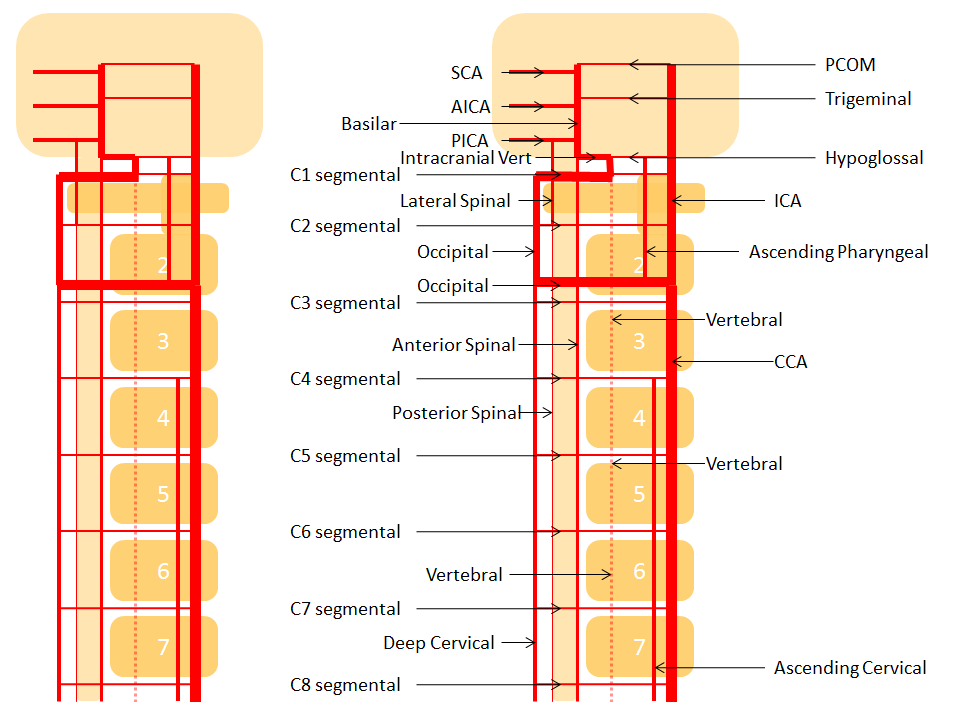
This one is less commonly seen, but is no different than C1 in principle — just a lower origin lateral spinal homolog. Again, it is key to understand that 1) lateral medullary supply will not come from the PICA, but will arise directly from the vert, and 2) the cervical PICA may contribute to supply of the lateral cord as the lateral spinal artery, and so must not be sacrificed without extreme caution. This image shows the C2 radicular branch (red) supplying the lateral spinal artery (white), with the tonsillar PICA segment in purple. Although the whole thing is just called “PICA” it is critical to understand that it is a very special kind of PICA, where the proximal part is made of the radicular C2 branch and the lateral spinal artery.
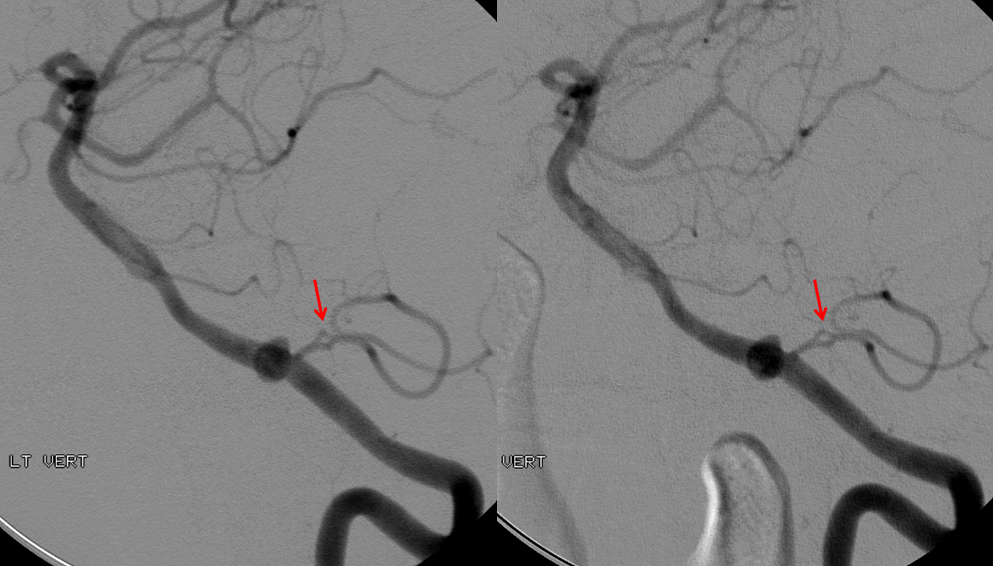
