-draining veins
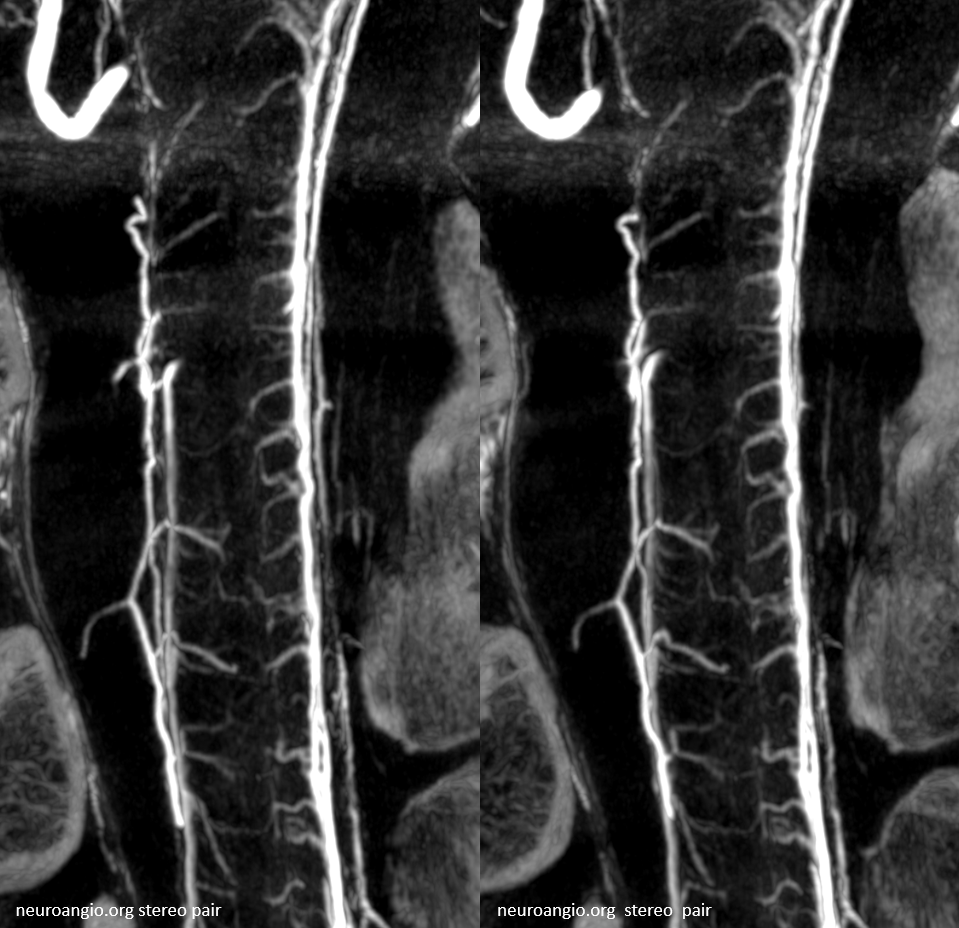
Another subject
Visualization is of course not limited to C-spine, though it is been seen there. Here is an angio of a normal Adamkiewicz, with corresponding CBCTA images below
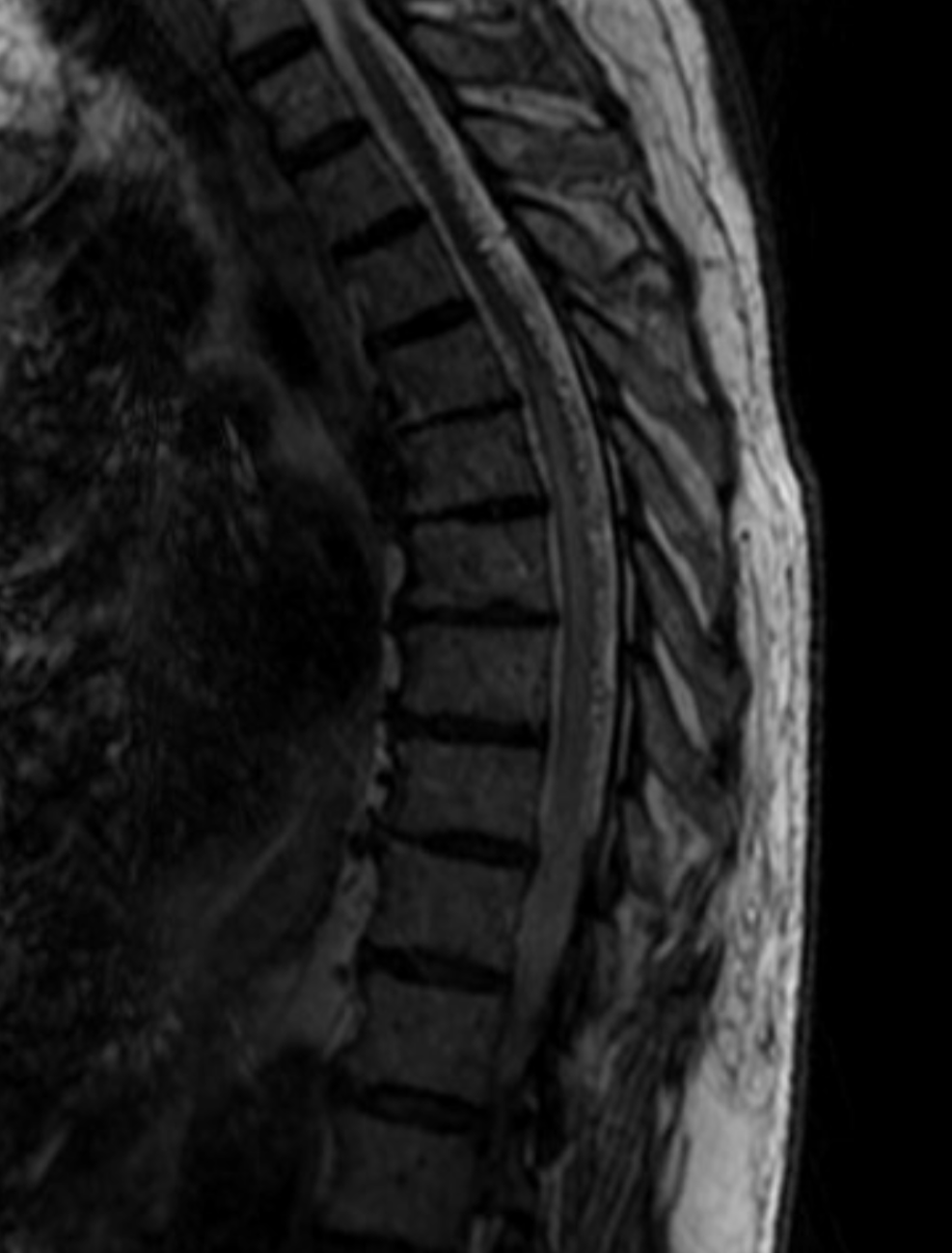
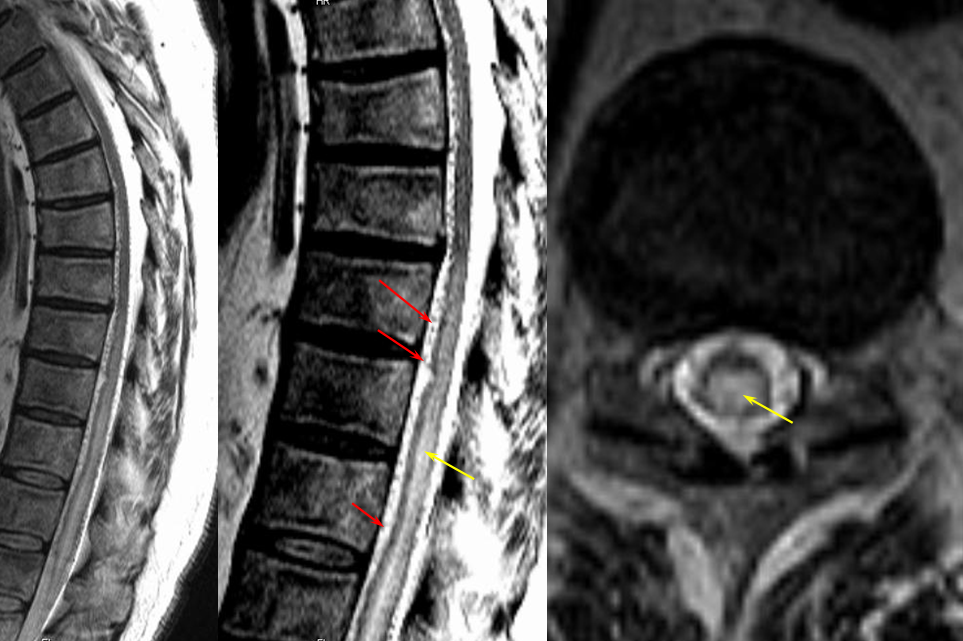
Contrast-enhanced MRI will often show a normal anterior median spinal vein, particularly in the sagittal plane where it weaves in and out of the field of view. It can be hard to differentiate a normally prominent longitudinal spinal vein on MRI from one congested by a fistula. Clinical correlation is important. If angiography is requested, the key is to not miss a level, and to visualize spinal cord veins in the venous phase of spinal artery injection — as above. Seeing veins is good evidence that there is no venous congestion.
Spinal cord venous infarcts, although likely underdiagnosed, are still quite rare, while awareness of their very existence is rarer still. Literature on subject understandably consists of individual cases. One good one is by: Niino M, Isu T, and Tashiro K. Nonhemorrhagic venous infarction of the spinal cord without spinal vascular malformation. Journal of Neurology 1999; 246 (9) 852-4 Link: http://www.springerlink.com/content/l412g0t09r8djl2g/
RADICULAR AND BRIDGING VEINS
Key section here. These veins are the link between the intradural (cord) and extradural (epidural) venous systems. It is the weakest point in the whole system. They are limited in number. Their paucity determines the extent of clinical symptomatology in spinal dural fistulas (see above). So, it is important to visualize and understand them.
Much has been written about the course of veins connecting the spinal cord and the extradural veins system. On the one hand, there are “radicular” veins which follow the nerve roots (dorsal and ventral) into the nerve root sleeve, through the dura, and into the foraminal or epidural venous systems. In addition, there is also the notion of “bridging” veins — which also connect the surface cord veins with extradural venous systems but NOT exiting via nerve root sleeve or, in a broad sense, somehow not connected to the radicular nerves — perhaps existing via their own foramen, etc. There is debate among those interested in venous cord drainage as to the relative prevalence of these two types.
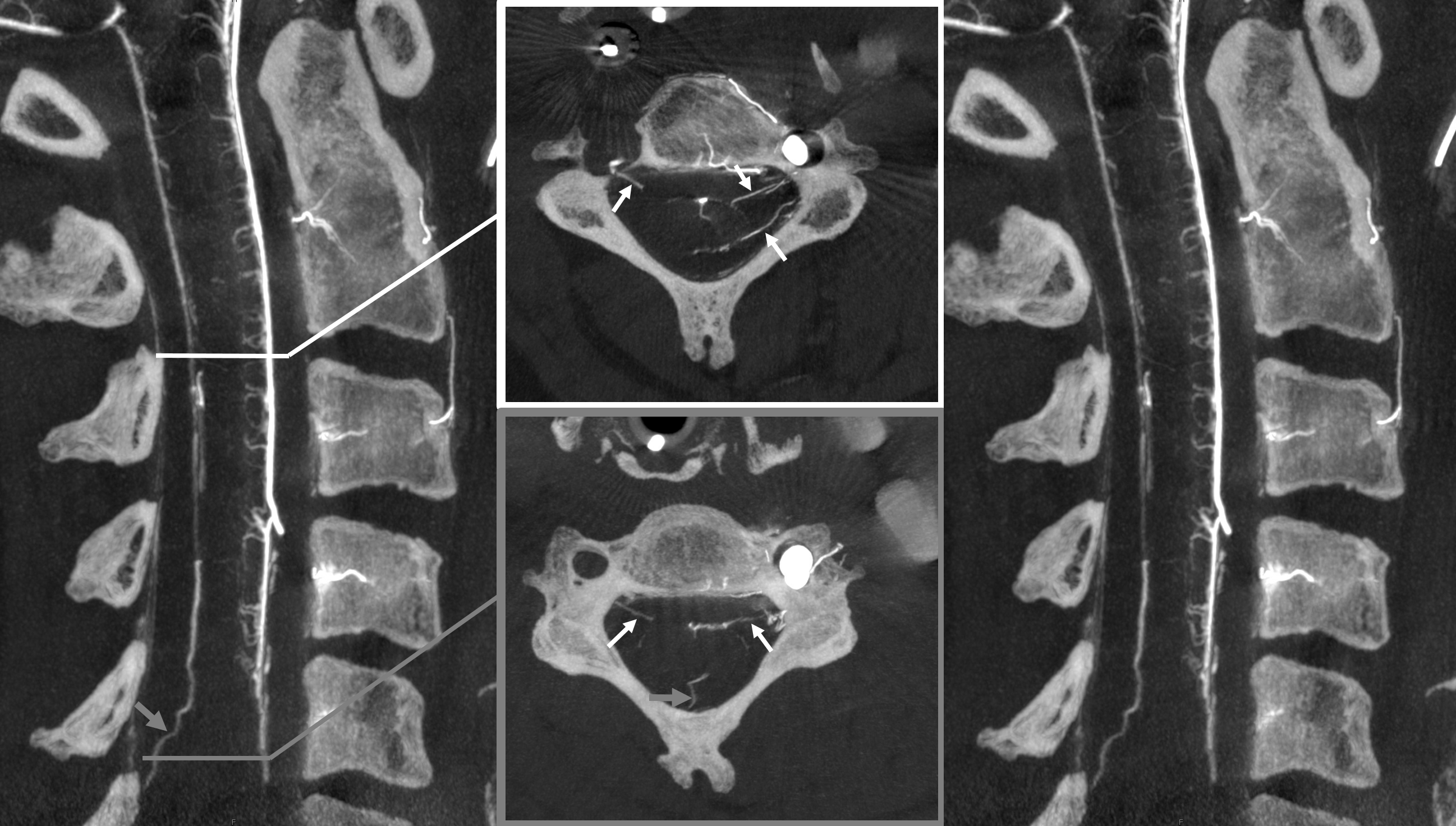
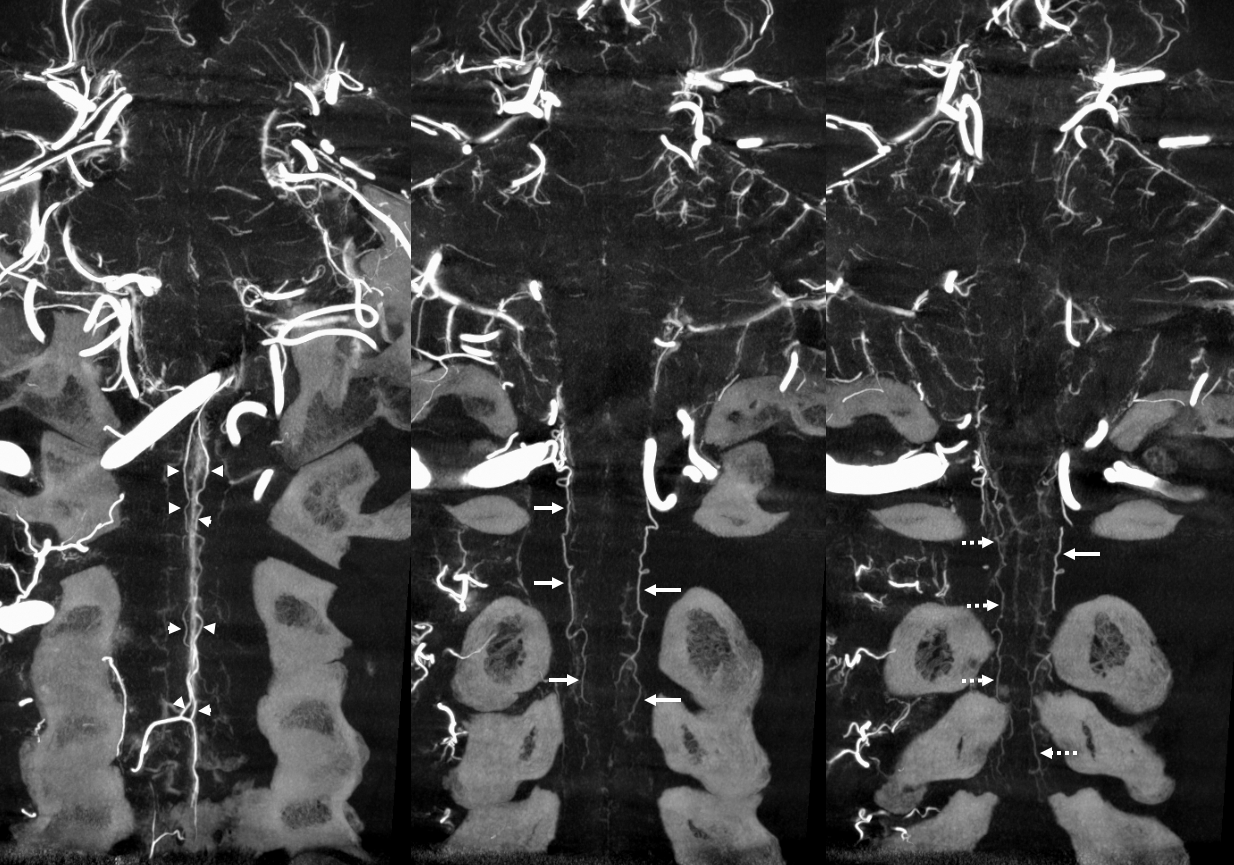
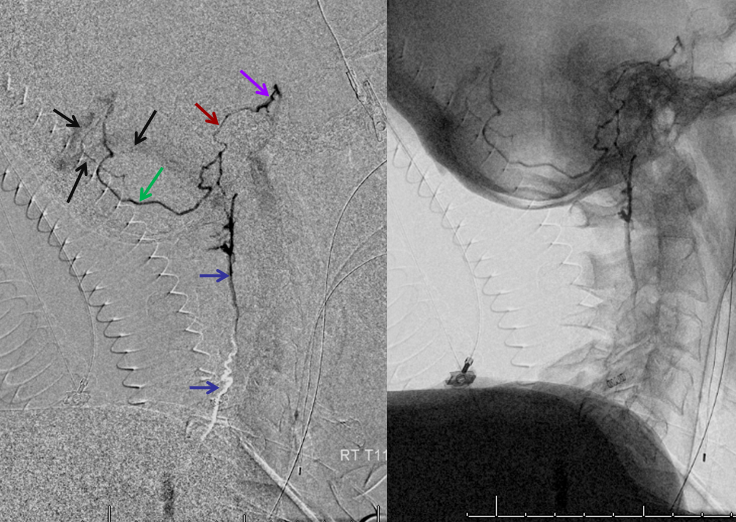
In our experience, broadly speaking, both types exist. The distinction or transition point between the two types is where the debate is. From a big picture perspective, there are undeniably both. Below is an example of cervical spine DYNA, where there is a very clear posterior midline vein draining the cord that has nothing to do with any foramen (gray lines, outline, arrow). The more numerous radicular veins are marked by white arrows.