Lateral stereo view shows the lateral medullary perforator (yellow) in location of the classical PICA origin. The arrows point to the same structures.
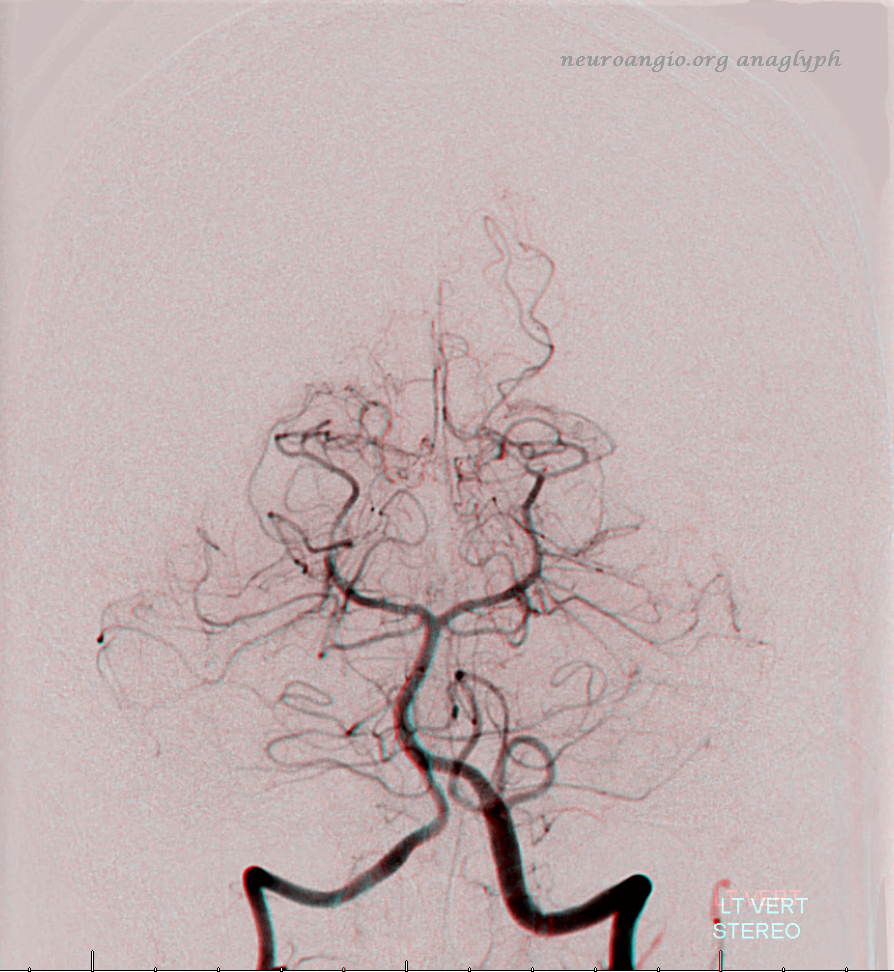
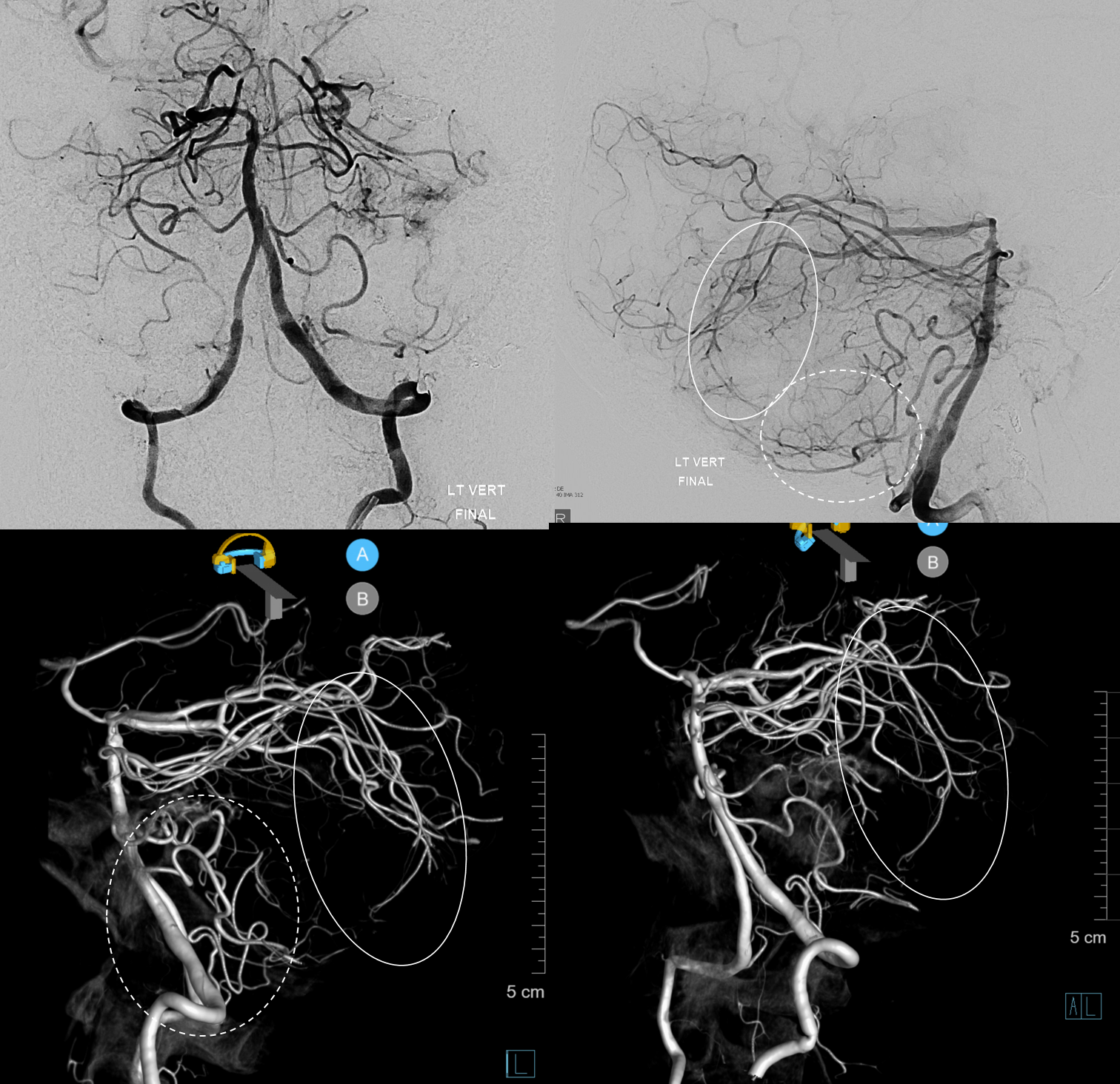
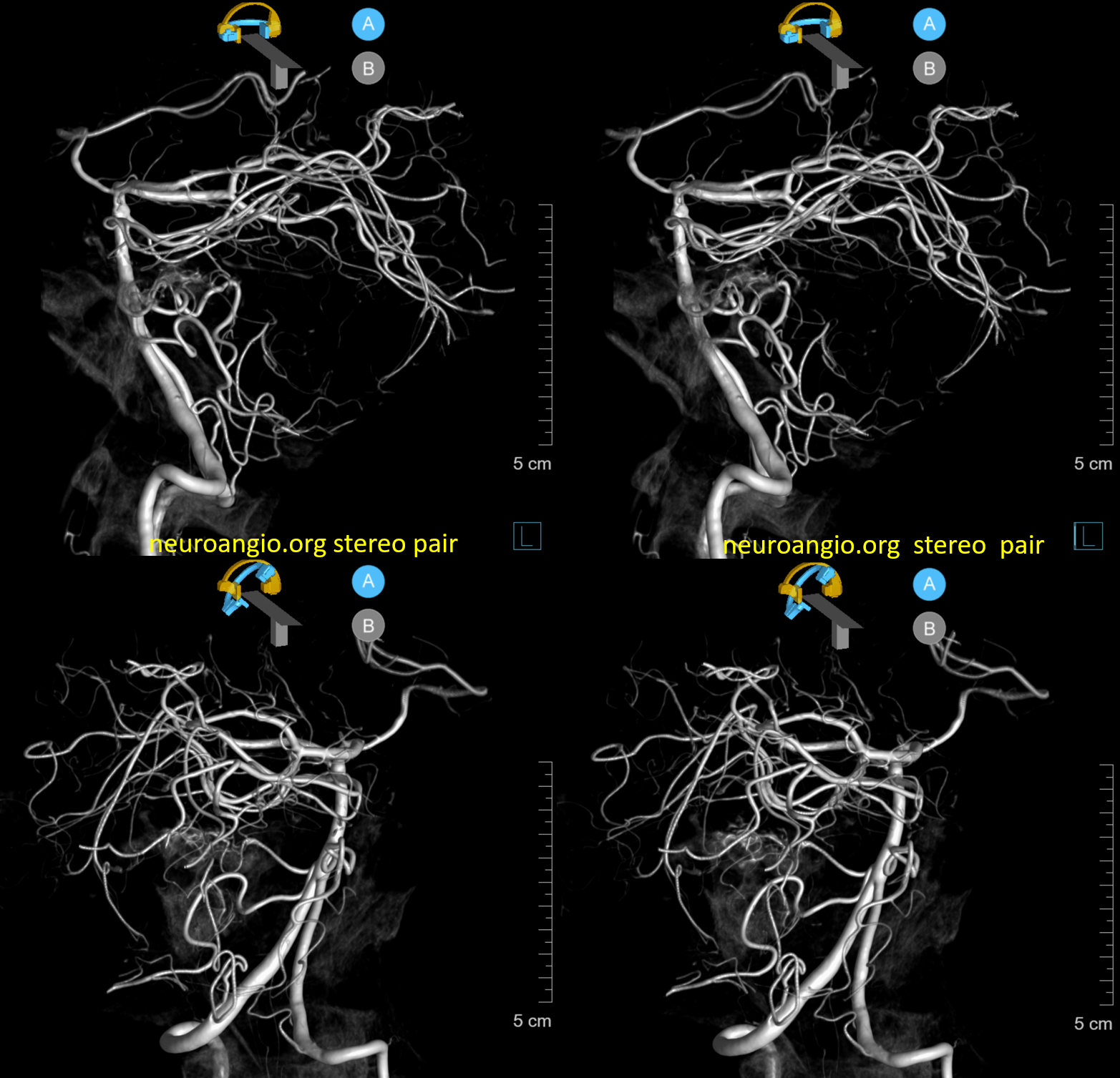
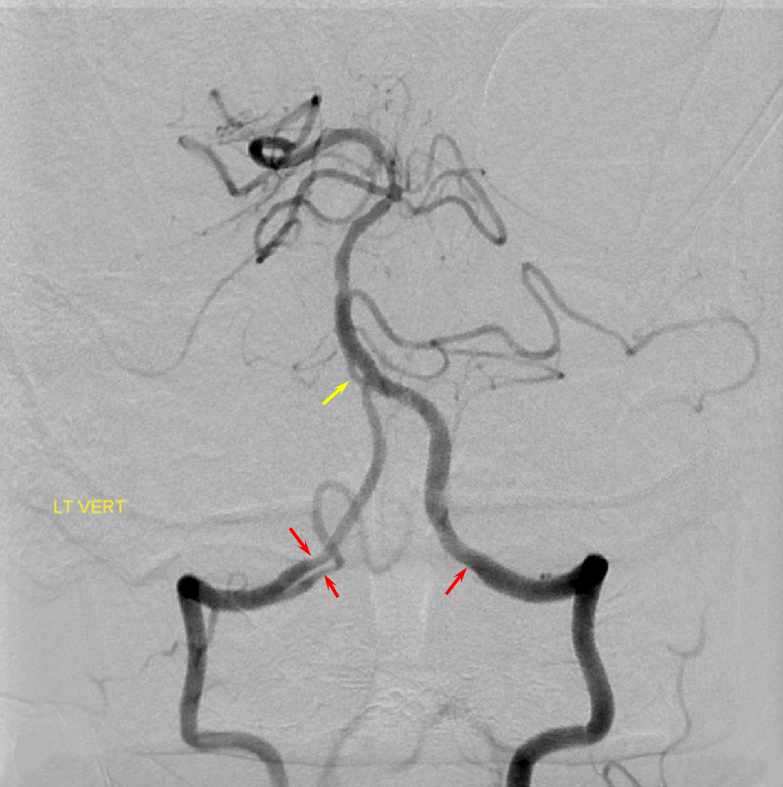
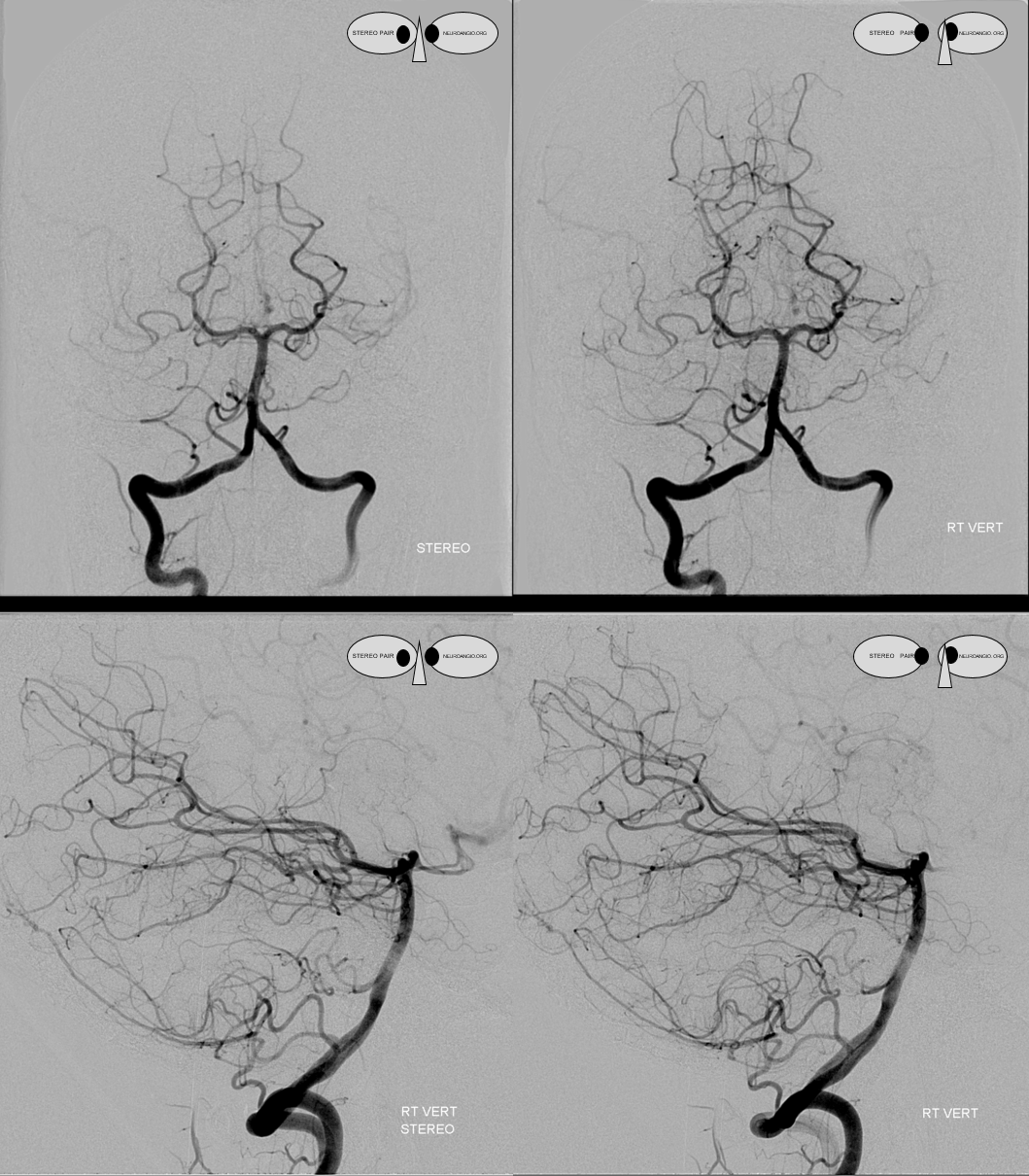
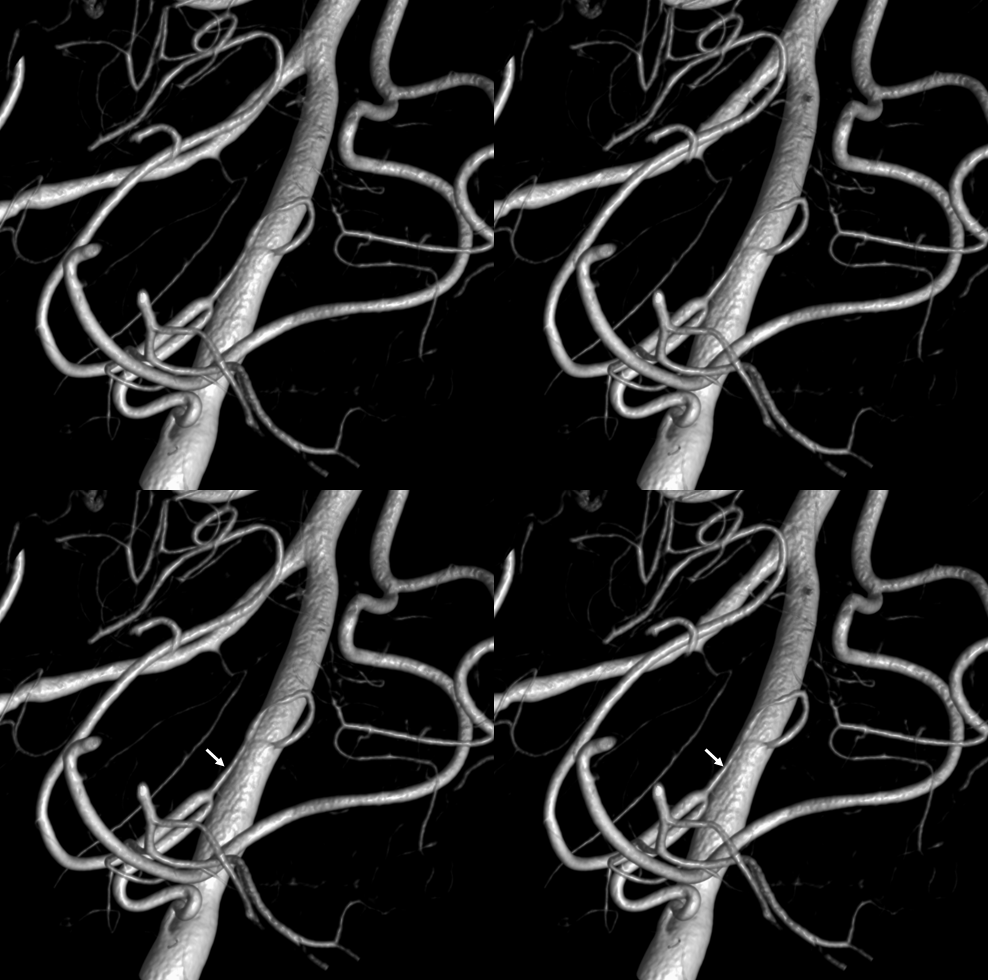
Stereo native image, demonstrating PICA arising below C1, at C2 segmental level
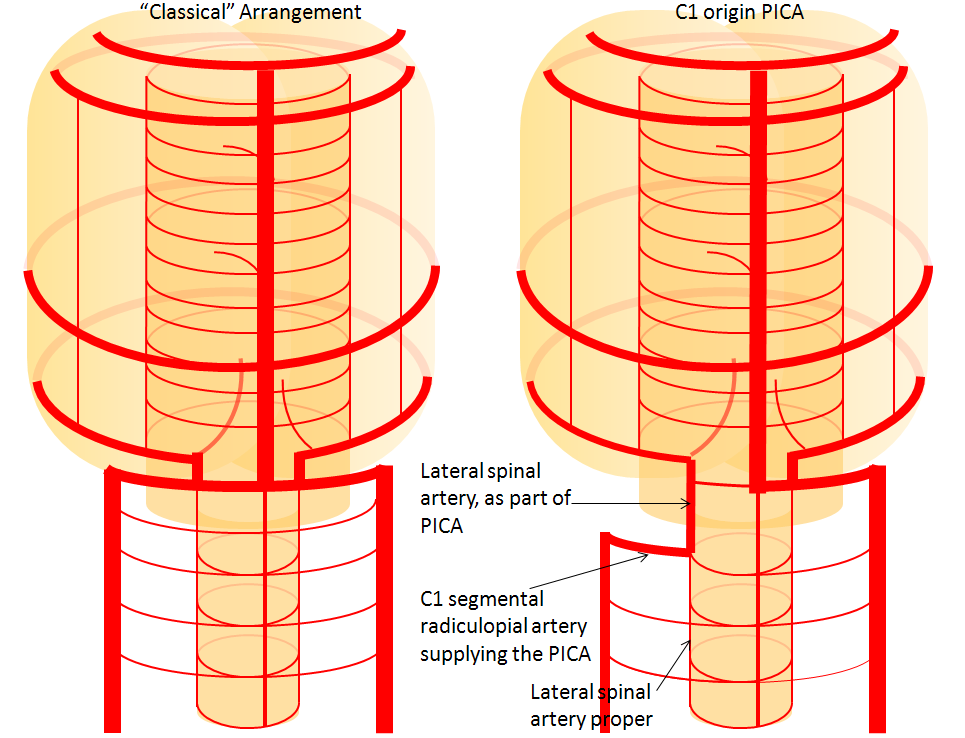
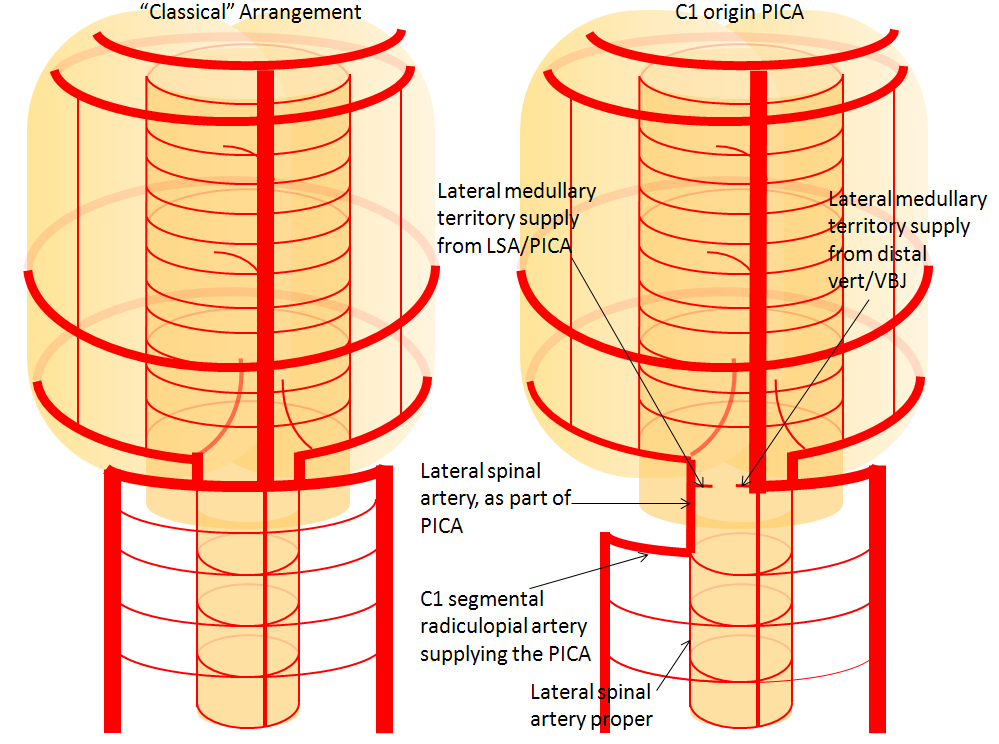
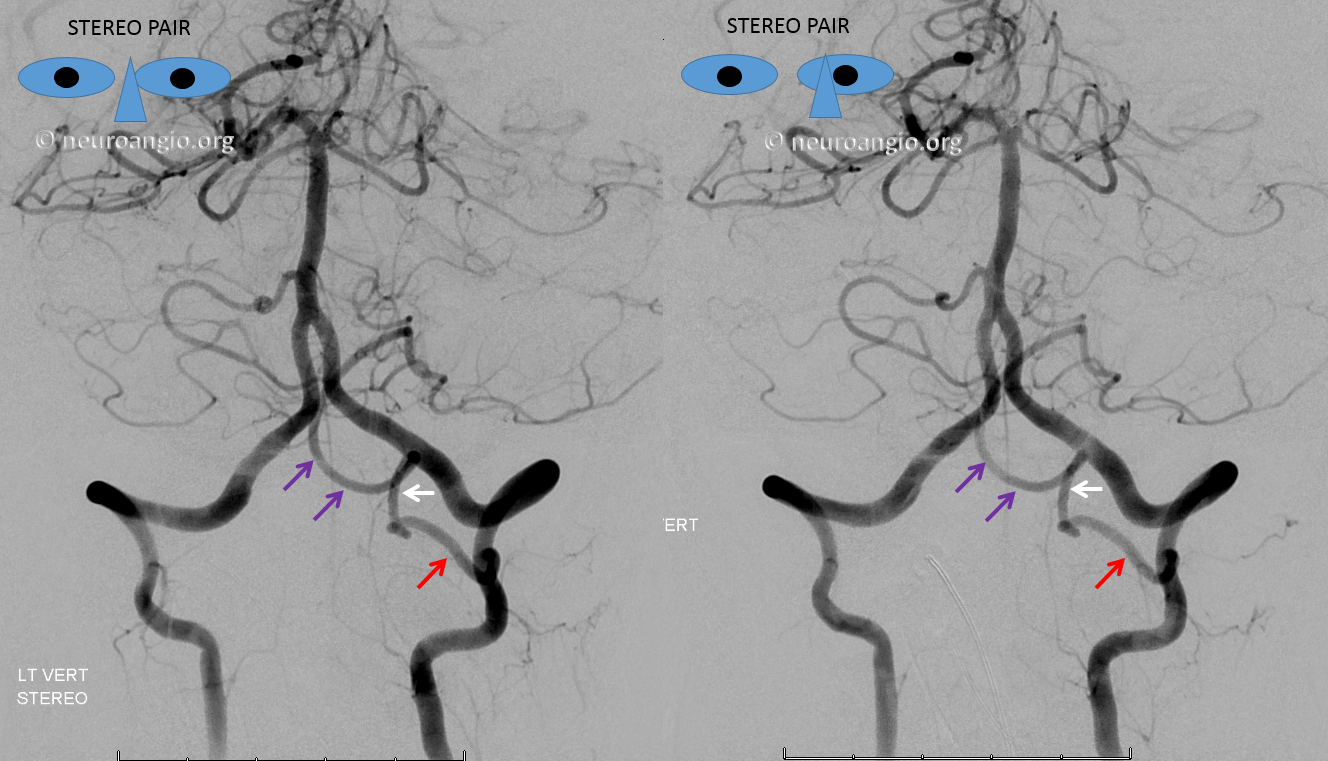
Lateral Spinal Artery / C1 radicular artery / PICA anastomosis
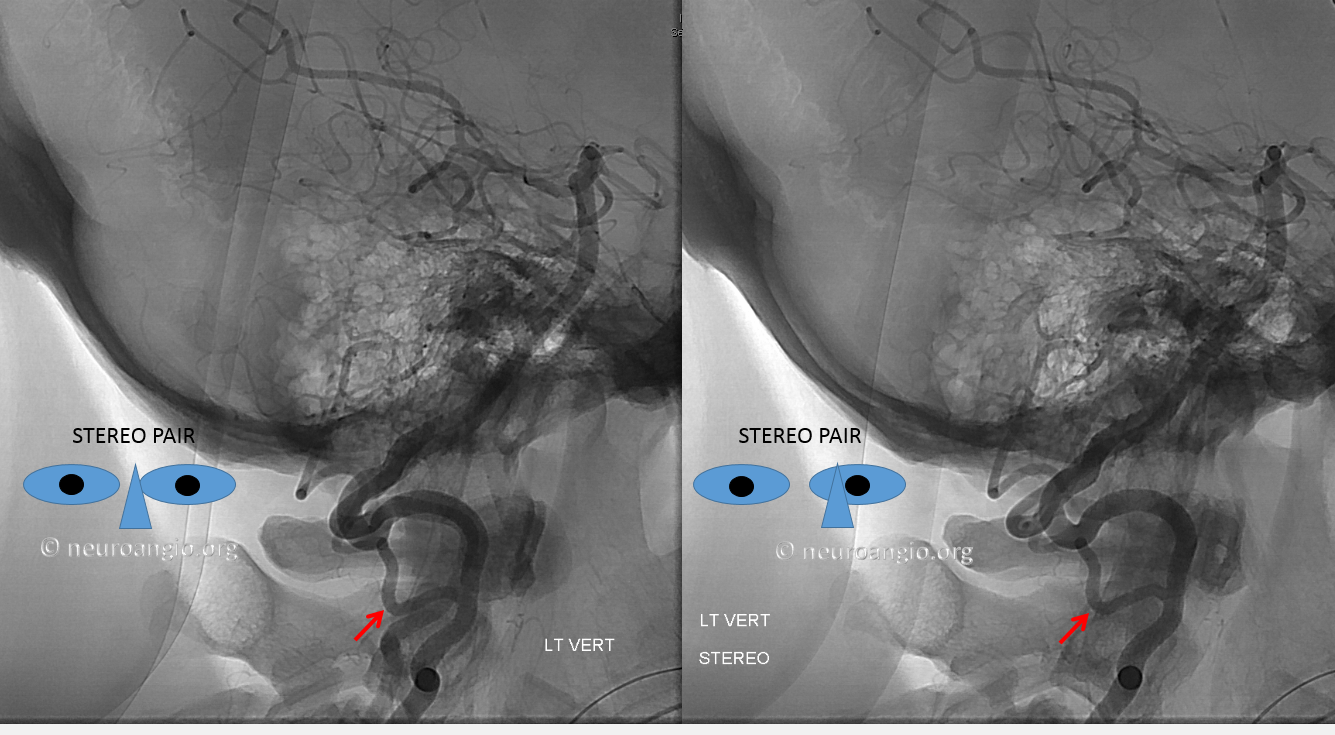
Lateral spinal artery is a pathway by which PICA can be reconstituted in cases of more proximal vertebral occlusion — again recognizing that PICA is a lateral spinal artery homolog — in effect an enlarged lateral spinal artery capturing territory of the cerebellum in addition to the lateral medulla. In this patient, the right vertebral artery is occluded just below the foramen magnum due to dissection. The C1 radiculopial artery (purple) connection to the lateral spinal artery (red) allows for reconstitution of the vermian branch of the PICA (black). The lateral spinal artery inferior to the C1 radiculopial artery is marked with a pink arrow. Notice also presence of the anterior spinal artery (yellow) perfectly contrasting its anterior and medial position to that of the spinal artery. The C1 radicular branch (purple) is in effect the radiculopial artery is acting as a radiculopial artery, homologous to radiculopial supply of the posterior spinal arteries at the thoracic and lumbar levels (see Spinal Arterial Anatomy) The C1 muscular branch (green) opacifies the occipital artery (white) and deep cervical artery (blue).