Without arrows
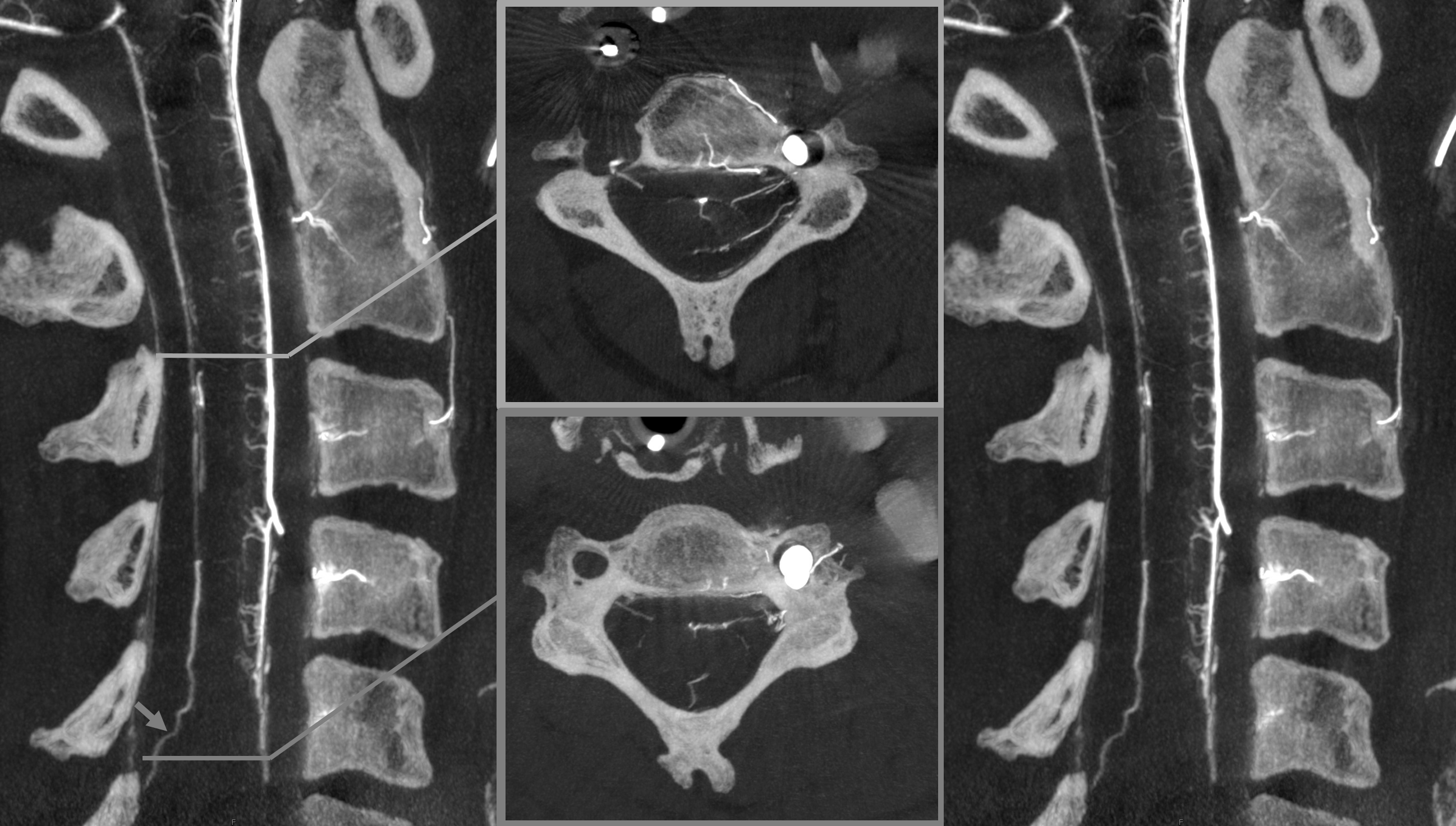
In our opinion, the “radicular” veins — meaning in our definition a vein that basically, or loosely at least, follows the radicular nerve, are more common. Bridging veins are less common, but certainly exist.
Note, in same case, duplicated left ventral C3 veins
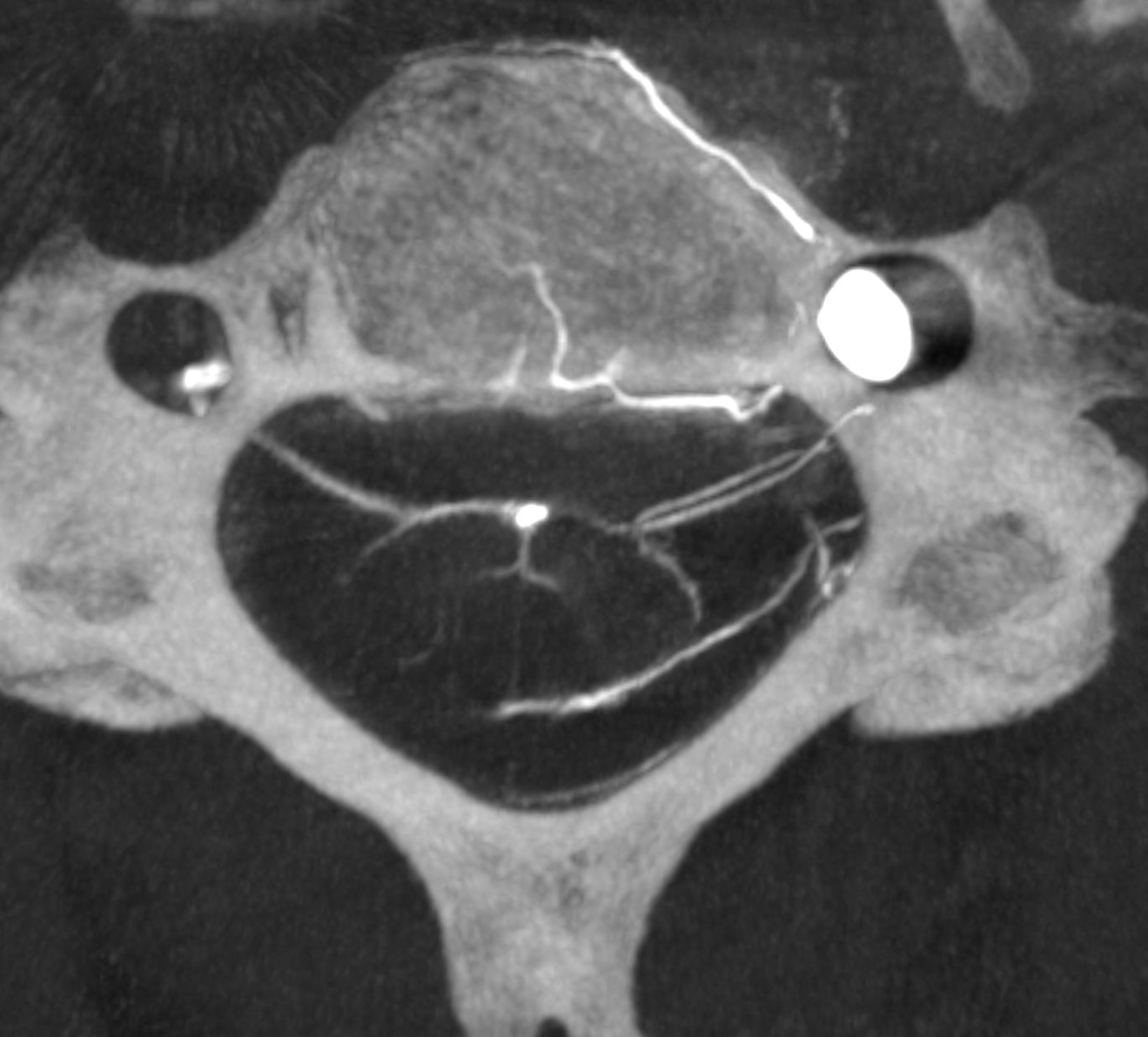
Obliques showing all types of veins. Black arrow is on the radiculomedullary artery
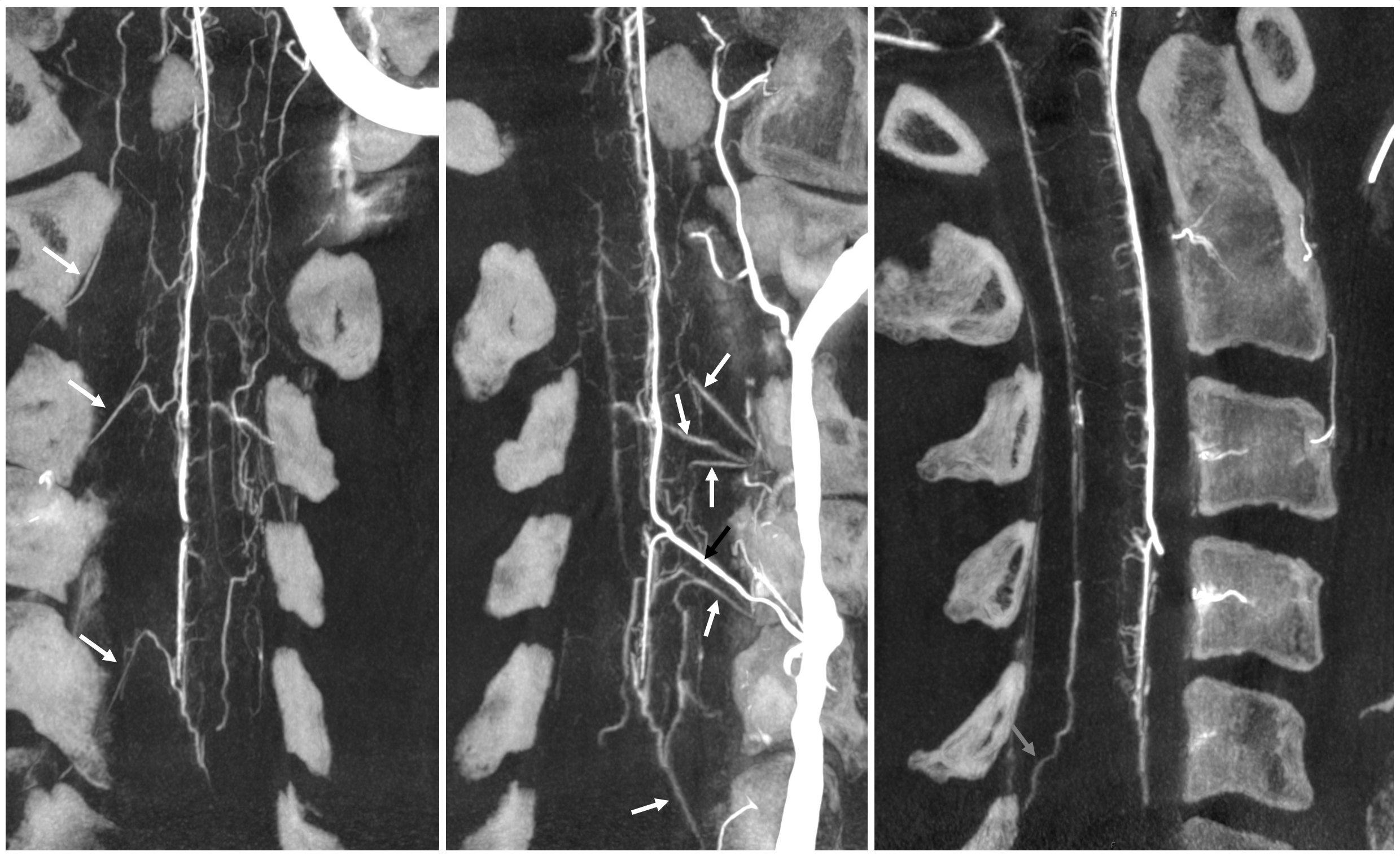
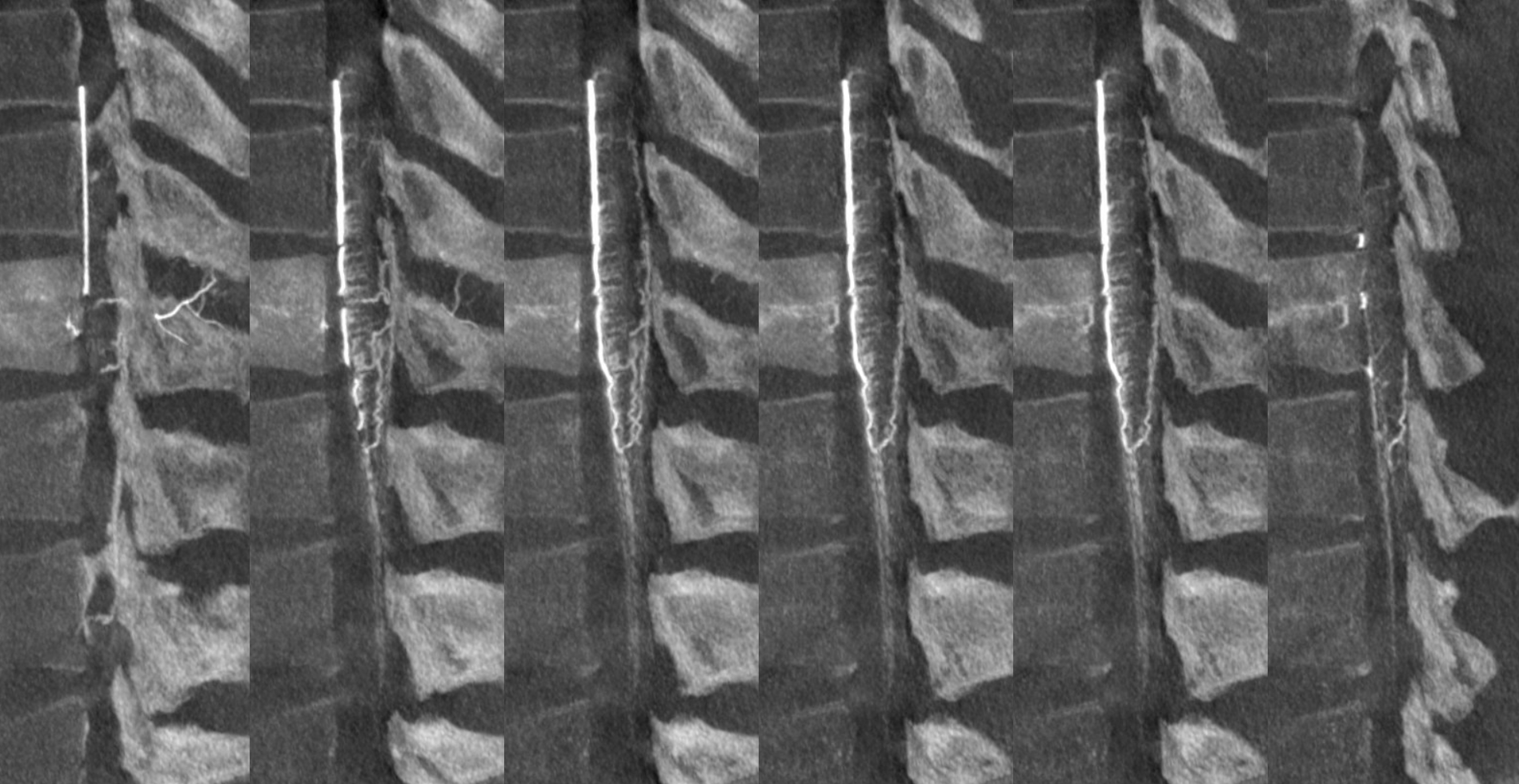
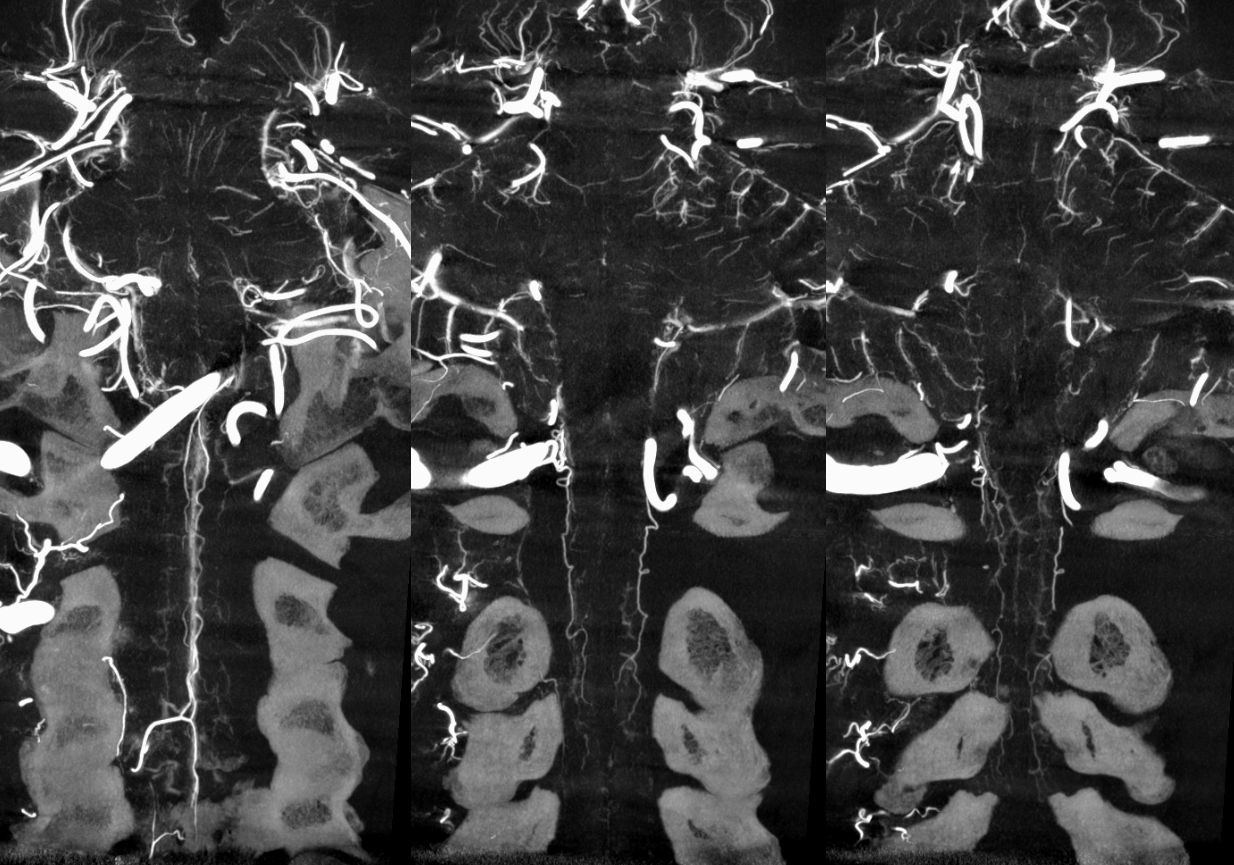
There is a lot of variability in the extent of normal radicular venous drainage. This likely plays a role in symptomatology of spinal dural fistulas for example. Below is another example of a cervical spinal DYNA, in a normal subject, where essentially no radicular or bridging veins are present.
The anterior spinal (arrowheads, fenestrated, lateral spinals (arrows) and posterior spinal (dashed arrows) have been filled, so its not a matter of poor injection.
Without errors below
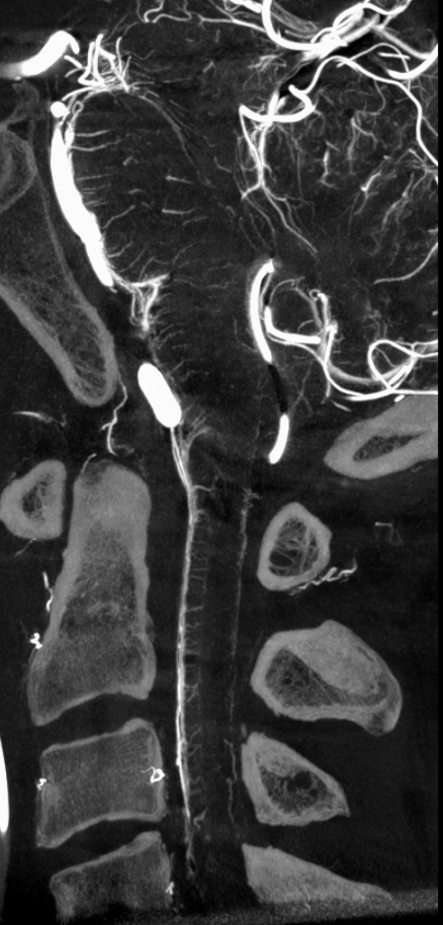
Sagittal view shows amazing sulco-comissural veins (and arteries)
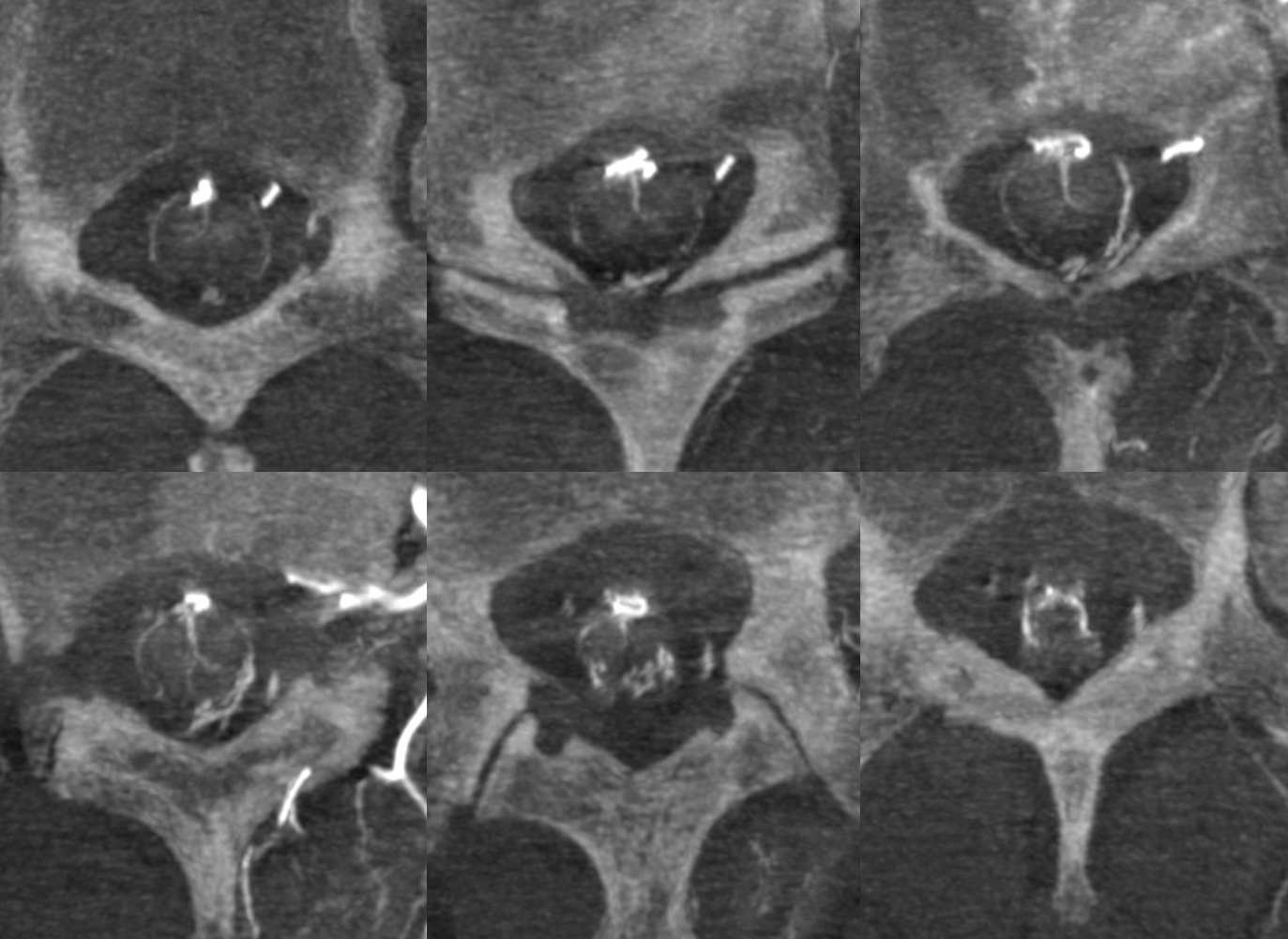
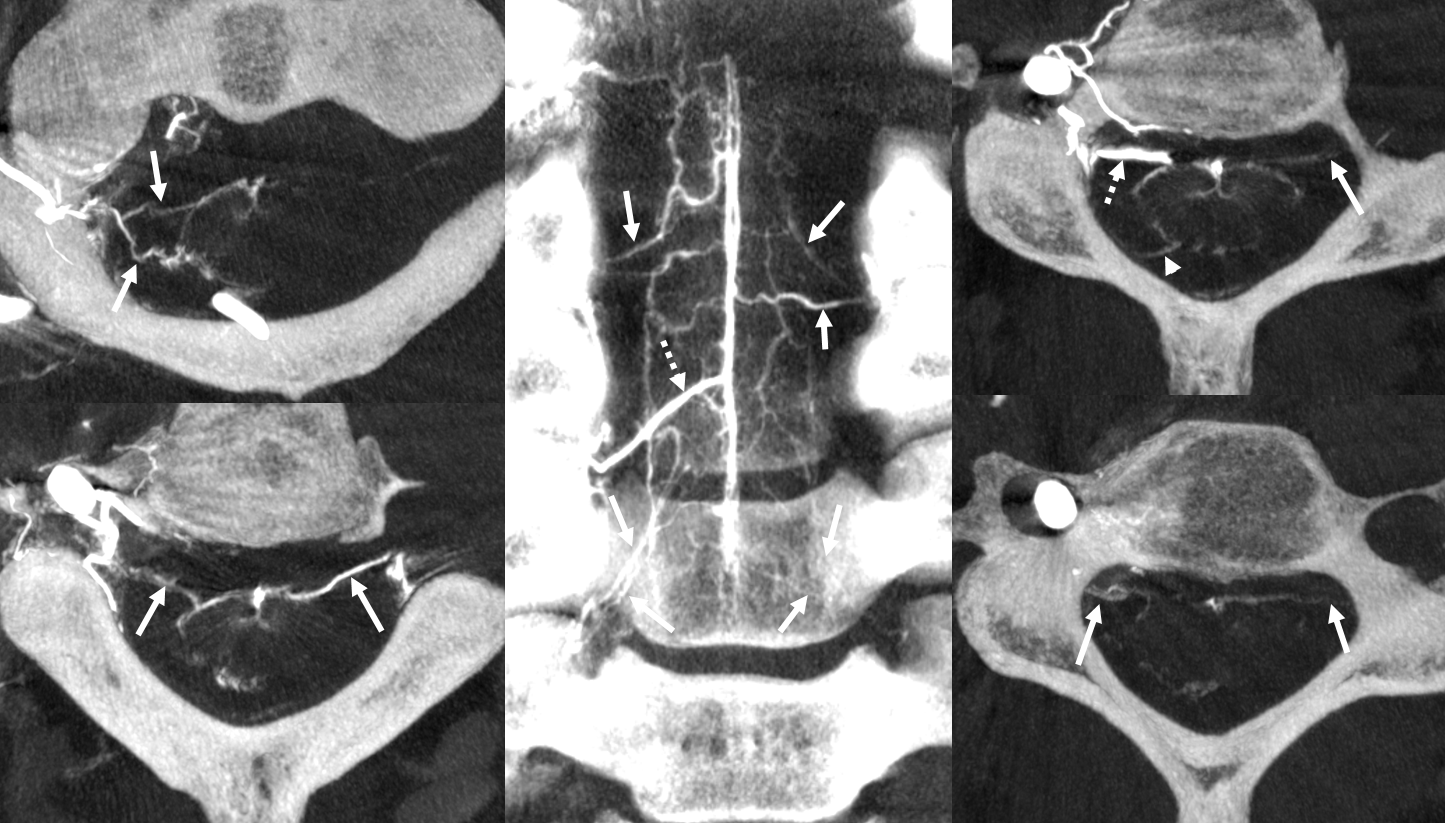
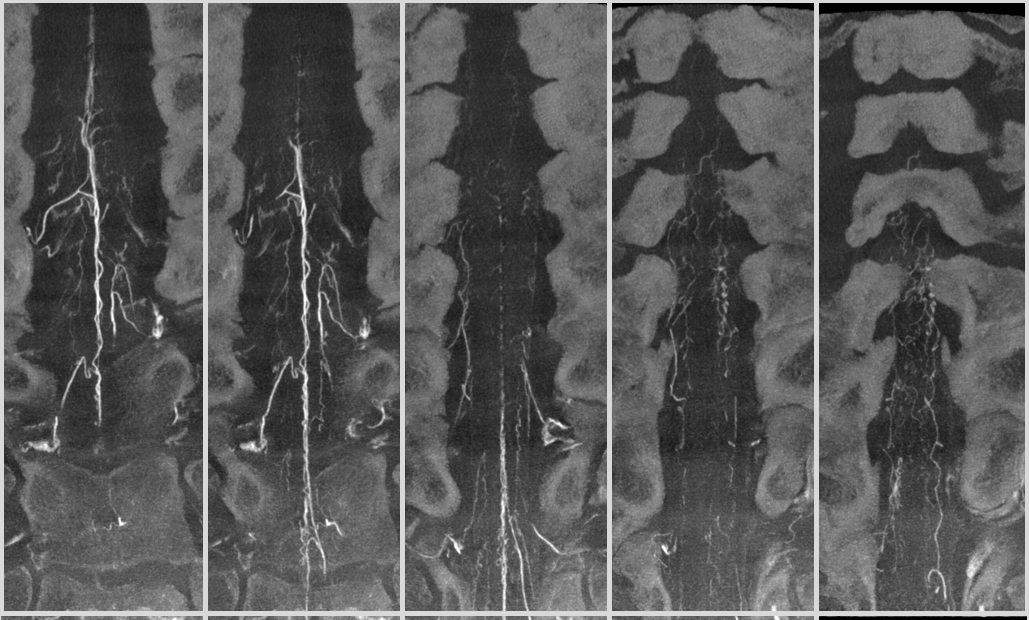
Below is a cervical segment with tons of radicular veins (arrows), one bridging one (arrowhead), and radiculomedullary artery (dashed arrow). Middle image is an unusual Average Intensity Projection. Kind of looks like a vintage ex vivo xray cast. This is very much in vivo though — and normal too.