The indispensible STEREO pair
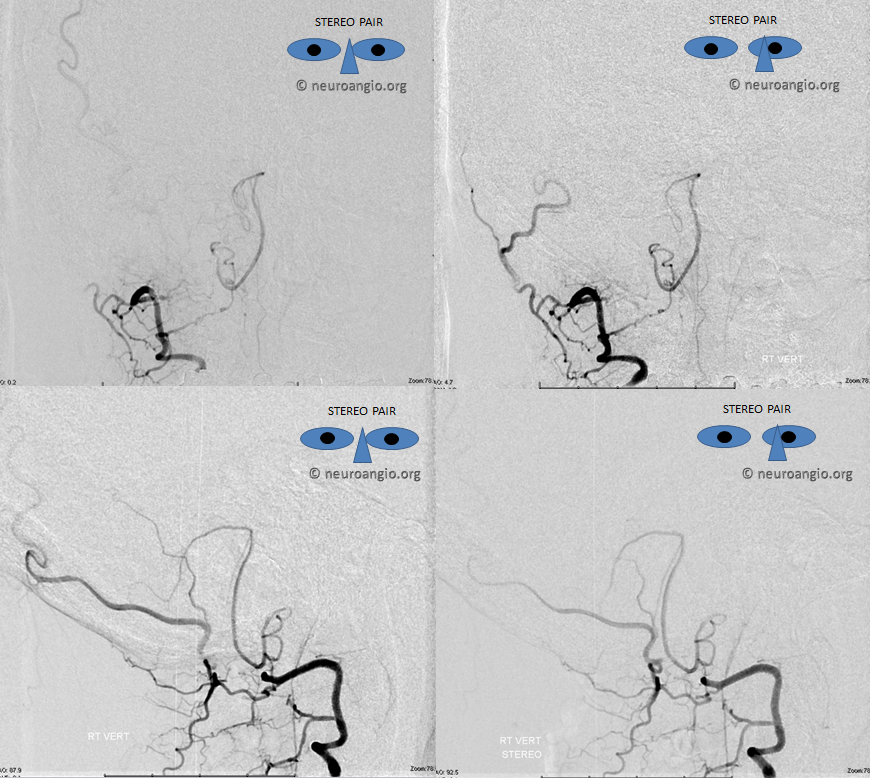
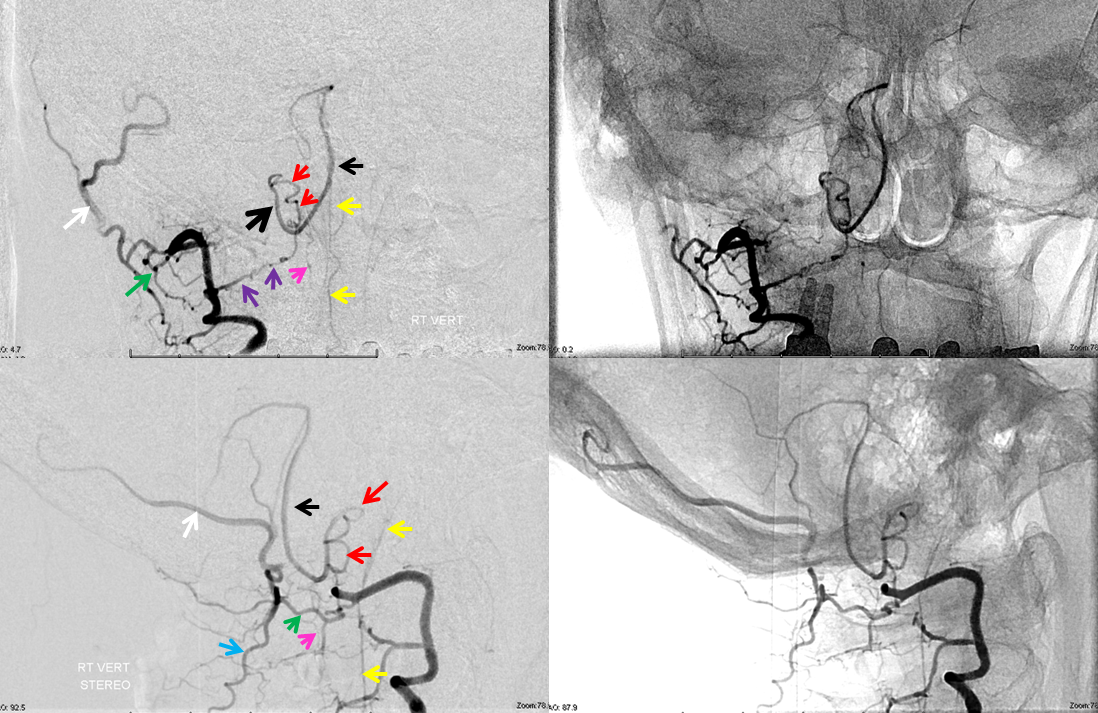
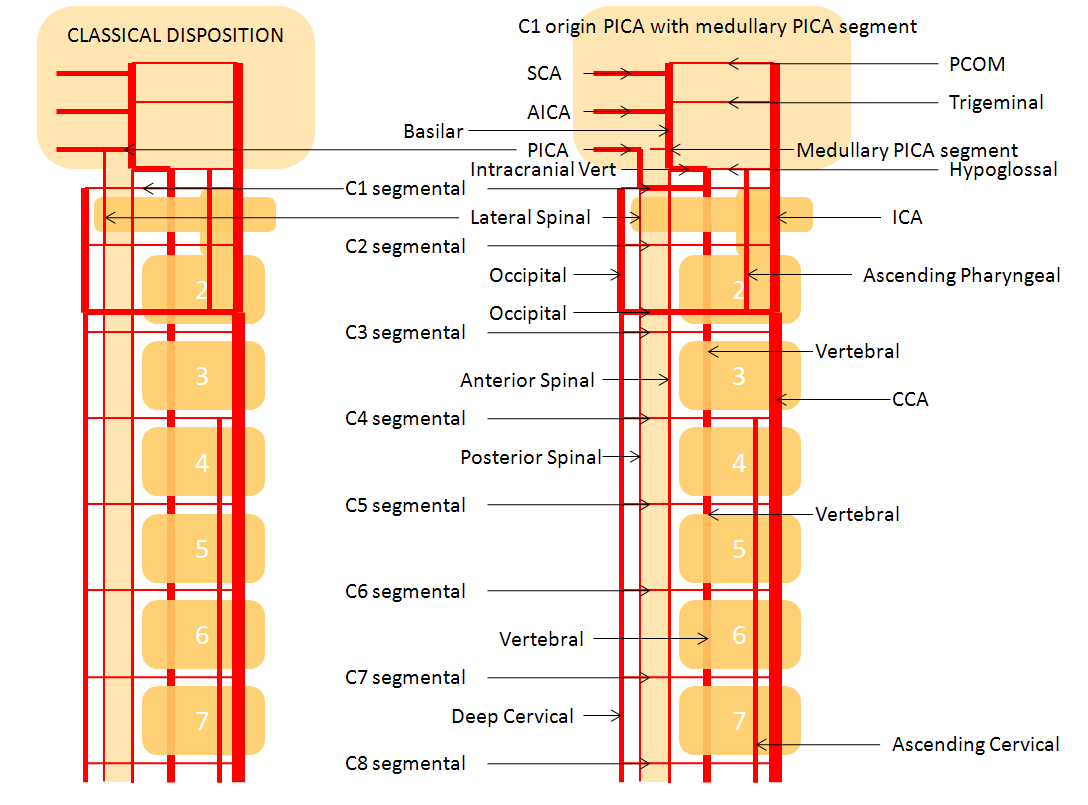
Inferior Extension of the PICA into the Cervical Spinal Canal
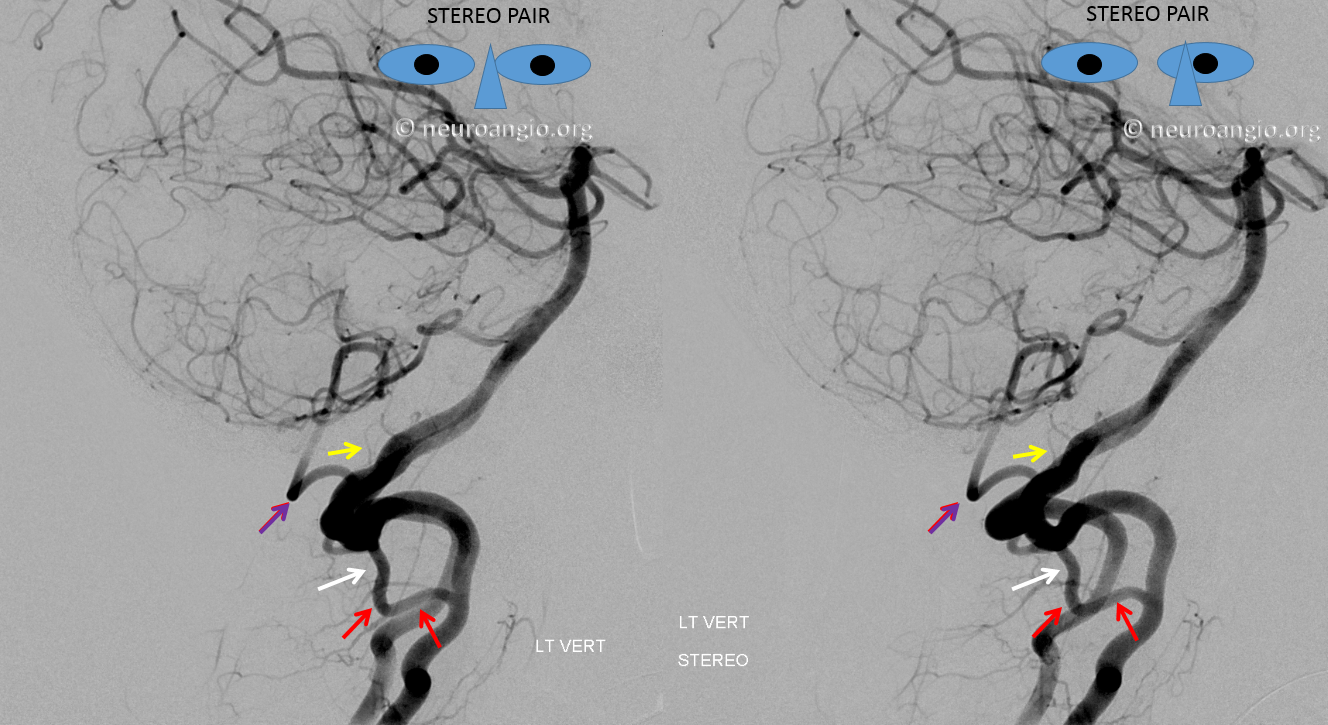
Just as the PICA can have an intradural course with a cervical origin, the same embryonic route can be used for the vessel to loop down into the cervical canal (medullary loop) and then head back up into the head. Here is one such example, in stereo, of bilateral such PICA dispositions.