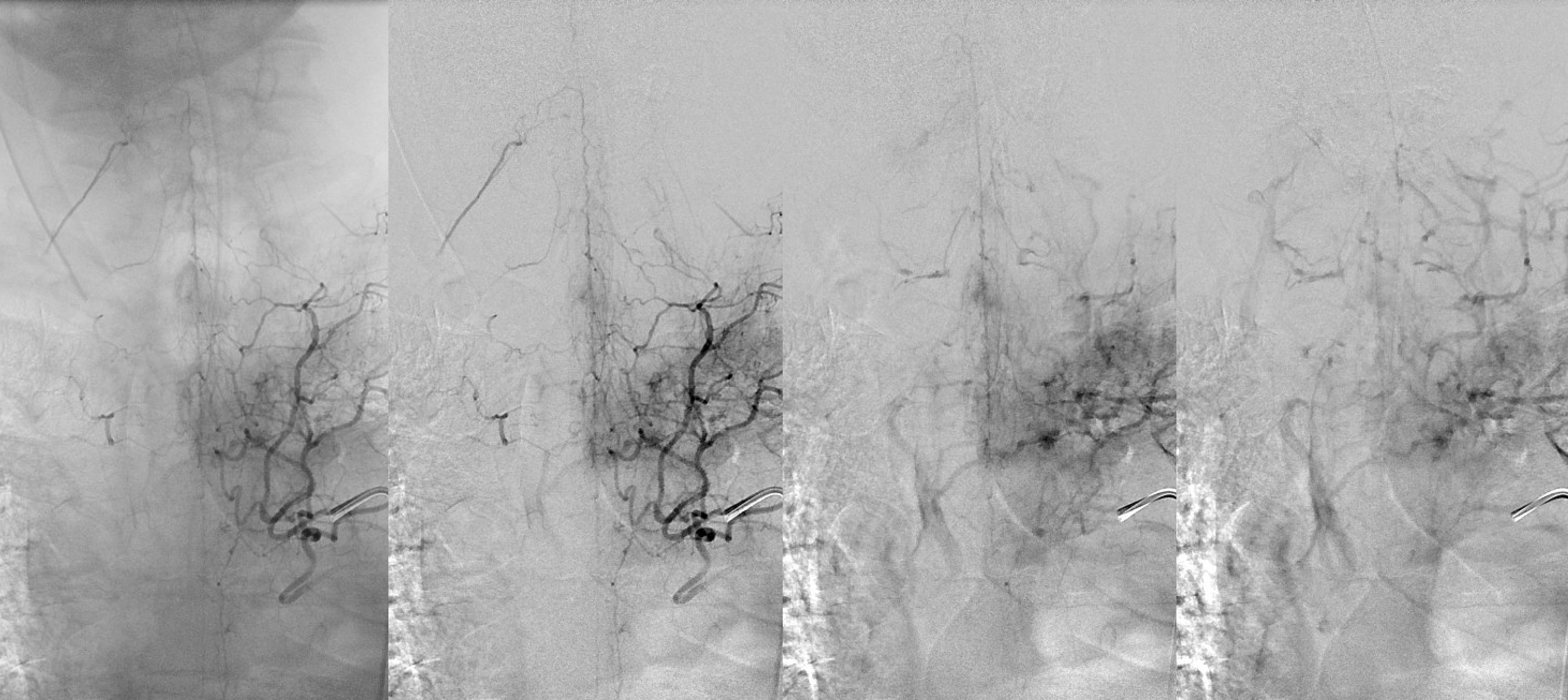
Below is an example of multiple radicular veins in a normal subject, following injection of the supreme intercostal artery
DSA of the same patient
In summary, the radicular and bridging veins are the key link between intradural and extradural venous systems. Their normal frequency and distribution are very poorly understood, but are likely to be highly variable. Some people just have more of these at baseline than others. Check out Armin Thron for what we know. The less those things one has, the more the chance that a fistula or something else congesting the cord may not be as well tolerated. Much more basic research is needed here…
Pathologic Correlation — Dural Fistula and Cord Venous Congestion
Another dural fistula, same lesson — the less radicular veins, the more congestion, the more symptoms. See this one for example — tons of congestion and cord edema. Why — no radicular outflow
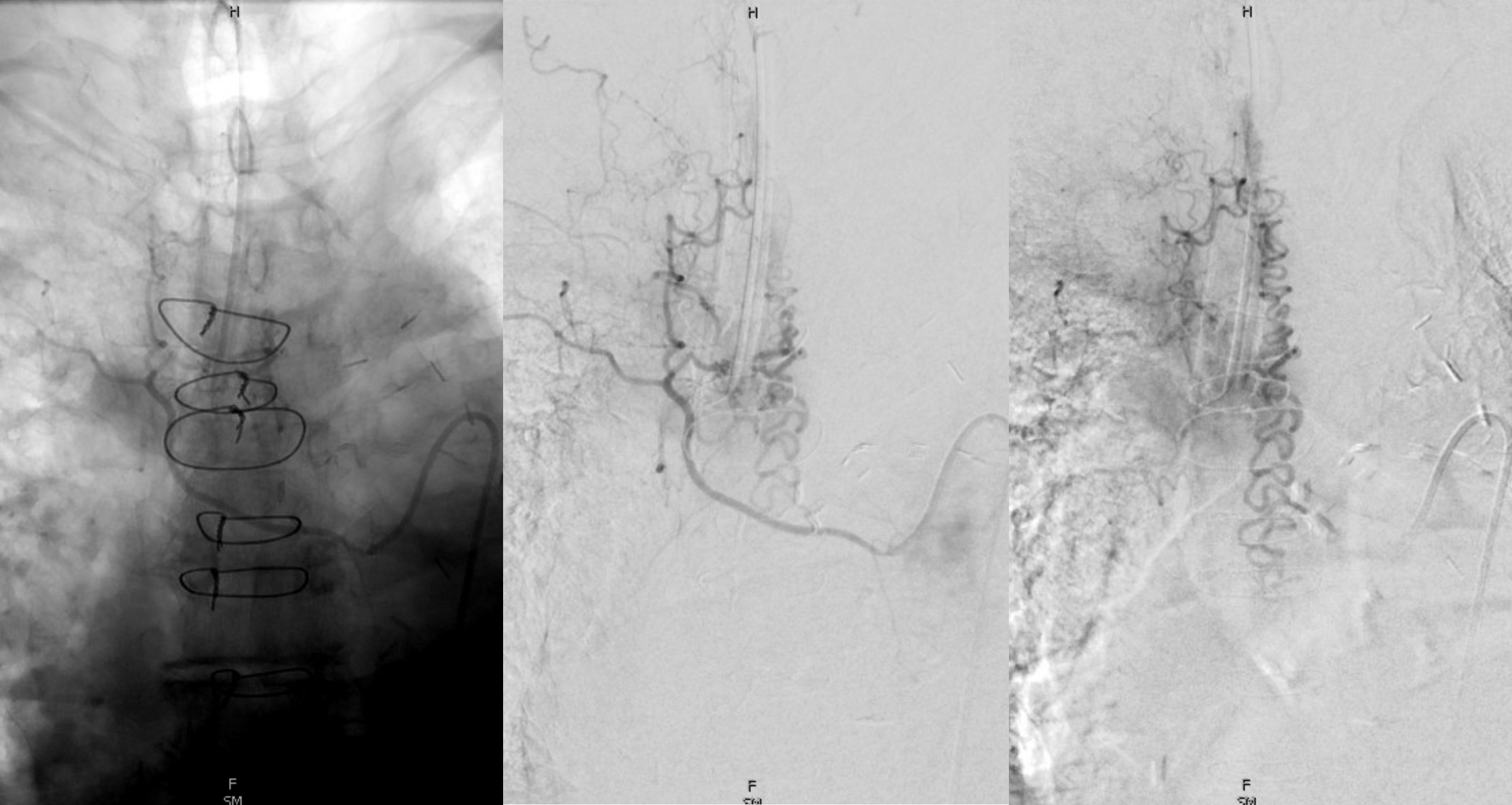
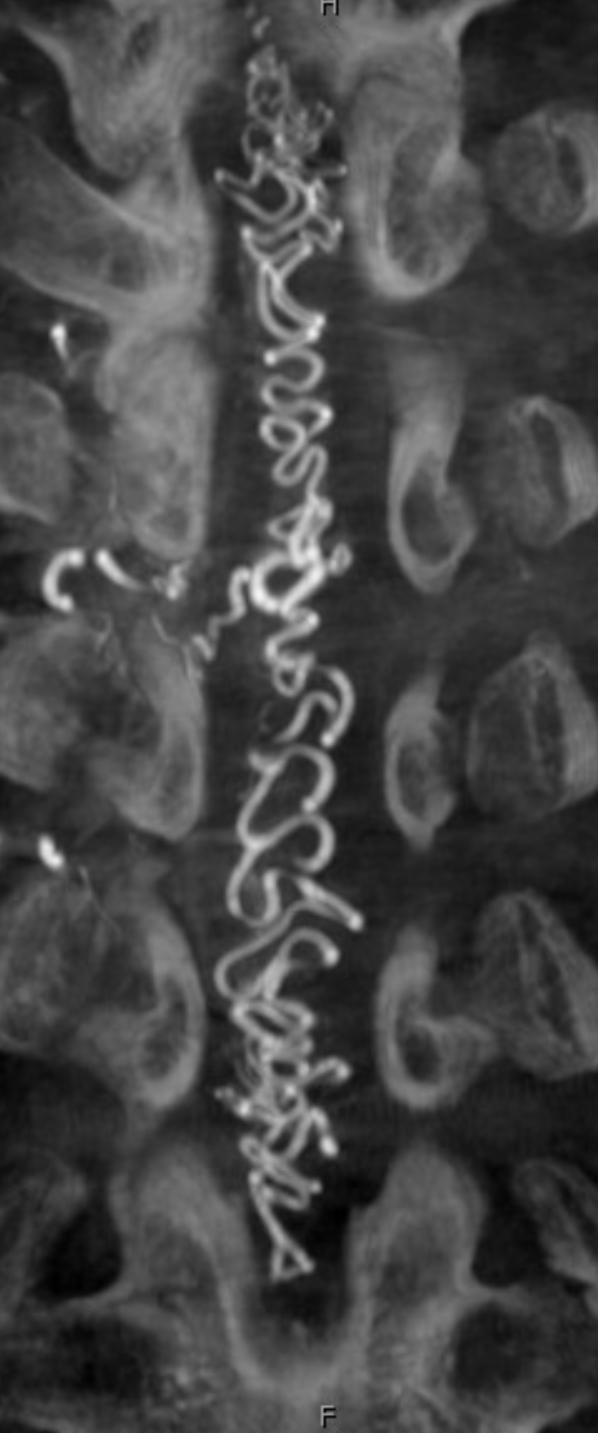
Below is an old case of a dural fistula — no outflow except much higher up at c-spine
Corresponding lack of venous phase following Adamkiewicz injection. The internal reference is bone, which is in venous phase on the right (black arrows). The cord blush is there (purple), but no radicular veins.
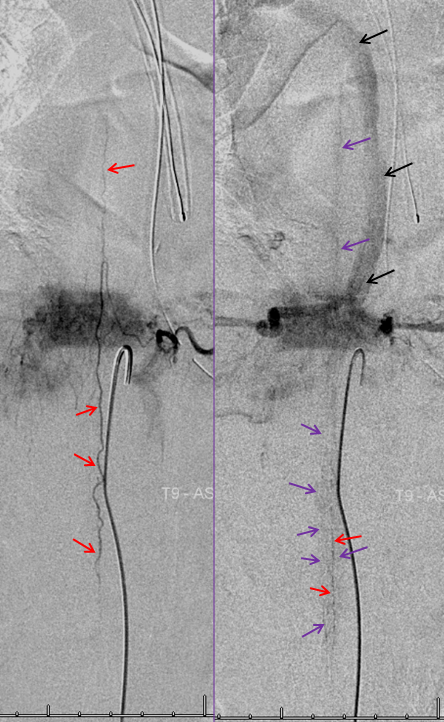
Another case — extreme congestion, with low thoracic fistula decompressing into the sigmoid and cavernous sinuses…
Lateral view
Pathologic Correlation — Hemangioblastoma
A hypervascular tumor like this can present with venous congestion much like a dural fistula. It does not have to be mass effect. Look at the congestion this hemangioblastoma is causing. Fortunately there are several radicular veins still in play
Extrinsic Venous Network and Batson’s Venous Plexus
Normal anatomy arterial injections are generally not good for visualizing this venous system — too many arterial tributaries, and only or two can be injected at a time. We will show some exceptions — C1 jugular venous stenosis for example results in suboccipital / epidural venous compensation, and thus can be seen well. Pathology (IVC thrombosis, fistulas) congest the plexus and allow visualization, but these are not “normal”. Phlebograms were a thing of the past, but thankfully no more. Because of the recent recognition and development of endovascular techniques for treatment of CSF venous fistulas, there is a lot of material available, as well as practical use of it. We are collecting it to have a comprehensive presentation.
EXAMPLES
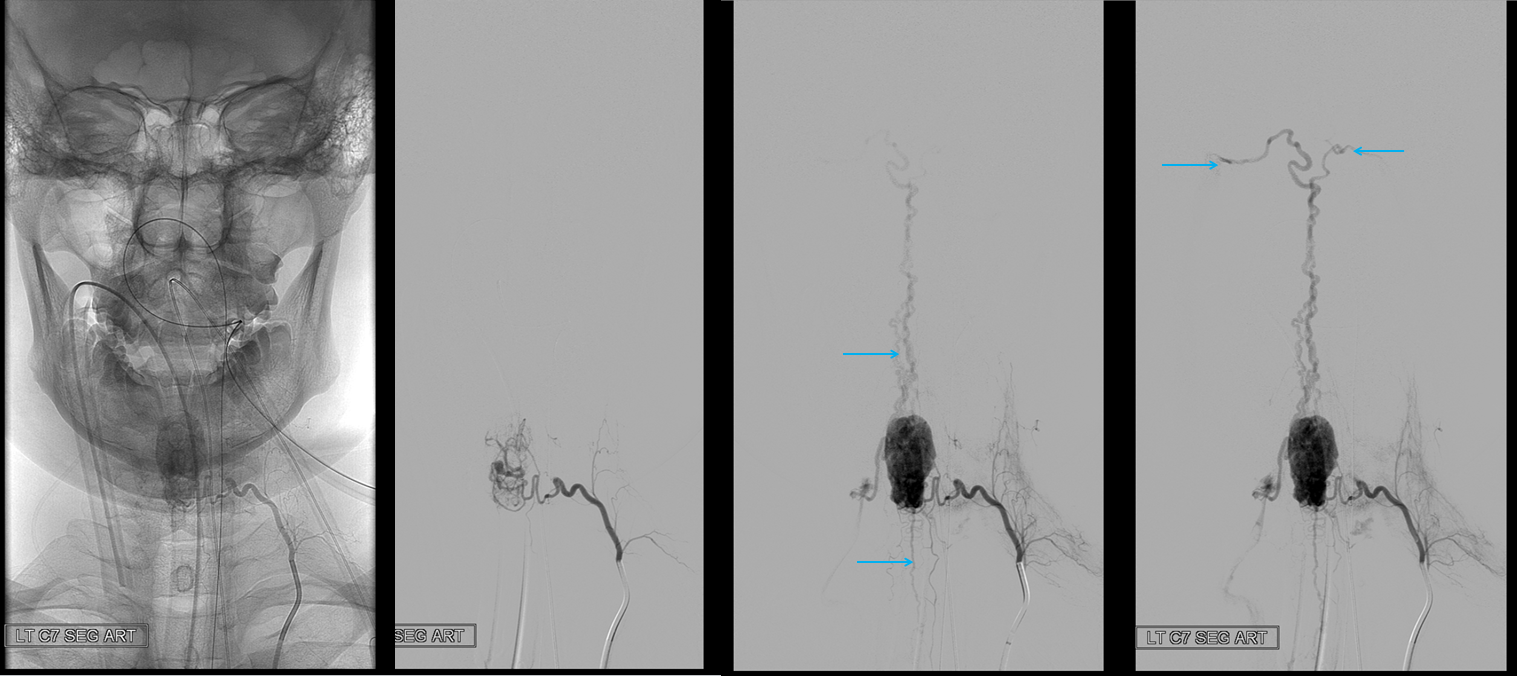
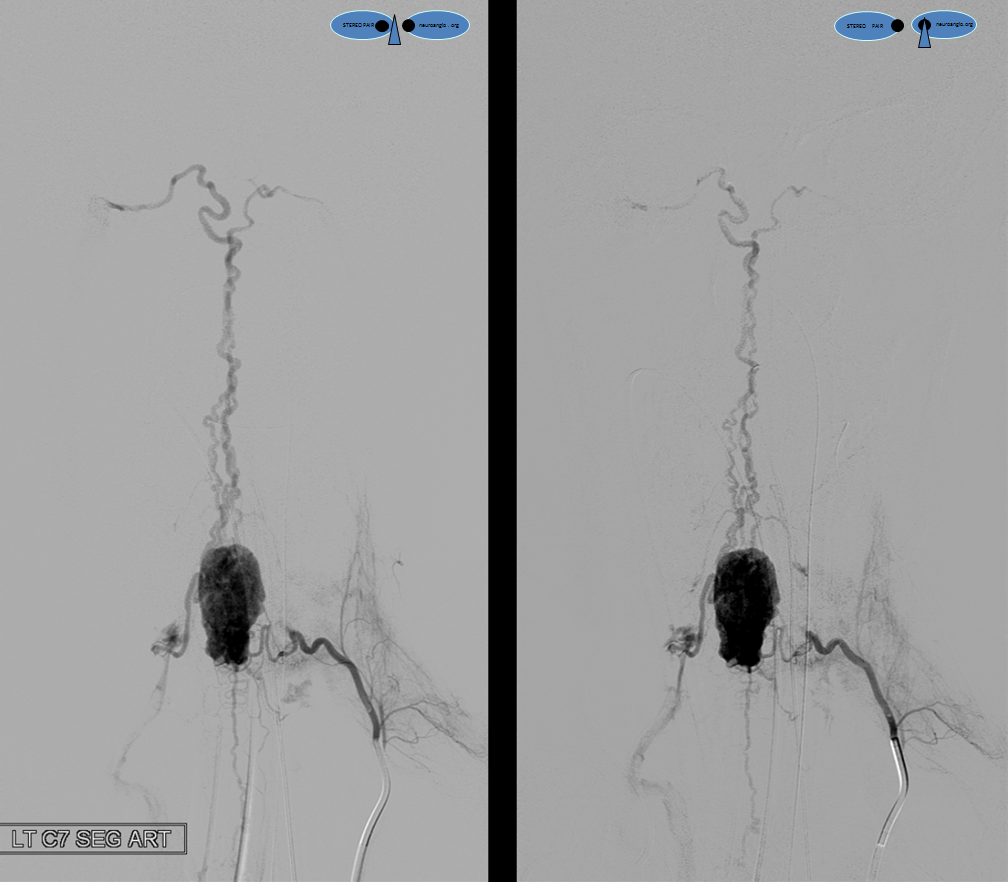
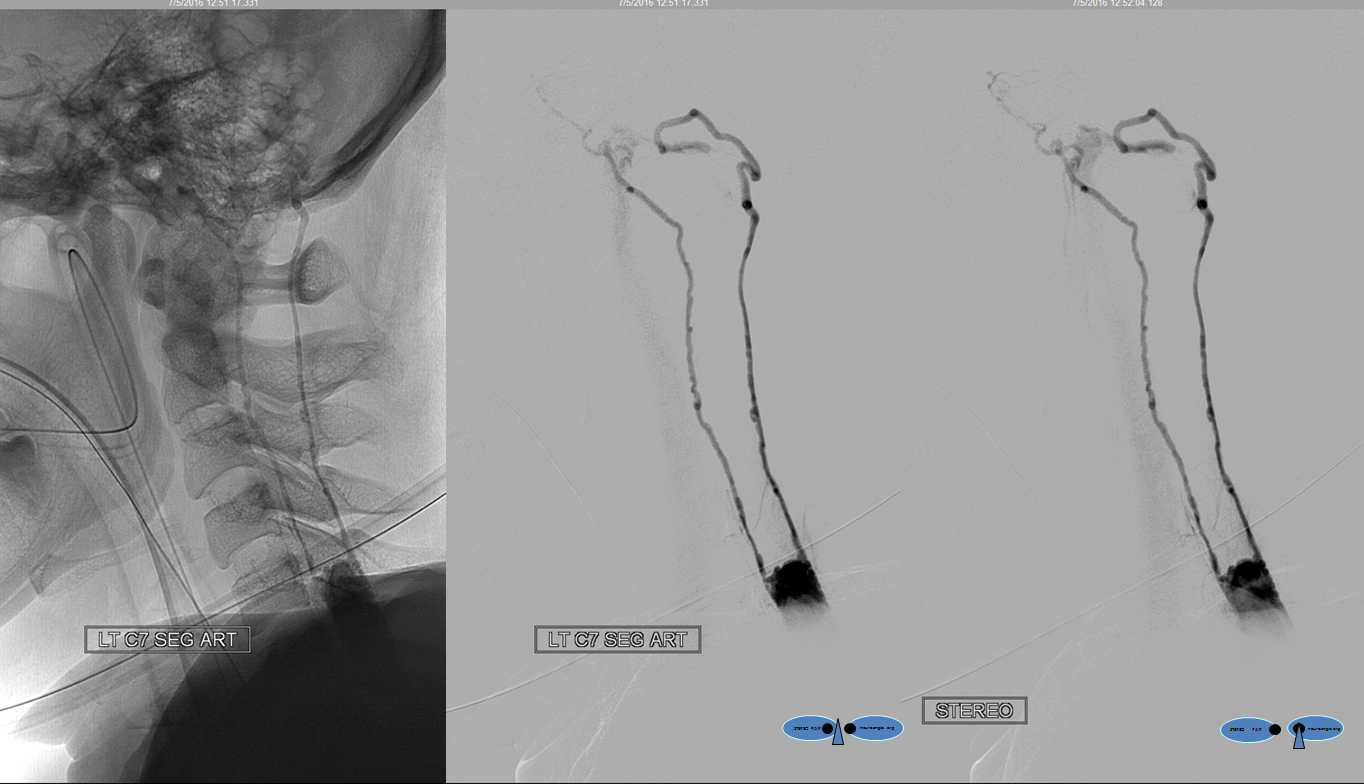
A segmental vessel injection can visualize only a part of the network subserving the arterial distribution of the interrogated vessel. For anatomical demonstration purposes, one can resort to some unusual methods, such as reflux of IV contrast administered into an upper extremity in an individual with distal venous outflow obstruction — such as this patient with subclavian stenosis (orange) status post pacemaker placement (red).
The extrinsic network consists of anterior epidural plexus (purple) and a generally smaller posterior epidural plexus (not seen). The medullary veins drain into a venous network within and around the nerve sheath (dark blue) which is named “emissary veins”, and classically consists of doral and ventral (with respect to the nerve root sheath) emissary vein. These emissary veins connect the dorsal (posterior) and ventral (anterior) epidural plexi with the longitudinal efferent veins (white and green arrows, not well demonstrated) which run craniocaudally along the vertebral body. At cervical level, the vertebral venous network which envelops the vertebral artery is particularly well seen (light blue). An extensive paraspinal network subserving the posterior elements and adjacent musculature is present (pink), which is of little clinical consequence. The longitudinal efferents eventually drain into the azygous system (light green) or ven cava.
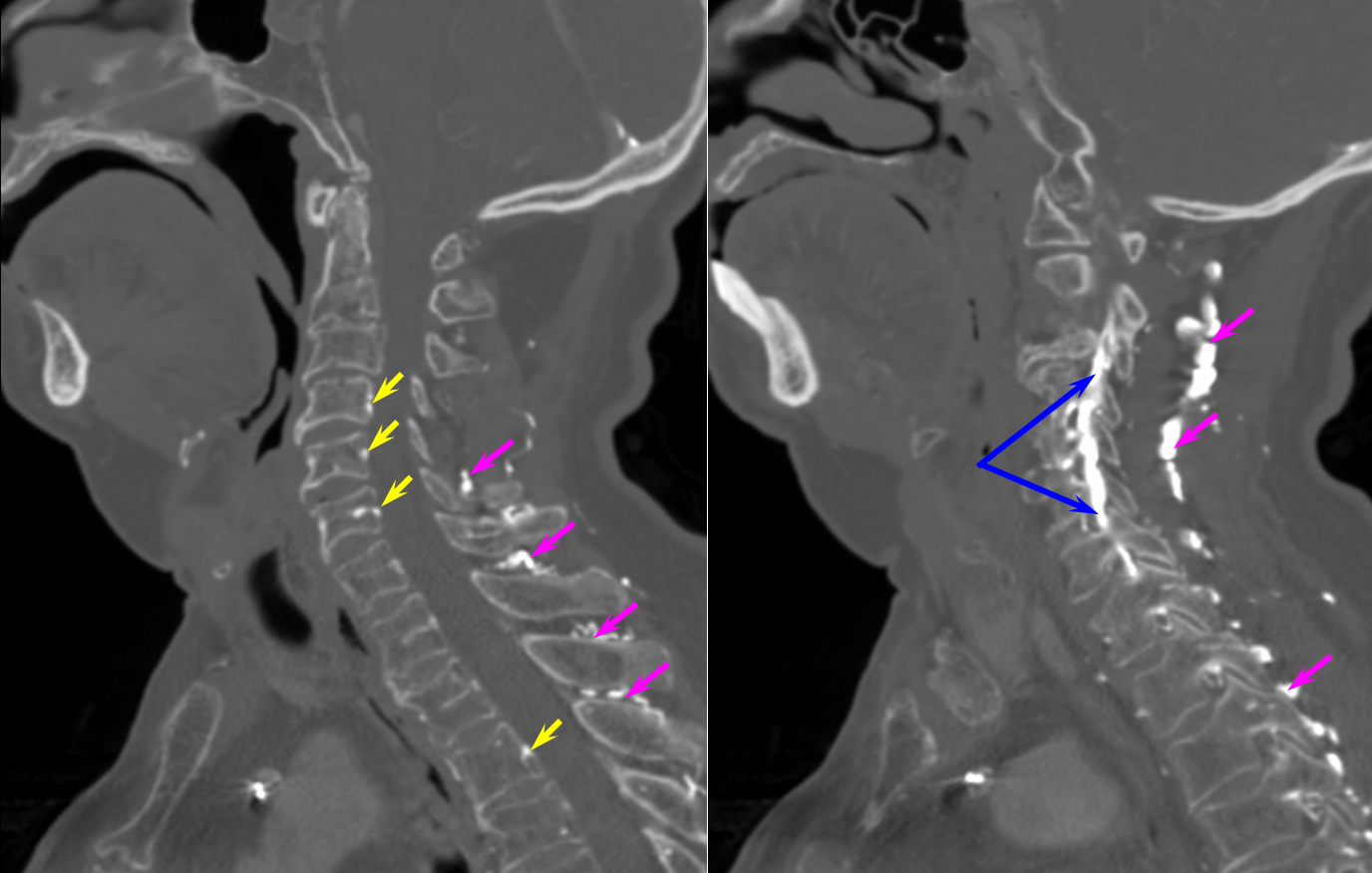
THYROCERVICAL TRUNK FISTULA
Here is a case of an unusual thyrocervical trunk AV fistula — probably iatrogenic from remote subclavian or IJ line attempt. Full case is here.