Native image, stereo:
PICA occlusion and the lateral medullary syndrome
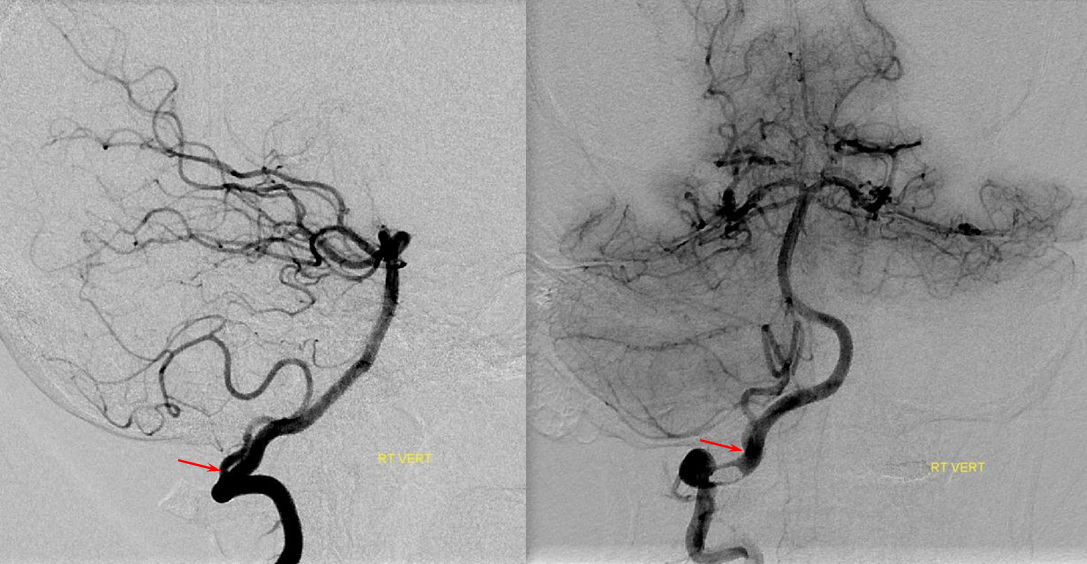
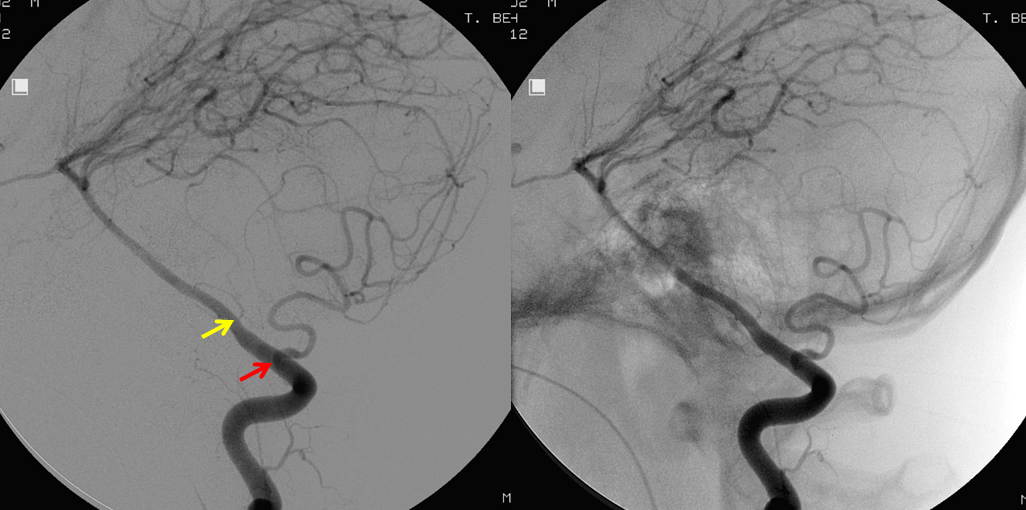
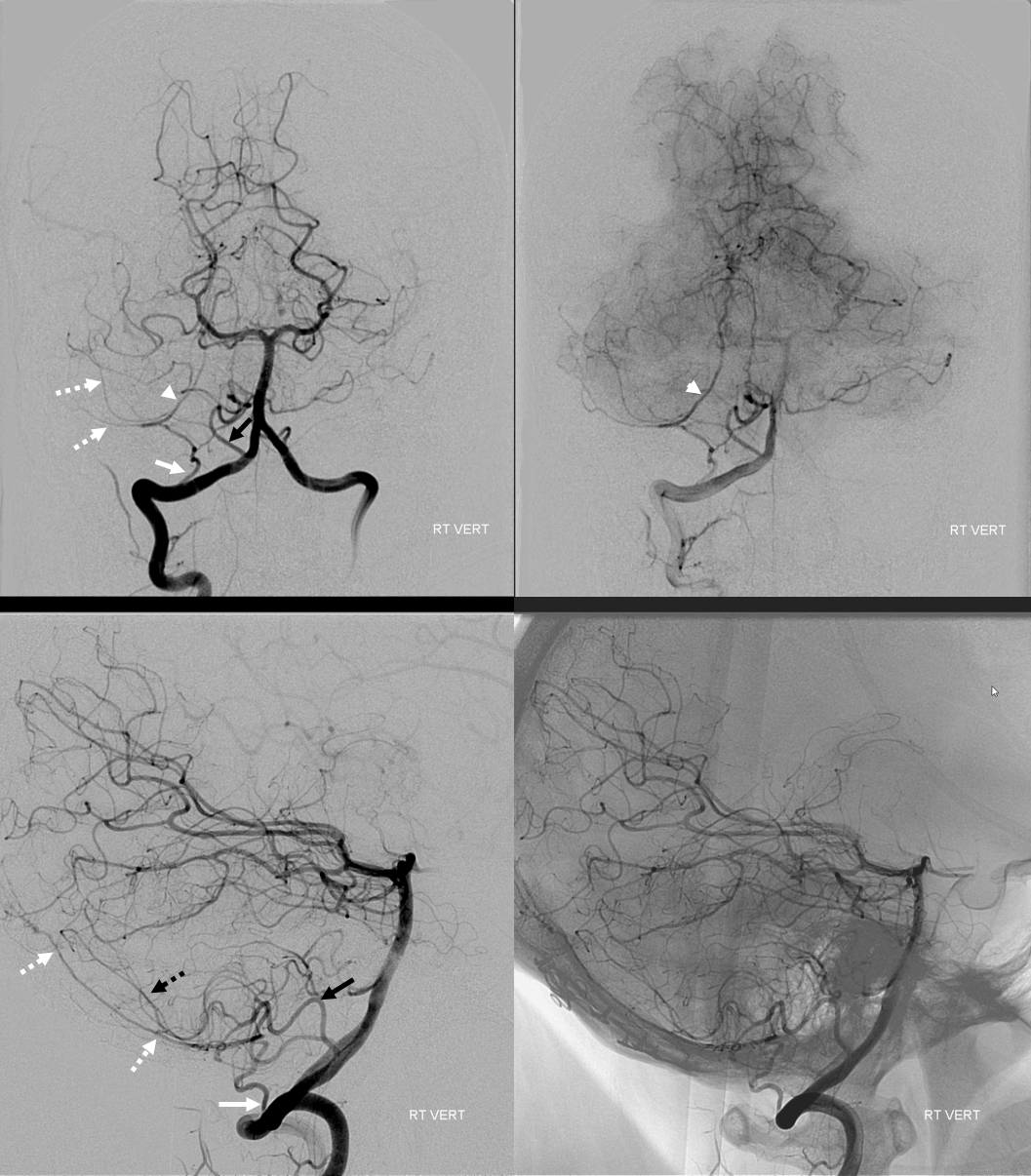
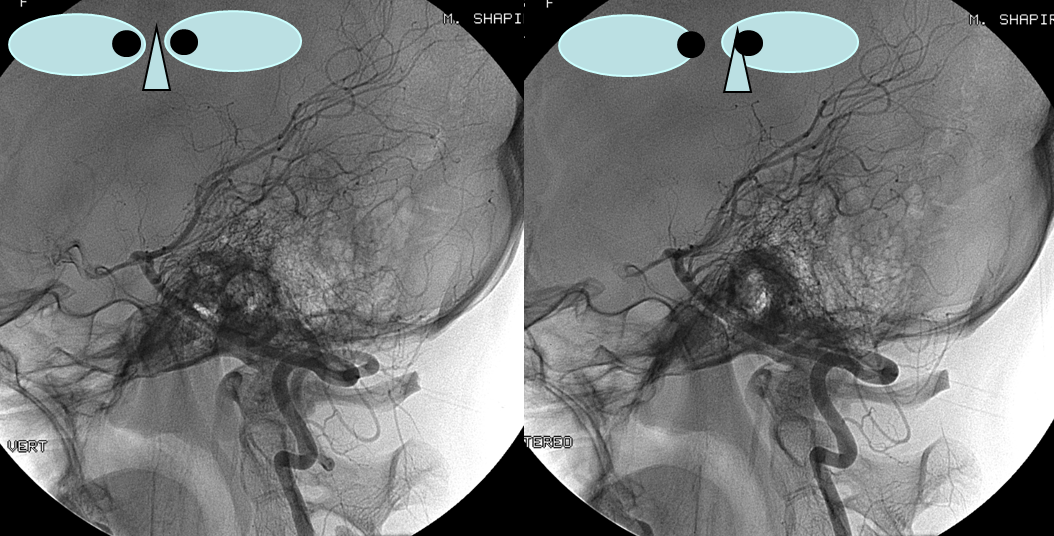
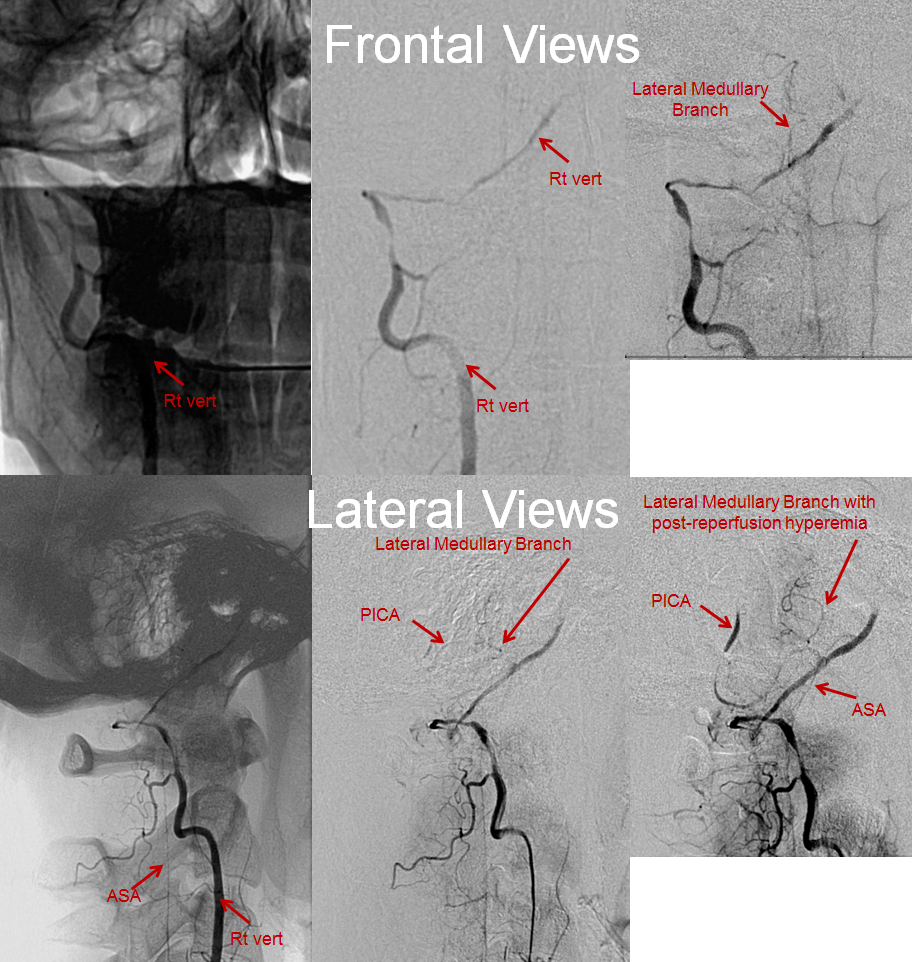
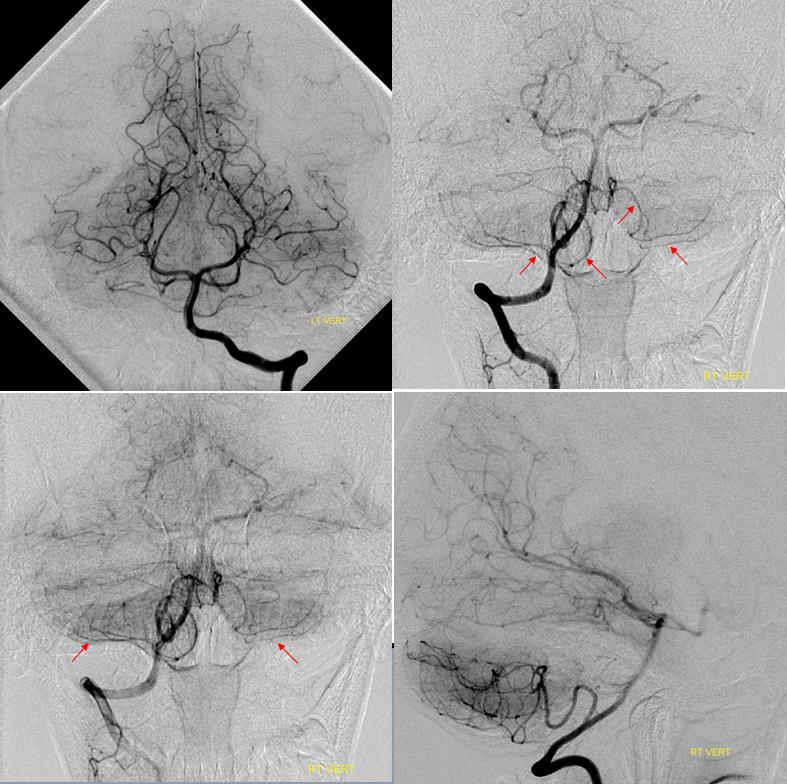
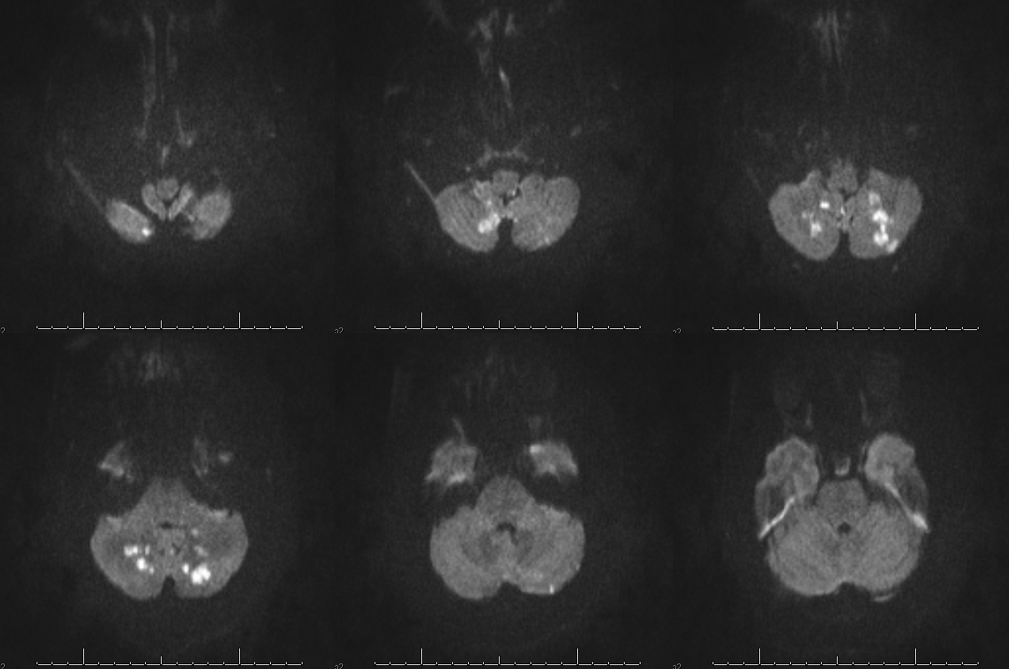
The clinical syndrome of PICA occlusion (Wallenberg and its various partial forms), a.k.a. the lateral medullary syndrome, is a clinical testament to PICA association with the lateral spinal artery. The syndrome is occasioned by occlusion of the PICA ostium (usually by thromboembolus lodging in the vert against PICA origin, or via vertebral dissection). The very proximal medullary portion