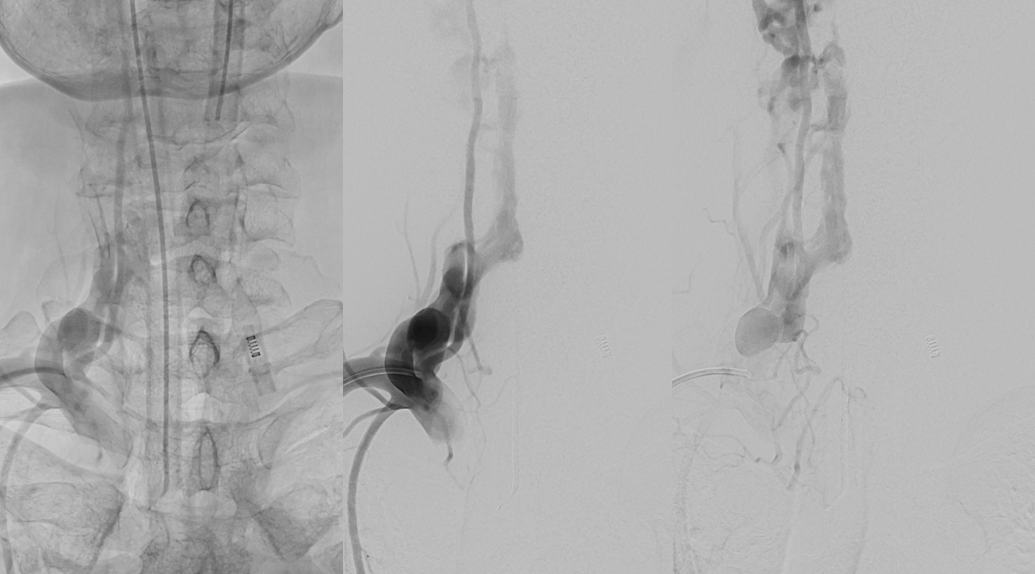
The reason for presentation (arm weakness/sensory deficit) is because the extraspinal veins for some reason do not drain antegradely into the subclavian or jugular systems. Instead the proximal post-fistulous vein (dashed arrow) drains via the C6 foramen (open arrow) retrogradely into the epidural space. Thanks to multiple patent foraminal venous outflows higher up (arrows), the congested epidural space can decompress into the paravertebral cervical veins, so that symptoms are mostly of nerve root congestion, and not (yet) cord congestion. See details here.
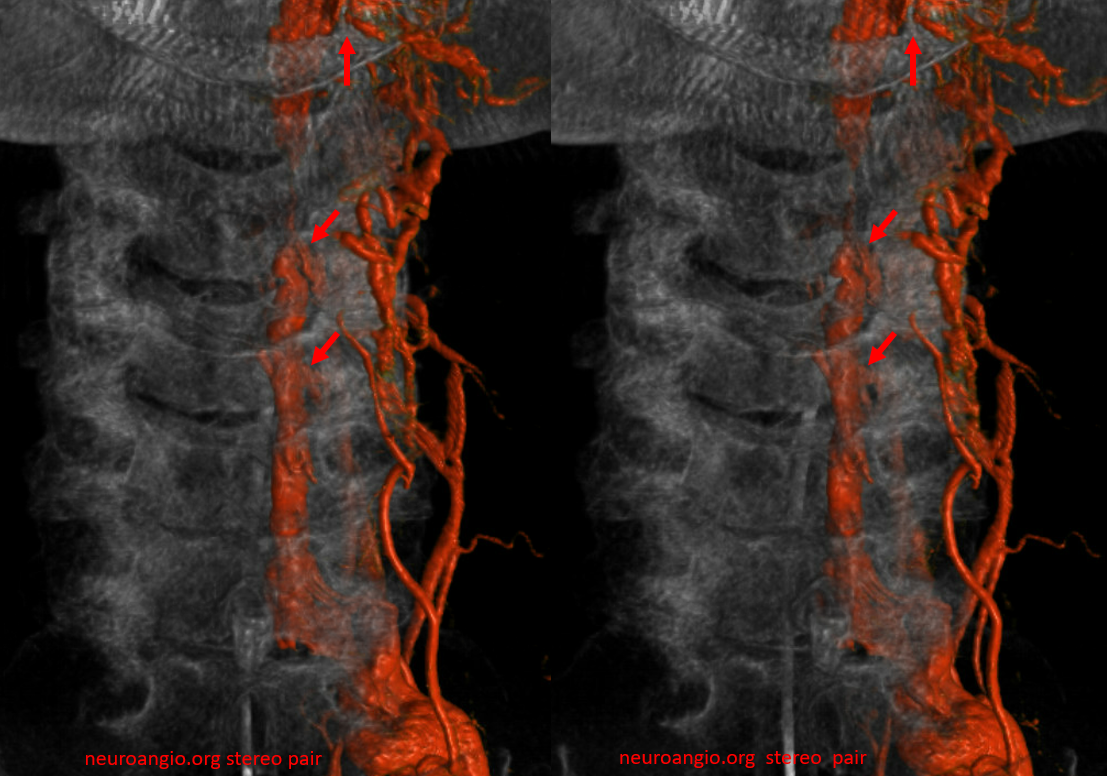
VR images. outflow foraminal veins are marked by arrows. stereo pair
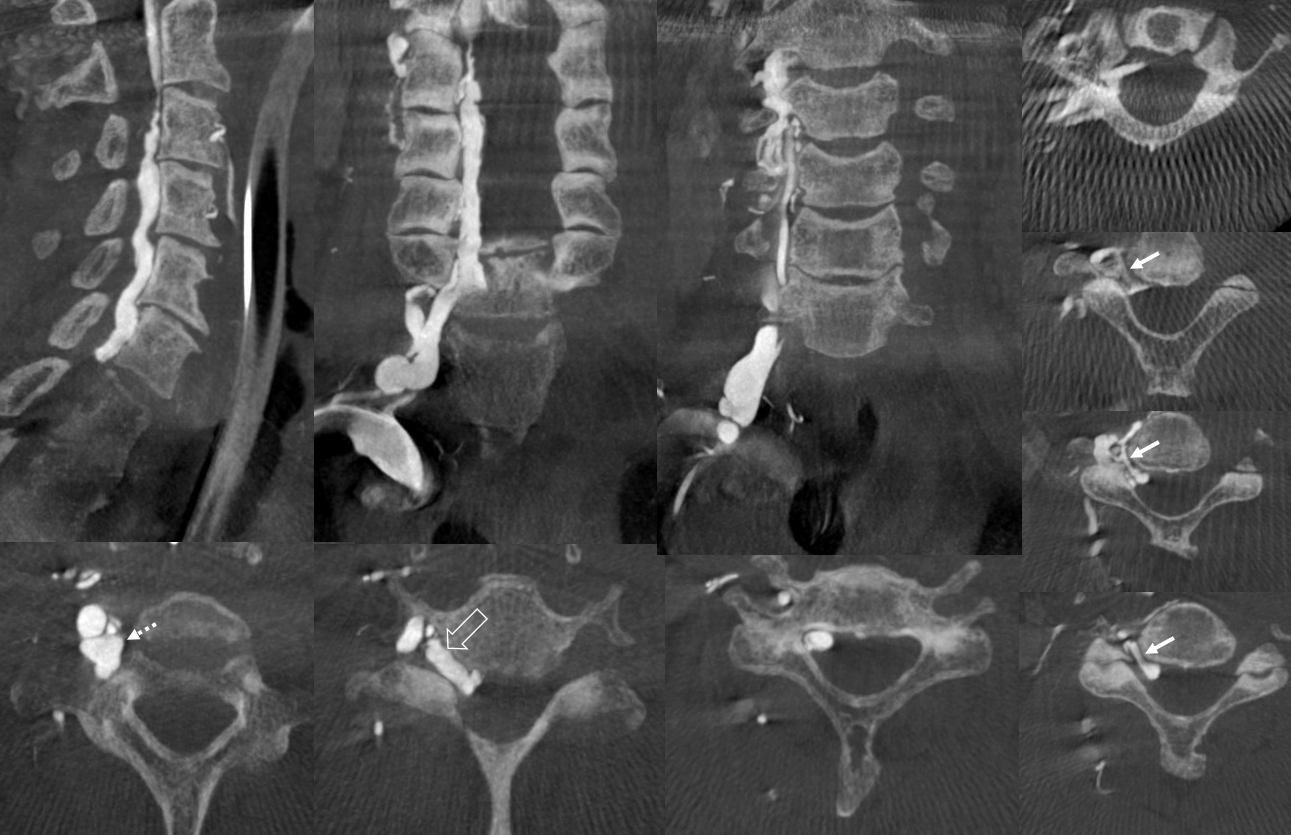
The same epidural plexus can be seen on MRI

C1 stenosis is often associated with venous compensation (prominent condylar plexus, epidural and cervical drainage). It is important to realize that C1 stenosis is often asymptoatic, though cases of pathology exist. Below is a view down the C-spine of a patient with C1 stenosis, showing promient epidural venous plexus. This is adaptive drainage, not pathology
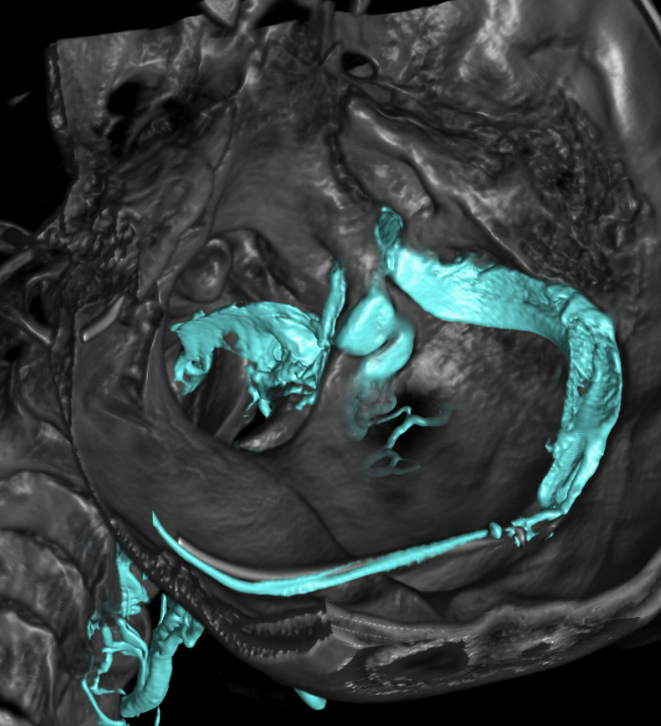
CTV (venous phase CTA) is an excellent way of looking at the epidural and foraminal venous plexi. Below are images of the same patient. Bilateral C1 jugular stenoses between lateral mass of C1 and styloid process — arrowheads (see another representative case here). In this one there is well-visualized epidural plexus (arrows) which is larger on the top and becomes progressively less congested towards the bottom of C-spine, as venous outflow is decompressed via multiple foraminal veins (dashed arrows)
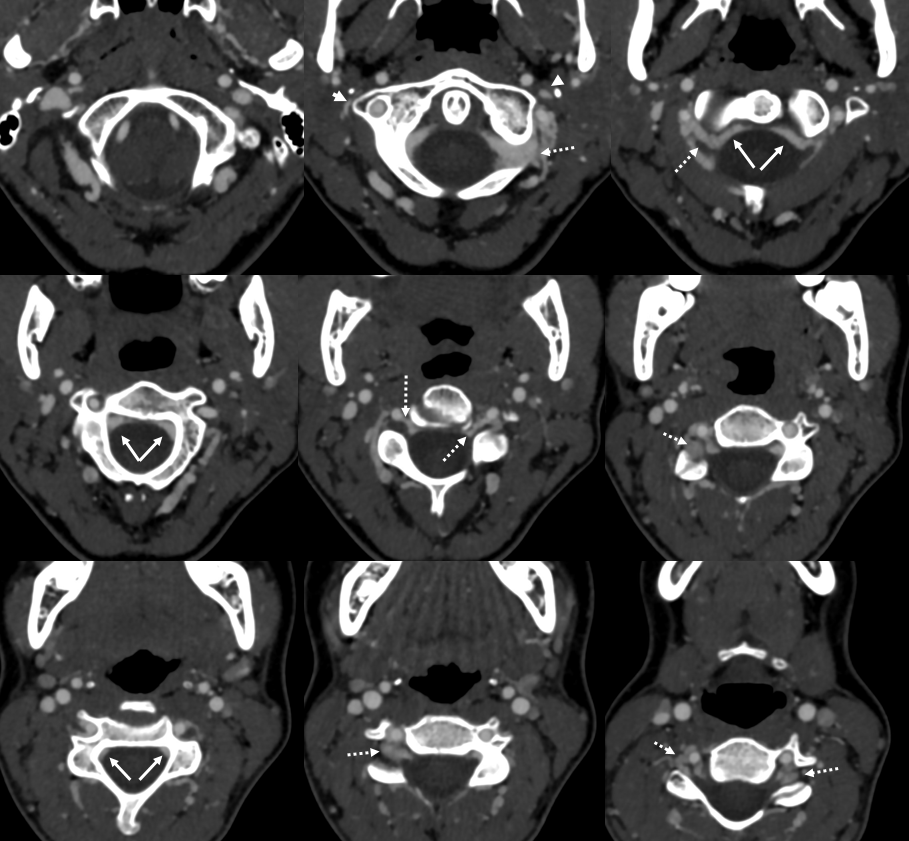
Paravertebral and Epidural Venous Plexi — Batson’s Plexus
The rich network of veins all around the vertebral column (prevertebral, paravertebral, posterior vertebral) and ventral and dorsal epidural venous plexi constitute a highly capacious and quite effective drainage system which parallels that of the Inferior Vena Cava. This functional network of paravertebral veins and epidural plexi is known as the Batson Venos Plexus, after Oscar Batson who described it as a way of tumor spread from prostate and rectum (see Batson’s Venous Plexus case). This system is now also understood to be the primary way of blood return to the heart from the lower body when the IVC is occluded (for any reason, not just malignancy). Today we rarely do angiography to diagnose IVC occlusion of course, so most cases are seen in cross-sectional imaging.
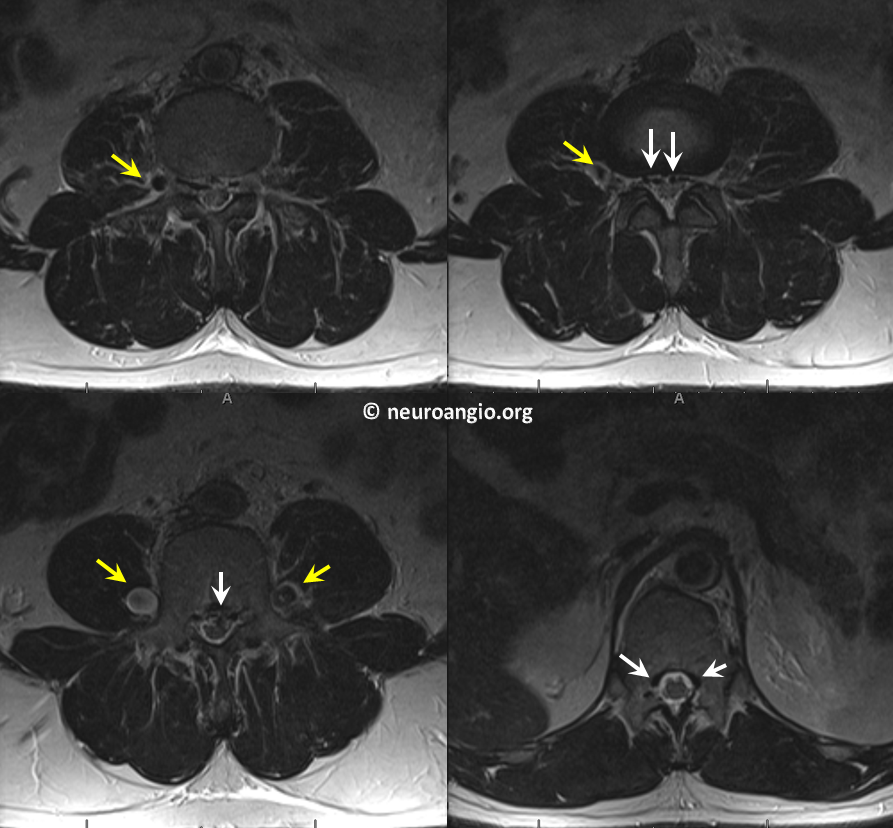
In this patient with long-standing feeling of heaviness and pain in the legs, the occlusion is due to retroperitoneal fibrosis. Axial MRI shows engorged paraspinal veins (yellow) and ventral epidural venous plexus (white)
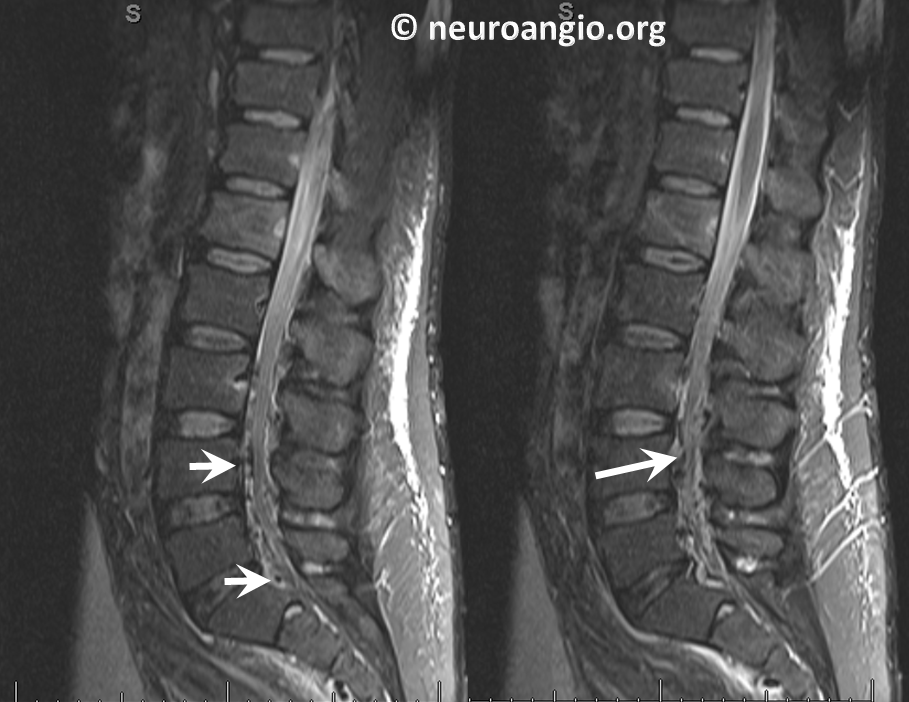
Sagittal images of the same
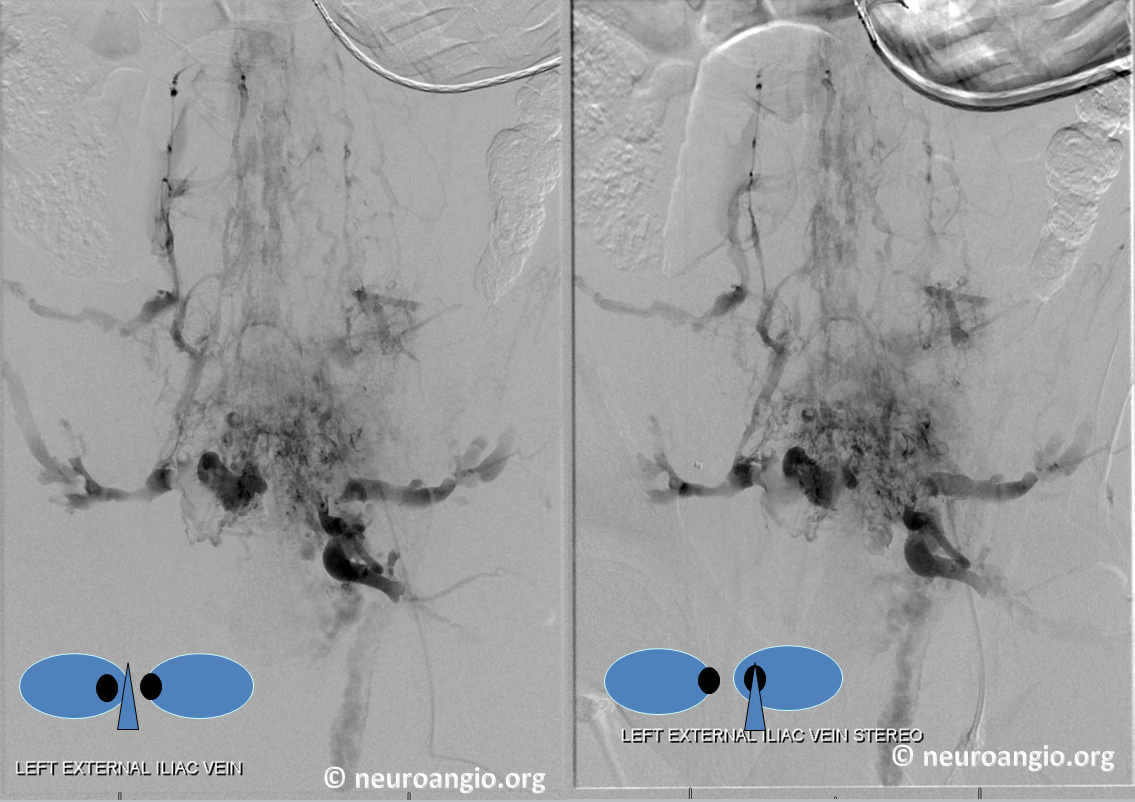
Frontal views of left internal iliac vein injection demonstrating occlusion of the IVC and drainage via Batson’s plexus of paravertebral (black) and epidural plexus (white) routes
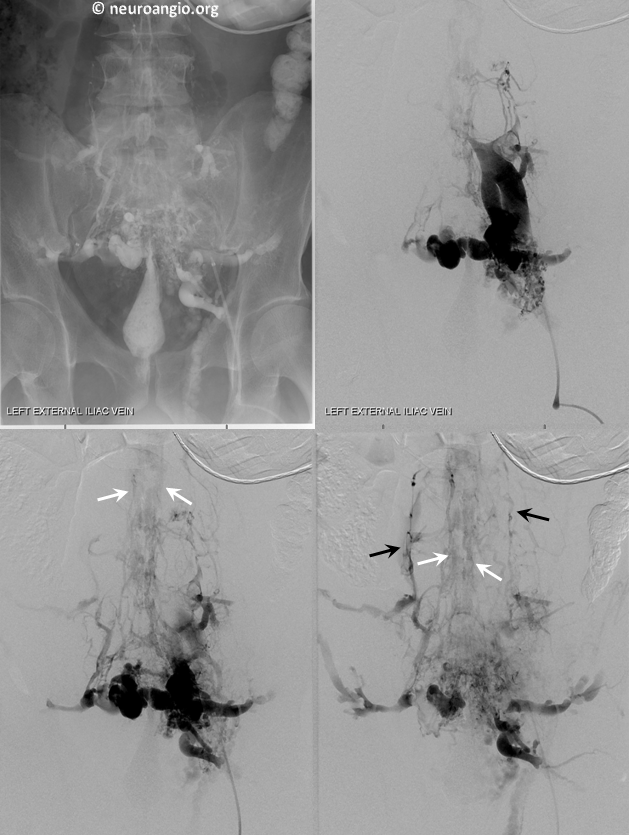
Stereo views of the same
Lateral views of the lumbar region
This drainage pathway has been stable in this person for many years.
Paravertebral and Epidural (Batson’s) Plexus visualization due to multiple fistulas
In this patient, an unusual fistula between branches of the internal iliac artery and anterior epidual venous plexus allows for visualization of the venous anatomy from an arterial injection. Unlike most spinal dural fistulas, this one does NOT drain via the radicular veins into the intrinsic venous plexus system directly related to the cord, but instead congests the extradural networks and impairs nerve root drainage.
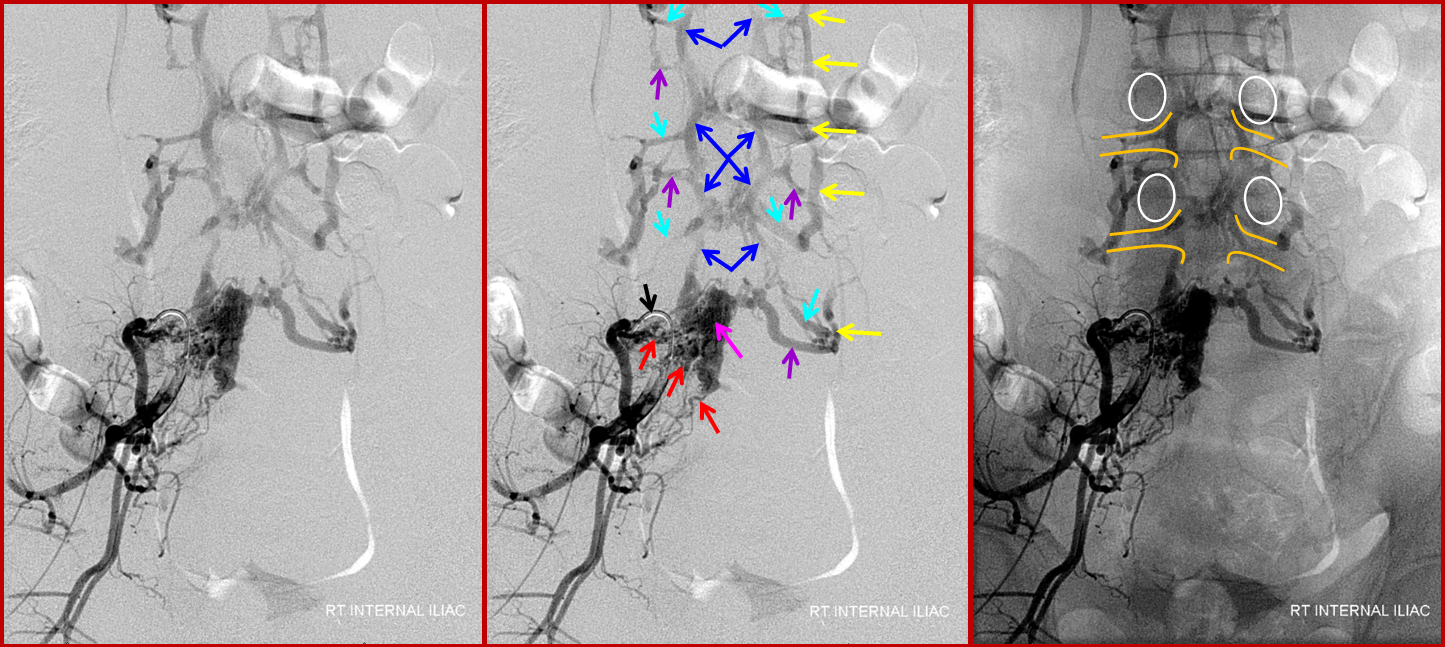
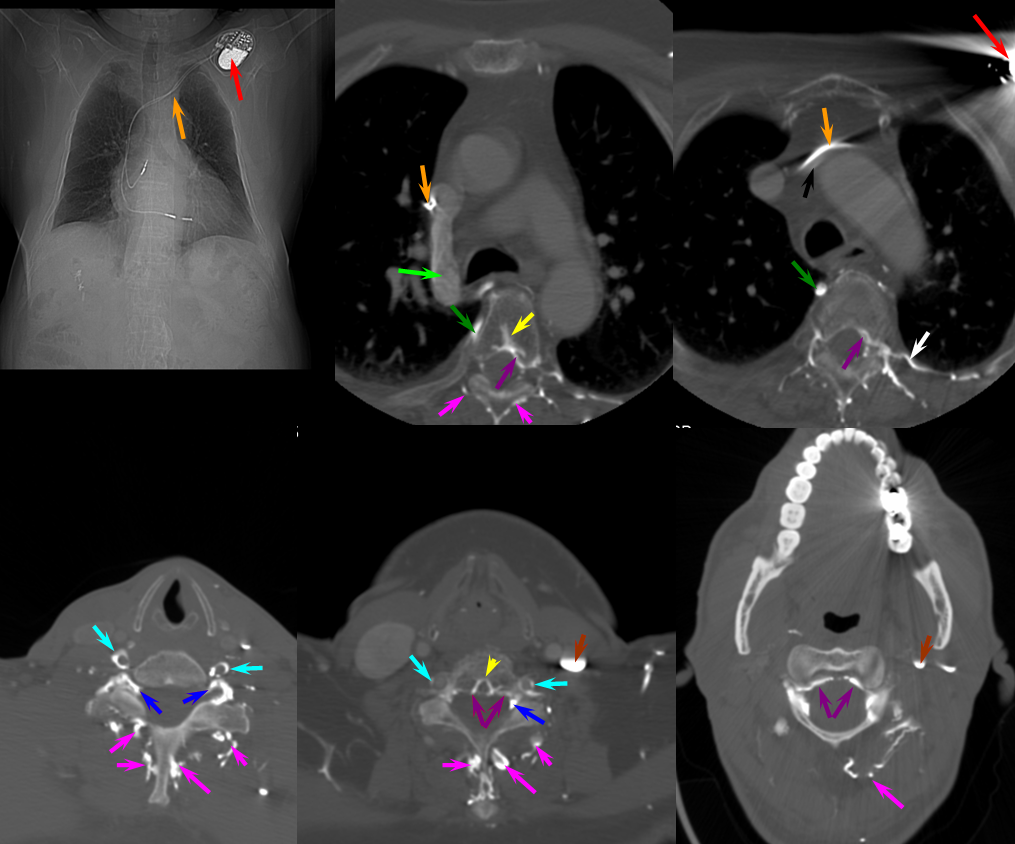
A 4F RDC catheter (black arrow) is engaged in the proximal right internal iliac artery. The center image is a labeled version of the otherwise identical left image. The right “native” image is enhanced with white ovals to mark the pedicles and orange lines to indicate expected boundaries of the nerve root sleeve. A number of arterial channels belonging to the L5 and S1 nerve root sleeves (red arrows) converge onto a fistula which opens into an epidural venous pouch (pink, part of “G” or anterior external venous plexus) behind the L5 vertebral body. The resultant congestion of the venous apparatus allows for angiographic visualization of the same venous anatomy which was illustrated on the CT scan above and diagram below. From the fistulous pouch (pink), contrast opacifies the diamond-shaped anterior extrinsic (ventral epidural) venous plexus (dark blue arrows, letter “G”). This network drains outside of the spinal column via emissary veins which surround the nerve root sleeve — venous channels superior (light blue, “F”) and inferior (purple, “F”) to the nerve root sleeve are visualized at multiple levels. The emissary veins empty into longitudinal efferent veins (yellow arrows, “I”), more prominent on the left, and located on the side of the vertebral body, usually anterior to the nerve root. The spinal cord veins would normally drain into radicular veins on the inside of the nerve root sleeve (not visible from this injection) which would then empty into the emissary veins.