of the PICA typically sends a lateral medullary branch to the lateral medulla, corresponding to the lateral spinal artery of the cervical spine. Here is a classical lateral medullary MRI/MRA, demonstrating acute infarction and apparent distal vertebral occlusion on MRA
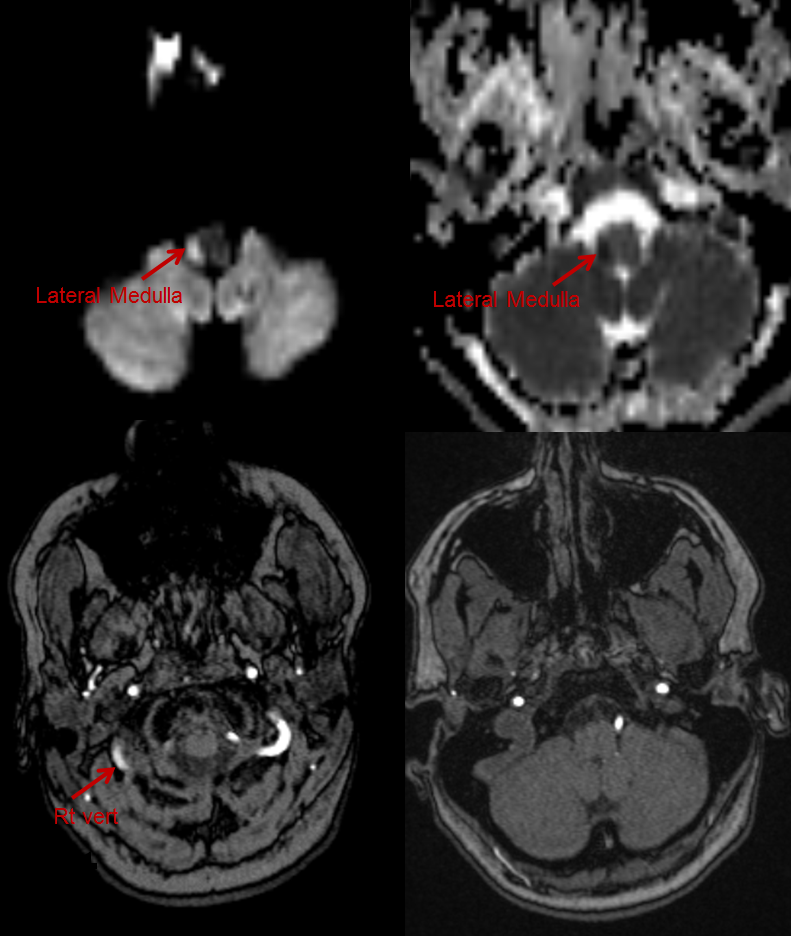
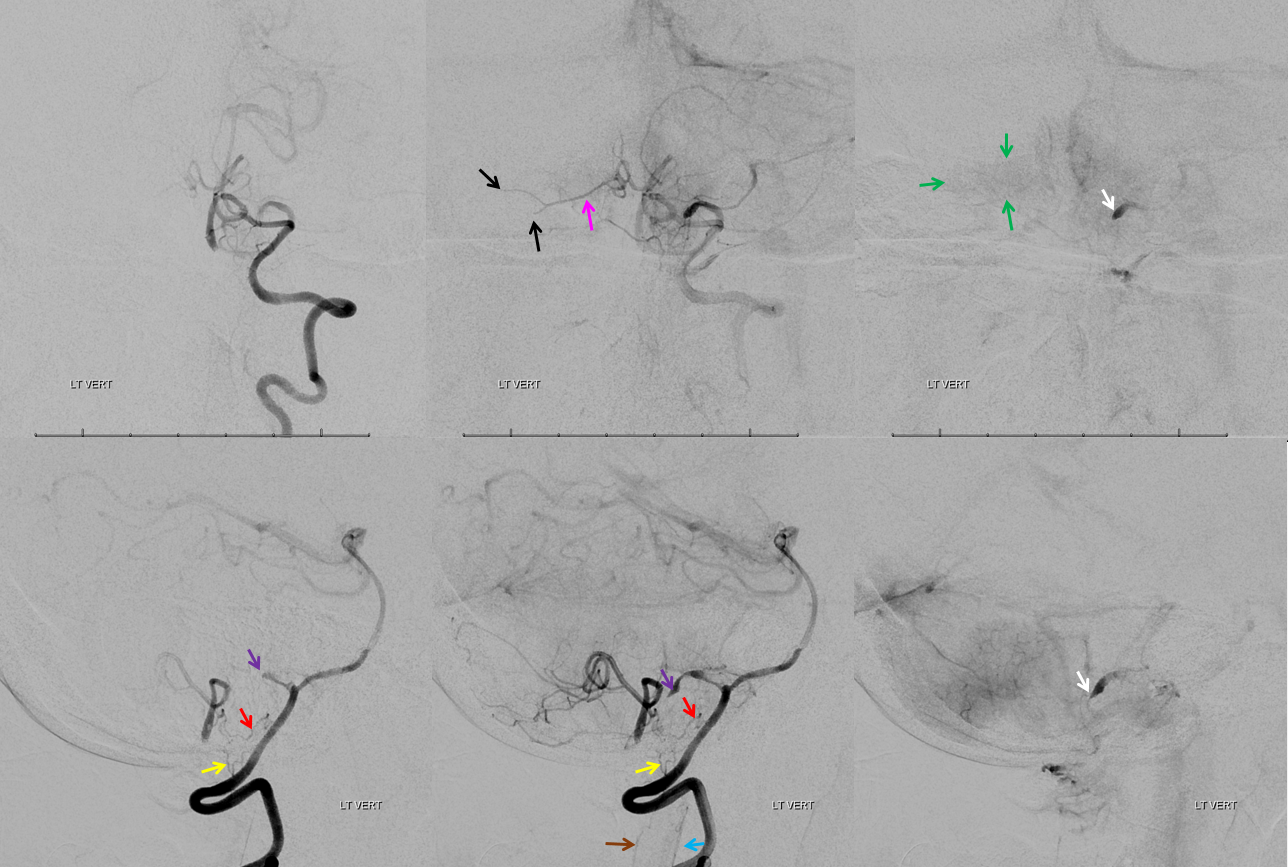
Angiogram of the same patient shows that the vertebral artery is, in fact, open, with a faintly seen stump of the lateral medullary artery on left and center frontal and lateral projection angiographic views. Paired images on the right, post intra-arterial heparin, show more fully the distribution of the lateral medullary artery, with post-reperfusion hyperemia. The patient did very well.