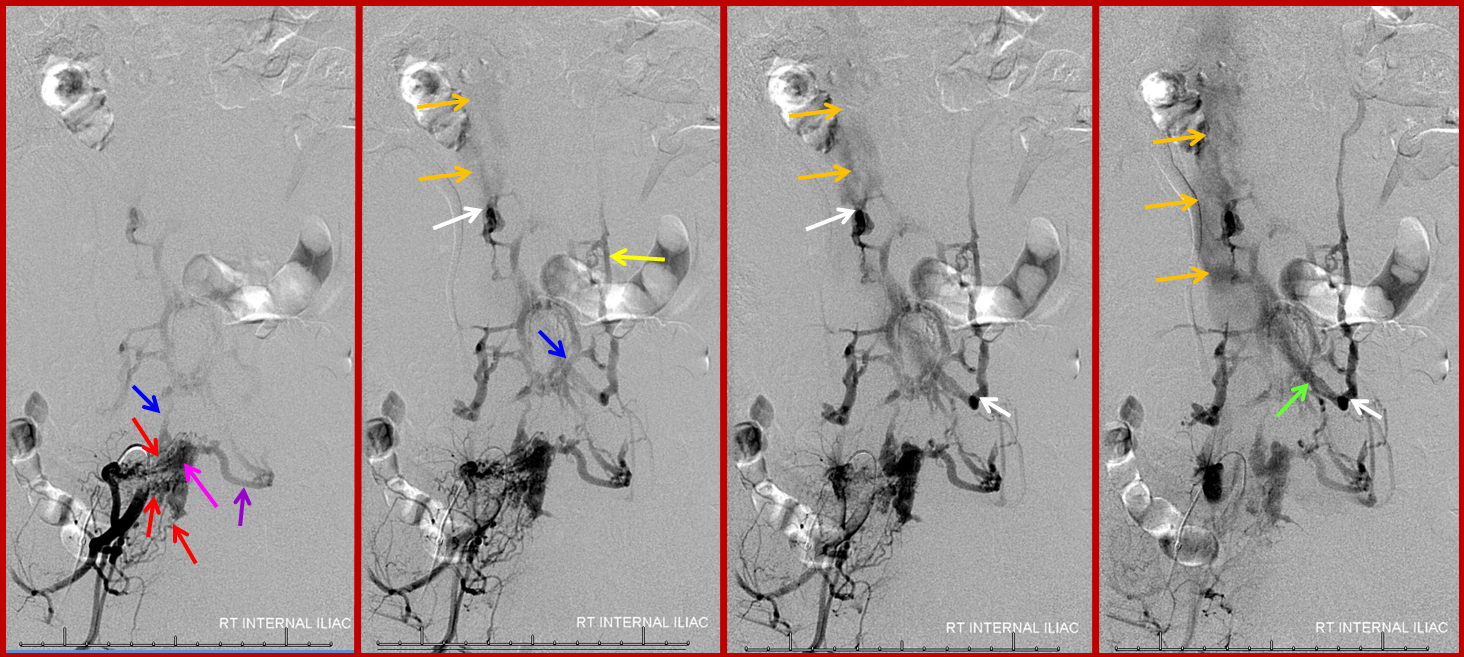
The same fistula in time sequence, from left to right, corresponding to earlier to later phases. The labels in leftmost picture are the same. The images are correlated with the diagram above. Notice particular congestion of the left L5 emissary vein (purple, “F” in diagram). The second to left image shows early appearance of the inferior vena cava (orange), which is progressively better filled on subsequent images. The common iliac vein (green) is also labeled on the rightmost injection, but first seen on the second to left image. Both the IVC and internal iliac vein are opacified from the longitudinal efferents (yellow, “I” in diagram) via dorsoventrally oriented veins (white arrows) which are markedly foreshortened on the AP projections, and whose depth is better appreciated on the stereoscopic images below. Blue arrow, or “G”, visualizes the anterior extrinsic venous plexus, into which the fistula drains.
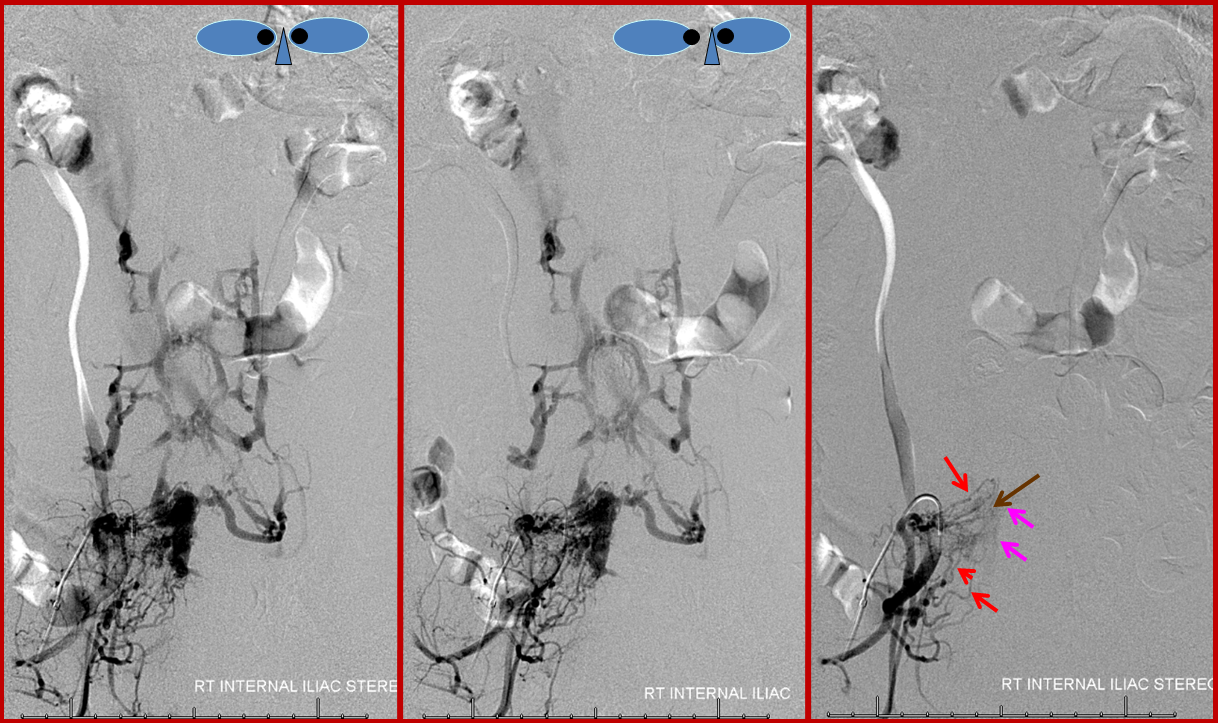
Stereo pair of the fistula and its recepient venous network, for enhanced appreciation. An earlier phase image (right) better shows the arterial network (red) leading into the venous pouch (pink). The arteries seem to converge on a single point (brown), which is the fistula itself.
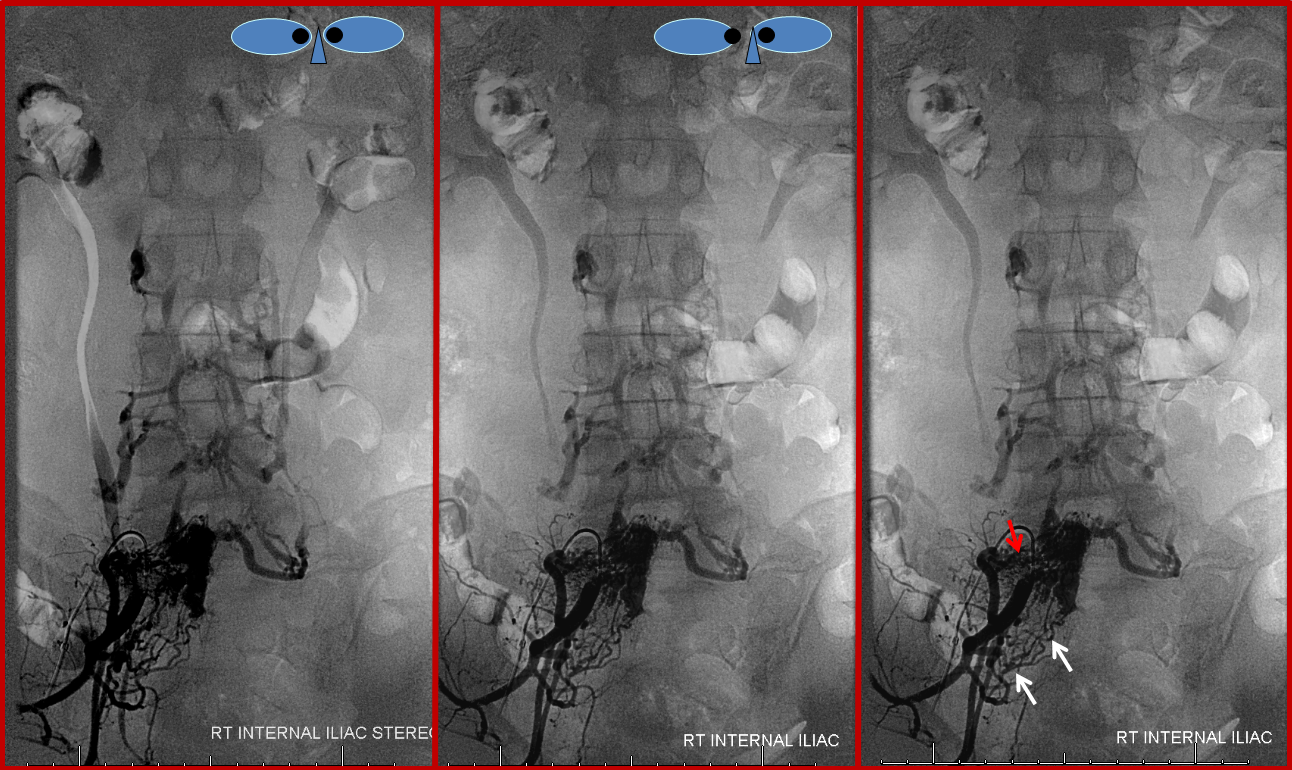
Stereo pair and corresponding labeled image, helping demonstrate the nature of the fistula, which consists of innumerable arterial channels (red) running predominantly towards the right L5 foramen, but also with significant contribution via the right S1 radicular arteries (white). The fistula itself is therefore located distal to both of these structures, in the ventral epidural space.
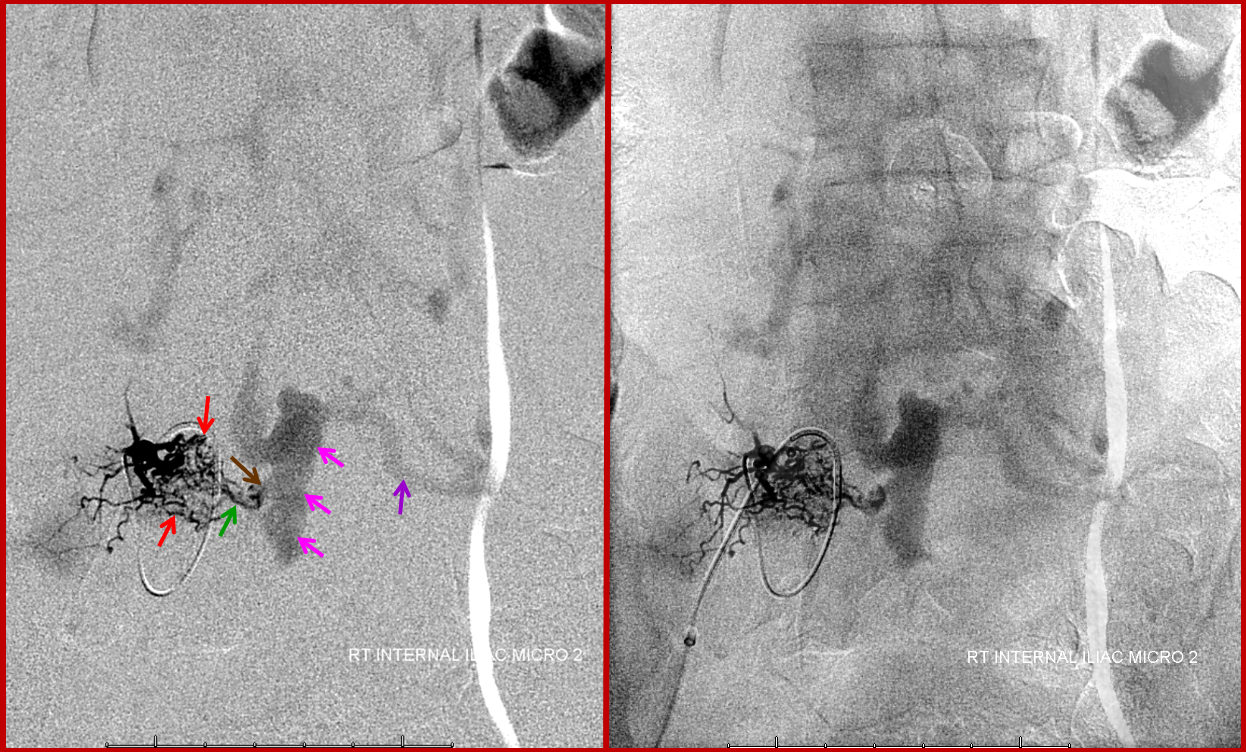
Finally, a microcatheter injection of the dominant L5 contributor elegantly demonstrates how innumerable arterialized channels (red) ultimately converge onto several common arterial conduits (green), which traverse the neural foramen to supply what is, here and almost ALWAYS, a single hole fistula (brown). For embolization to be curative, the n-BCA (or Onyx) must completely fill the fistula (which, in this case, would be evidenced by visualization of the large epidural venous pouch (pink) on the glue shot