Occipital origin of PICA
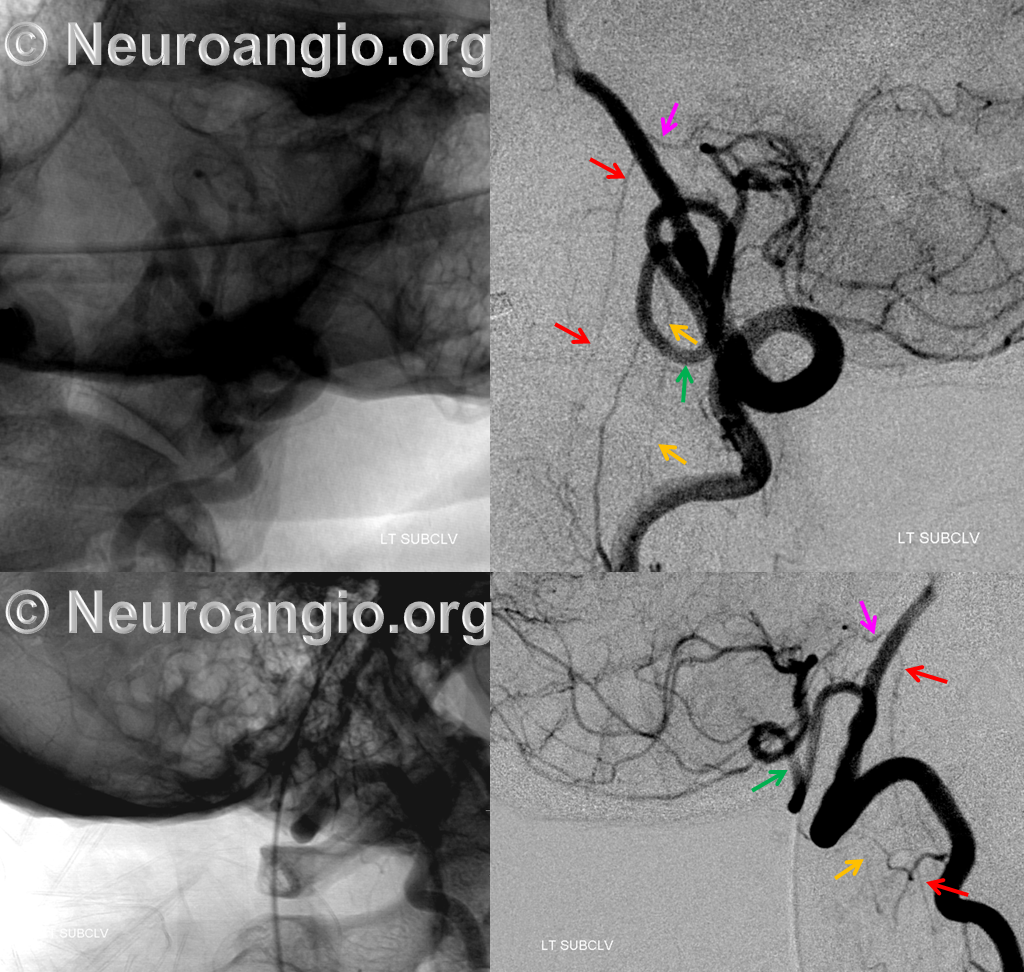
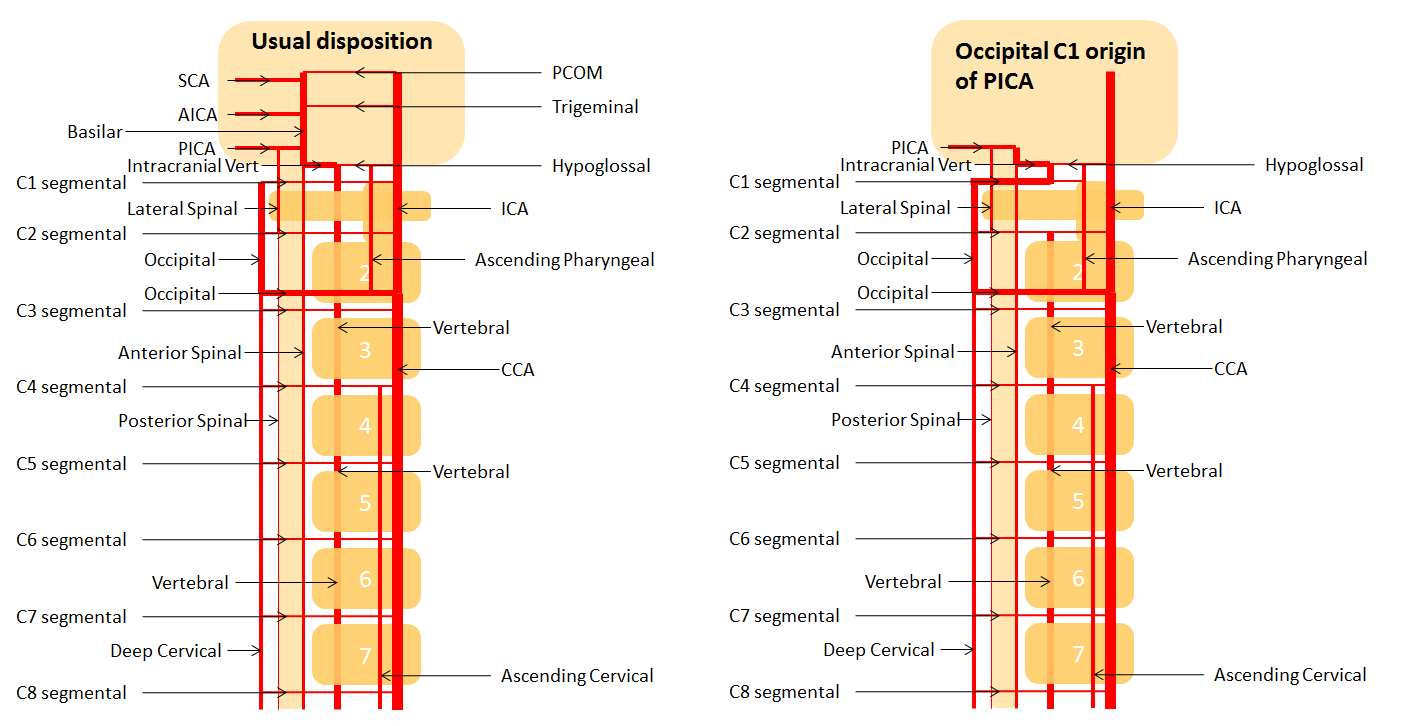
Just as in the above diagrams the extradural origin of PICA is part of a connection between the vert and occipital artery at C1, in the same way the extradural PICA can originate from the occipital artery just as well as it can from the vert. It is far less common, but the important point is that both dispositions are predictable based on the embryonic connections between the occipital and vertebral arteries. In fact, both occipital and vert origins of PICA are, in a way, extremes of a continuum. Functionally we often see reflux of contrast into the vertebrobasilar system during occipital injections, and more frequently C1 origin of the occipital artery from the vert. All of these are possible variations on the theme of transverse/longitudinal organization.
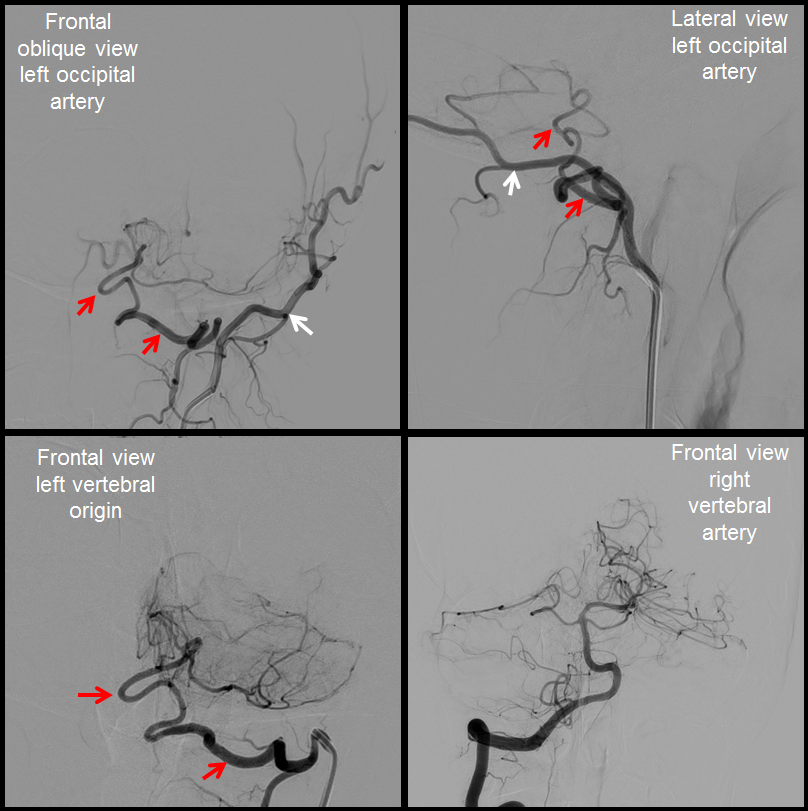
In this case, courtesy of Dr. Antonio López-Rueda from Hospital Clínic i Provincial de Barcelona (alrueda81@hotmail.com), an isolated PICA (red arrows) is opacified via injections of the left occipital artery (white arrows).
A diagram of this disposition is shown below:
Note please that the above case and diagram are exactly the same as that of the Proatlantal artery disposition, except that the PICA is isolated and therefore there is no occpital supply to the basilar. The proatlantal artery is just an occipital artery with a persistent connection to the vertebrobasilar circulation, Type 1 at C1, and Type 2 at C2. Below is a diagram of the C1 type.
AICA-PICA balance
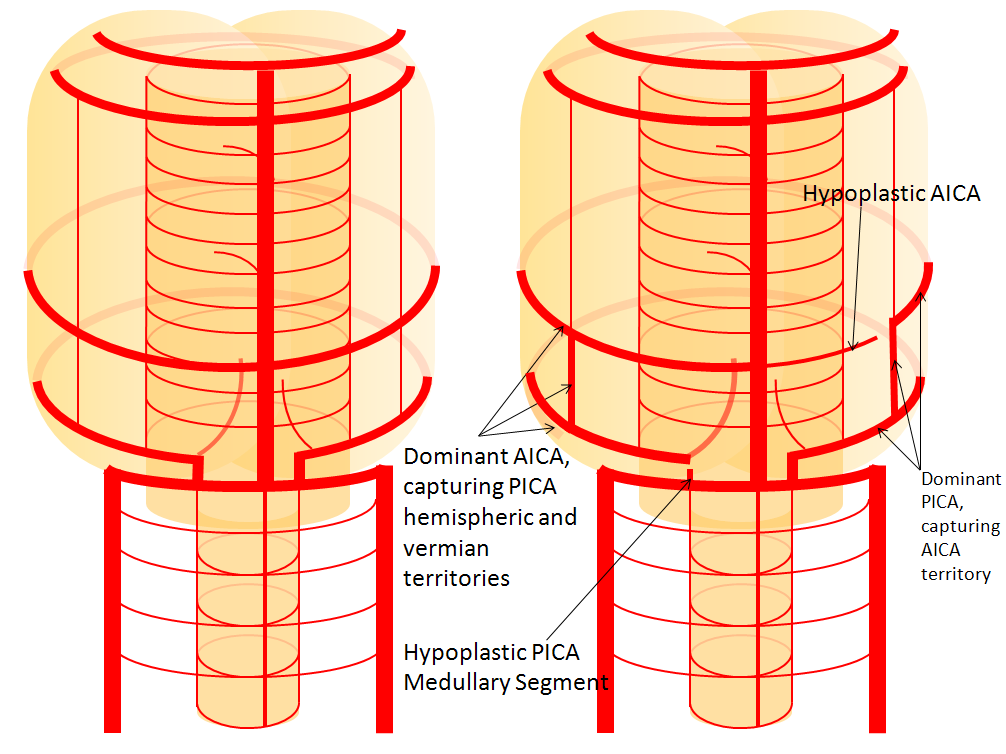
Probably the best known variation of the entire cerebral circulation (excepting, maybe, the fetal PCOM), and perfectly illustrative of the above concepts. In the simplest form, the AICA is big, and PICA is nowhere to be found, and so an “AICA-PICA” is called. The same is not said, however, when the reverse is true, and the AICA is “missing”, where a “PICA-AICA” would be equally appropriate. In fact, there is a continuous distribution (probably Gaussian) in the AICA-PICA balance. Extremes of left and right are represented by apparently “missing” AICA or PICA, whereas the contiuum is reflected in the relative sizes of the two vessels, as required by the relative size of the territories they supply. For example, the AICA may capture more or less of the hemispheric territory of the PICA, or may take over both hemispheric and vermian areas. But there is NEVER a situation where the is no PICA, or no AICA — ALWAYS there is a small PICA, though perhaps too small to be seen on MRA or CTA. When does an AICA get so dominant as to capture the lateral medullary territory of the true PICA — I have never seen a lateral medullary infarct due to AICA occlusion. In the same fasion, when does occlusion of dominant PICA produce a ventral pontine infarction, as would be expected in case of its compete capture of the AICA domain? And, to make things more complicated, there is not infrequently a situation where one PICA takes over the hemispheric and vermian territories of its contalateral homolog, so that one sees a very large ipsilatearal PICA, and hypoplastic contralateral PICA and AICA, as an incidental variation with no clinical deficit. All of these dispositions illustrate highly variabe arrangement in regard to cerebellar vascularization, and relative rigidity in control of the brainstem territory.
In this diagram, the right AICA is dominant and supports PICA hemispheric and vermian territories; only the lateral medullary territory stays constant to a small PICA, which is not often appreciated as such on angio, and even less so on CTA/MRA. But there is ALWAYS some part of the PICA present, even if tiny. On the left, PICA dominance is depicted, with a relatively hypoplastic AICA.
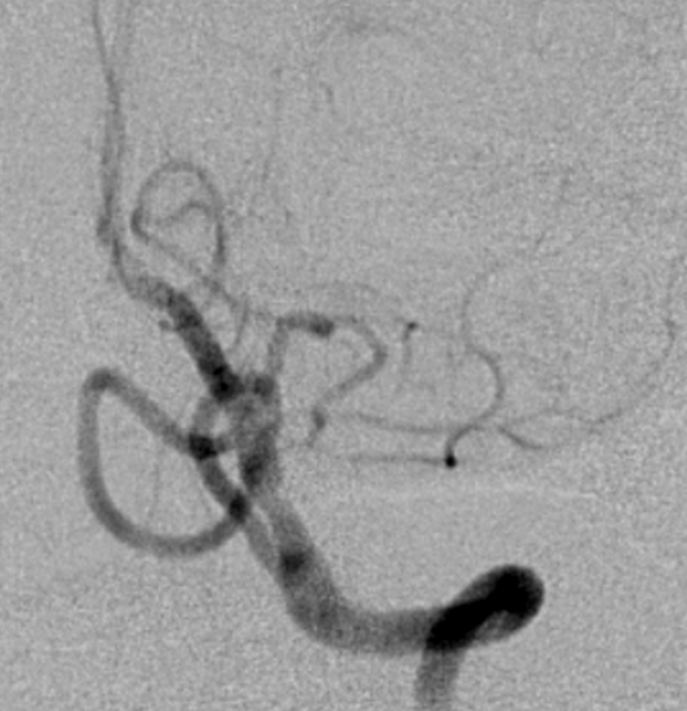
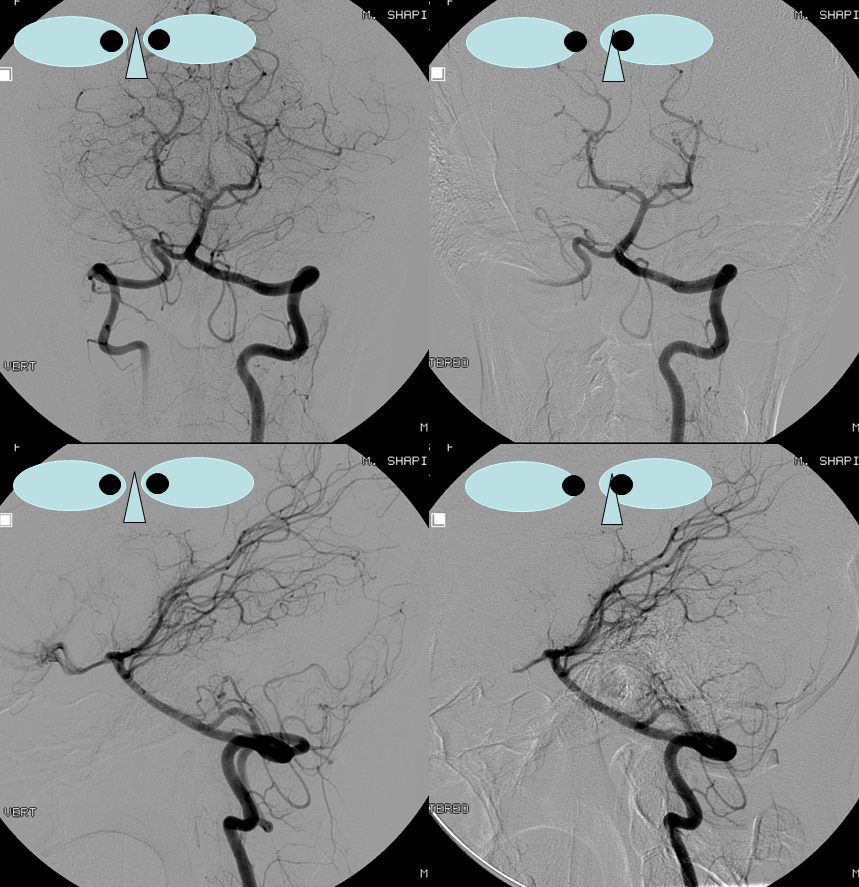
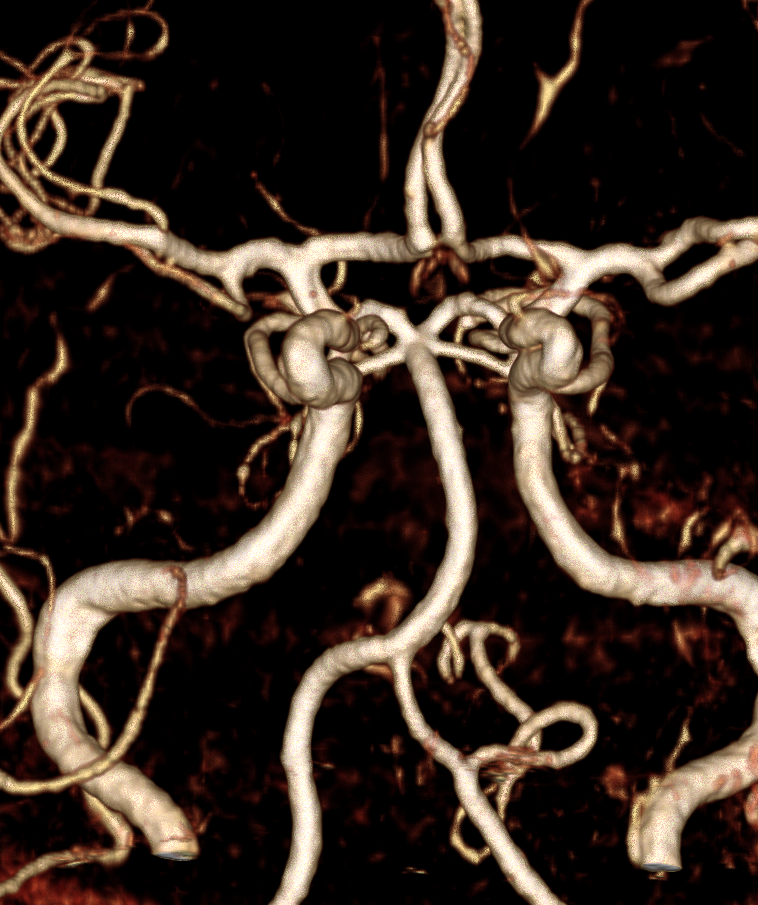
“Azygous” PICA supplying both cerebellar hemispheres
Right PICA (red) supplying both cerebellar hemispheres. The left PICA is not visualized (top left image)





![]() bumper sticker, and everyone here is actually happy
bumper sticker, and everyone here is actually happy